Sources of Variation in Food-Related Metabolites during Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Participants
2.2. Maternal Serum Metabolome Analyses
2.3. Assessment of Dietary Intake
2.4. Non-Dietary Factors
2.5. Statistical Analysis
β0j = γ00 + u0j
β0j = γ00 + u0j
β1j = γ10
β0j = γ00 + u0j
β1j = γ10
β2j = γ20
3. Results
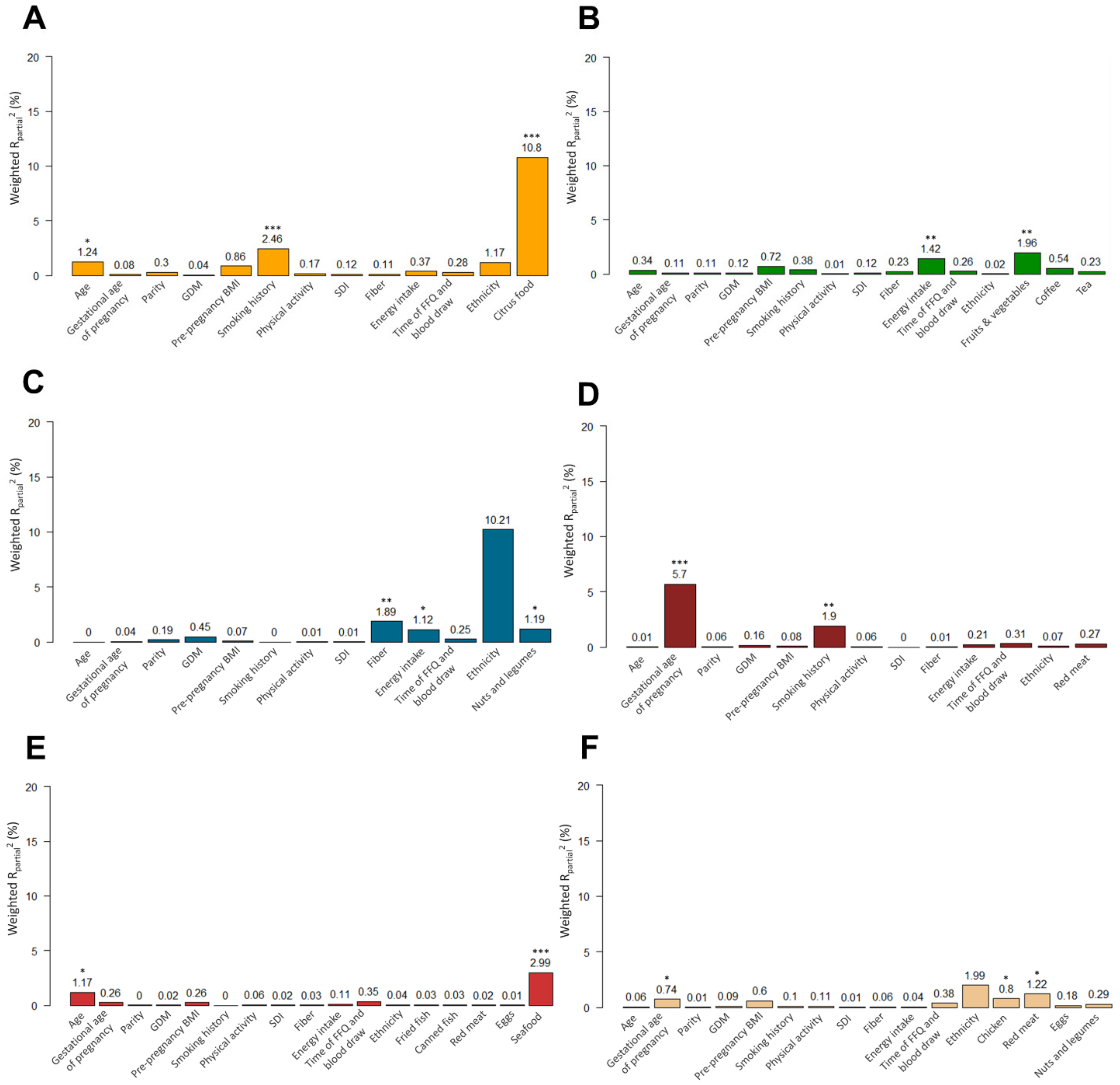
3.1. Association of Dietary and Non-Dietary Factors with Food-Related Metabolites
3.2. Results from PC-PR2 Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kipnis, V.; Midthune, D.; Freedman, L.; Bingham, S.; Day, N.E.; Riboli, E.; Ferrari, P.; Carroll, R.J. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002, 5, 915–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafiq, T.; Azab, S.M.; Teo, K.K.; Thabane, L.; Anand, S.S.; Morrison, K.M.; de Souza, R.J.; Britz-McKibbin, P. Nutritional Metabolomics and the Classification of Dietary Biomarker Candidates: A Critical Review. Adv. Nutr. 2021, 12, 2333–2357. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Freisling, H.; Assi, N.; Zamora-Ros, R.; Achaintre, D.; Affret, A.; Mancini, F.; Boutron-Ruault, M.C.; Flögel, A.; Boeing, H.; et al. Identification of Urinary Polyphenol Metabolite Patterns Associated with Polyphenol-Rich Food Intake in Adults from Four European Countries. Nutrients 2017, 9, 796. [Google Scholar] [CrossRef] [PubMed]
- Cuparencu, C.; Praticó, G.; Hemeryck, L.Y.; Sri Harsha, P.S.C.; Noerman, S.; Rombouts, C.; Xi, M.; Vanhaecke, L.; Hanhineva, K.; Brennan, L.; et al. Biomarkers of meat and seafood intake: An extensive literature review. Genes Nutr. 2019, 14, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, H.M.; Olivier, L.; Wilson, L.A. Urinary biomarkers for assessing dietary exposure to caffeine. Food Addit. Contam. 2001, 18, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int. J. Mol. Sci. 2020, 21, 3688. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; von Ruesten, A.; Drogan, D.; Schulze, M.B.; Prehn, C.; Adamski, J.; Pischon, T.; Boeing, H. Variation of serum metabolites related to habitual diet: A targeted metabolomic approach in EPIC-Potsdam. Eur. J. Clin. Nutr. 2013, 67, 1100–1108. [Google Scholar] [CrossRef]
- Dogan, B.; Karaer, A.; Tuncay, G.; Tecellioglu, N.; Mumcu, A. High-resolution (1)H-NMR spectroscopy indicates variations in metabolomics profile of follicular fluid from women with advanced maternal age. J. Assist. Reprod. Genet. 2020, 37, 321–330. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Donovan, B.M.; Fleener, D.K.; Bedell, B.; Borowski, K.S. Pregnancy-Related Changes of Amino Acid and Acylcarnitine Concentrations: The Impact of Obesity. AJP Rep. 2016, 6, e329–e336. [Google Scholar] [CrossRef] [Green Version]
- Canova, C.; Cantarutti, A. Population-Based Birth Cohort Studies in Epidemiology. Int. J. Environ. Res. Public Health 2020, 17, 5276. [Google Scholar] [CrossRef]
- Li, P.Y.; Zhong, C.R.; Li, S.Z.; Sun, T.P.; Huang, H.; Chen, X.; Zhu, Y.L.; Hu, X.L.; Peng, X.B.; Zhang, X.; et al. Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2018, 108, 603–610. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [Green Version]
- Morrison, K.M.; Atkinson, S.A.; Yusuf, S.; Bourgeois, J.; McDonald, S.; McQueen, M.J.; Persadie, R.; Hunter, B.; Pogue, J.; Teo, K. The Family Atherosclerosis Monitoring In earLY life (FAMILY) study: Rationale, design, and baseline data of a study examining the early determinants of atherosclerosis. Am. Heart J. 2009, 158, 533–539. [Google Scholar] [CrossRef]
- Anand, S.S.; Vasudevan, A.; Gupta, M.; Morrison, K.; Kurpad, A.; Teo, K.K.; Srinivasan, K. Rationale and design of South Asian Birth Cohort (START): A Canada-India collaborative study. BMC Public Health 2013, 13, 79. [Google Scholar] [CrossRef]
- Shanmuganathan, M.; Kroezen, Z.; Gill, B.; Azab, S.; de Souza, R.J.; Teo, K.K.; Atkinson, S.; Subbarao, P.; Desai, D.; Anand, S.S.; et al. The maternal serum metabolome by multisegment injection-capillary electrophoresis-mass spectrometry: A high-throughput platform and standardized data workflow for large-scale epidemiological studies. Nat. Protoc. 2021, 16, 1966–1994. [Google Scholar] [CrossRef]
- DiBattista, A.; McIntosh, N.; Lamoureux, M.; Al-Dirbashi, O.Y.; Chakraborty, P.; Britz-McKibbin, P. Metabolic Signatures of Cystic Fibrosis Identified in Dried Blood Spots For Newborn Screening Without Carrier Identification. J. Proteome Res. 2019, 18, 841–854. [Google Scholar] [CrossRef] [Green Version]
- de Souza, R.J.; Shanmuganathan, M.; Lamri, A.; Atkinson, S.A.; Becker, A.; Desai, D.; Gupta, M.; Mandhane, P.J.; Moraes, T.J.; Morrison, K.M.; et al. Maternal Diet and the Serum Metabolome in Pregnancy: Robust Dietary Biomarkers Generalizable to a Multiethnic Birth Cohort. Curr. Dev. Nutr. 2020, 4, nzaa144. [Google Scholar] [CrossRef]
- Wellington, N.; Shanmuganathan, M.; de Souza, R.J.; Zulyniak, M.A.; Azab, S.; Bloomfield, J.; Mell, A.; Ly, R.; Desai, D.; Anand, S.S.; et al. Metabolic Trajectories Following Contrasting Prudent and Western Diets from Food Provisions: Identifying Robust Biomarkers of Short-Term Changes in Habitual Diet. Nutrients 2019, 11, 2407. [Google Scholar] [CrossRef] [Green Version]
- Azab, S.; Ly, R.; Britz-McKibbin, P. Robust Method for High-Throughput Screening of Fatty Acids by Multisegment Injection-Nonaqueous Capillary Electrophoresis-Mass Spectrometry with Stringent Quality Control. Anal. Chem. 2019, 91, 2329–2336. [Google Scholar] [CrossRef]
- Azab, S.M.; de Souza, R.J.; Ly, R.; Teo, K.K.; Atkinson, S.A.; Morrison, K.M.; Anand, S.S.; Britz-McKibbin, P. Non-esterified fatty acids as biomarkers of diet and glucose homeostasis in pregnancy: The impact of fatty acid reporting methods: NEFA reporting methods affect dietary and cardiometabolic endpoints. Prostaglandins Leukot. Essent. Fat. Acids 2022, 176, 102378. [Google Scholar] [CrossRef]
- Kelemen, L.E.; Anand, S.S.; Vuksan, V.; Yi, Q.; Teo, K.K.; Devanesen, S.; Yusuf, S. Development and evaluation of cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J. Am. Diet. Assoc. 2003, 103, 1178–1184. [Google Scholar] [CrossRef]
- Chevli, P.A.; Hari, K.J.; Kanaya, A.M.; Talegawkar, S.A.; Needham, B.L.; Herrington, D. Association of Alcohol Consumption and Ideal Cardiovascular Health Among South Asians: The Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study. Alcohol. Clin. Exp. Res. 2020, 44, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Talegawkar, S.A.; Kandula, N.R.; Gadgil, M.D.; Desai, D.; Kanaya, A.M. Dietary intakes among South Asian adults differ by length of residence in the USA. Public Health Nutr. 2016, 19, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S–1231S. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Razak, F.; Davis, A.D.; Jacobs, R.; Vuksan, V.; Teo, K.; Yusuf, S. Social disadvantage and cardiovascular disease: Development of an index and analysis of age, sex, and ethnicity effects. Int. J. Epidemiol. 2006, 35, 1239–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudenbush, S.W.; Bryk, A.S. Hierarchical Linear Models: Applications and Data Analysis Methods, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2002; Volume 1. [Google Scholar]
- Heo, M.; Faith, M.S.; Mott, J.W.; Gorman, B.S.; Redden, D.T.; Allison, D.B. Hierarchical linear models for the development of growth curves: An example with body mass index in overweight/obese adults. Stat. Med. 2003, 22, 1911–1942. [Google Scholar] [CrossRef]
- Feldman, H.A. Families of lines: Random effects in linear regression analysis. J. Appl. Physiol. 1988, 64, 1721–1732. [Google Scholar] [CrossRef]
- Raftery, A.E. Bayesian Model Selection in Social Research. Sociol. Methodol. 1995, 25, 111–163. [Google Scholar] [CrossRef]
- Fages, A.; Ferrari, P.; Monni, S.; Dossus, L.; Floegel, A.; Mode, N.; Johansson, M.; Travis, R.C.; Bamia, C.; Sanchez-Perez, M.J.; et al. Investigating sources of variability in metabolomic data in the EPIC study: The Principal Component Partial R-square (PC-PR2) method. Metabolomics 2014, 10, 1074–1083. [Google Scholar] [CrossRef]
- Crozier, S.R.; Robinson, S.M.; Borland, S.E.; Godfrey, K.M.; Cooper, C.; Inskip, H.M. Do women change their health behaviours in pregnancy? Findings from the Southampton Women’s Survey. Paediatr. Perinat. Epidemiol. 2009, 23, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Skreden, M.; Bere, E.; Sagedal, L.R.; Vistad, I.; Øverby, N.C. Changes in fruit and vegetable consumption habits from pre-pregnancy to early pregnancy among Norwegian women. BMC Pregnancy Childbirth 2017, 17, 107. [Google Scholar] [CrossRef] [Green Version]
- Morrow, J.D.; Frei, B.; Longmire, A.W.; Gaziano, J.M.; Lynch, S.M.; Shyr, Y.; Strauss, W.E.; Oates, J.A.; Roberts, L.J., II. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995, 332, 1198–1203. [Google Scholar] [CrossRef]
- Eiserich, J.P.; van der Vliet, A.; Handelman, G.J.; Halliwell, B.; Cross, C.E. Dietary antioxidants and cigarette smoke-induced biomolecular damage: A complex interaction. Am. J. Clin. Nutr. 1995, 62, 1490S–1500S. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Ocké, M.C.; Smit, H.A.; Seidell, J.C. Do dietary and supplementary intakes of antioxidants differ with smoking status? Int. J. Epidemiol. 1996, 25, 70–79. [Google Scholar] [CrossRef]
- Marangon, K.; Herbeth, B.; Lecomte, E.; Paul-Dauphin, A.; Grolier, P.; Chancerelle, Y.; Artur, Y.; Siest, G. Diet, antioxidant status, and smoking habits in French men. Am. J. Clin. Nutr. 1998, 67, 231–239. [Google Scholar] [CrossRef]
- Wang, M.; Shu, Z.J.; Wang, Y.; Peng, W. Stachydrine hydrochloride inhibits proliferation and induces apoptosis of breast cancer cells via inhibition of Akt and ERK pathways. Am. J. Transl. Res. 2017, 9, 1834–1844. [Google Scholar]
- Cao, T.T.; Chen, H.H.; Dong, Z.; Xu, Y.W.; Zhao, P.; Guo, W.; Wei, H.C.; Zhang, C.; Lu, R. Stachydrine Protects Against Pressure Overload-Induced Cardiac Hypertrophy by Suppressing Autophagy. Cell. Physiol. Biochem. 2017, 42, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Heinzmann, S.S.; Brown, I.J.; Chan, Q.; Bictash, M.; Dumas, M.E.; Kochhar, S.; Stamler, J.; Holmes, E.; Elliott, P.; Nicholson, J.K. Metabolic profiling strategy for discovery of nutritional biomarkers: Proline betaine as a marker of citrus consumption. Am. J. Clin. Nutr. 2010, 92, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Hudgins, L.C.; Hellerstein, M.; Seidman, C.; Neese, R.; Diakun, J.; Hirsch, J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Investig. 1996, 97, 2081–2091. [Google Scholar] [CrossRef]
- Hellerstein, M.K.; Schwarz, J.M.; Neese, R.A. Regulation of hepatic de novo lipogenesis in humans. Annu. Rev. Nutr. 1996, 16, 523–557. [Google Scholar] [CrossRef]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid. Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Ratnayake, W.M. Concerns about the use of 15:0, 17:0, and trans-16:1n-7 as biomarkers of dairy fat intake in recent observational studies that suggest beneficial effects of dairy food on incidence of diabetes and stroke. Am. J. Clin. Nutr. 2015, 101, 1102–1103. [Google Scholar] [CrossRef] [PubMed]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv. Nutr. 2016, 7, 730–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepsch, J.; Farias, D.R.; Vaz Jdos, S.; de Jesus Pereira Pinto, T.; da Silva Lima, N.; Freitas Vilela, A.A.; Cunha, M.; Factor-Litvak, P.; Kac, G. Serum saturated fatty acid decreases plasma adiponectin and increases leptin throughout pregnancy independently of BMI. Nutrition 2016, 32, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, O.; Santaniemi, M. Adiponectin: A link between excess adiposity and associated comorbidities? J. Mol. Med. 2002, 80, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Aglago, E.K.; Biessy, C.; Torres-Mejía, G.; Angeles-Llerenas, A.; Gunter, M.J.; Romieu, I.; Chajès, V. Association between serum phospholipid fatty acid levels and adiposity in Mexican women. J. Lipid Res. 2017, 58, 1462–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaw, K.T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N.; Khaw, K.-T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.E. Carnitine metabolism in human subjects. I. Normal metabolism. Am. J. Clin. Nutr. 1978, 31, 293–306. [Google Scholar] [CrossRef]
- Talián, G.C.; Komlósi, K.; Decsi, T.; Koletzko, B.; Melegh, B. Determination of carnitine ester patterns during the second half of pregnancy, at delivery, and in neonatal cord blood by tandem mass spectrometry: Complex and dynamic involvement of carnitine in the intermediary metabolism. Pediatr. Res. 2007, 62, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Oey, N.A.; van Vlies, N.; Wijburg, F.A.; Wanders, R.J.; Attie-Bitach, T.; Vaz, F.M. L-carnitine is synthesized in the human fetal-placental unit: Potential roles in placental and fetal metabolism. Placenta 2006, 27, 841–846. [Google Scholar] [CrossRef]
- Grube, M.; Schwabedissen, H.M.Z.; Draber, K.; Präger, D.; Möritz, K.U.; Linnemann, K.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Expression, localization, and function of the carnitine transporter octn2 (slc22a5) in human placenta. Drug Metab. Dispos. 2005, 33, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Van Duynhoven, J.; van der Hooft, J.J.; van Dorsten, F.A.; Peters, S.; Foltz, M.; Gomez-Roldan, V.; Vervoort, J.; de Vos, R.C.; Jacobs, D.M. Rapid and sustained systemic circulation of conjugated gut microbial catabolites after single-dose black tea extract consumption. J. Proteome Res. 2014, 13, 2668–2678. [Google Scholar] [CrossRef]
- Williams, H.R.T.; Cox, I.J.; Walker, D.G.; Cobbold, J.F.L.; Taylor-Robinson, S.D.; Marshall, S.E.; Orchard, T.R. Differences in gut microbial metabolism are responsible for reduced hippurate synthesis in Crohn’s disease. BMC Gastroenterol. 2010, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ivey, K.L.; Wang, D.D.; Wilkinson, J.E.; Franke, A.; Lee, K.H.; Chan, A.; Huttenhower, C.; Hu, F.B.; et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: Findings from a longitudinal cohort of US men. Gut 2021, 71, 724–733. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Kärkkäinen, O.; Borewicz, K.; Zarei, I.; Jokkala, J.; Micard, V.; Rosa-Sibakov, N.; Auriola, S.; Aura, A.M.; Smidt, H.; et al. Contribution of gut microbiota to metabolism of dietary glycine betaine in mice and in vitro colonic fermentation. Microbiome 2019, 7, 103. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Sharma, S.; Sheehy, T.; Kolonel, L.N. Ethnic differences in grains consumption and their contribution to intake of B-vitamins: Results of the Multiethnic Cohort Study. Nutr. J. 2013, 12, 65. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Hatcher, D.W.; Tyler, R.T.; Toews, R.; Gawalko, E.J. Effect of cooking on the composition of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L.). Food Res. Int. 2010, 43, 589–594. [Google Scholar] [CrossRef]
- Temellini, A.; Mogavero, S.; Giulianotti, P.C.; Pietrabissa, A.; Mosca, F.; Pacifici, G.M. Conjugation of benzoic acid with glycine in human liver and kidney: A study on the interindividual variability. Xenobiotica 1993, 23, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Pallister, T.; Jackson, M.A.; Martin, T.C.; Zierer, J.; Jennings, A.; Mohney, R.P.; MacGregor, A.; Steves, C.J.; Cassidy, A.; Spector, T.D.; et al. Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Sci. Rep. 2017, 7, 13670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017, 105, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef]
- Picó, C.; Serra, F.; Rodríguez, A.M.; Keijer, J.; Palou, A. Biomarkers of Nutrition and Health: New Tools for New Approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef] [Green Version]
- Maruvada, P.; Lampe, J.W.; Wishart, D.S.; Barupal, D.; Chester, D.N.; Dodd, D.; Djoumbou-Feunang, Y.; Dorrestein, P.C.; Dragsted, L.O.; Draper, J.; et al. Perspective: Dietary Biomarkers of Intake and Exposure—Exploration with Omics Approaches. Adv. Nutr. 2020, 11, 200–215. [Google Scholar] [CrossRef]

| Factor | Overall n = 600 | White European n = 300 | South Asian n = 300 | p-Value |
|---|---|---|---|---|
| Age (years), mean (SD) | 31.20 (4.50) | 32.35 (4.89) | 30.01 (3.73) | <0.0001 |
| Gestational age (weeks), mean (SD) | 28.06 (3.27) | 29.50 (3.76) | 26.61 (1.75) | <0.0001 |
| Pre-pregnancy BMI (kg/m2), mean (SD) | 25.35 (5.63) | 26.77 (6.39) | 23.94 (4.33) | <0.0001 |
| Parity, n (%) | ||||
| 0 | 240 (42.33) | 145 (48.33) | 95 (35.58) | 0.0528 |
| 1 | 229 (40.39) | 110 (36.67) | 119 (44.57) | |
| 2 | 76 (13.40) | 34 (11.33) | 42 (15.73) | |
| ≥3 | 22 (3.88) | 11 (3.67) | 11 (4.12) | |
| Gestational diabetes (GDM), n (%) a | 169 (28.94) | 50 (17.54) | 119 (39.80) | <0.0001 |
| Smoking history (ever smoked), n (%) | 104 (17.48) | 104 (35.25) | 0 (0.00) | <0.0001 |
| Physical activity (moderate/vigorous), n (%) | 144 (24.04) | 84 (28.00) | 60 (20.07) | 0.0231 |
| Social disadvantage index, mean (SD) b | 1.31 (1.37) | 0.85 (1.22) | 1.84 (1.35) | <0.0001 |
| Fiber intake (g/day), mean (SD) | 22.52 (10.24) | 20.66 (9.23) | 24.38 (10.85) | <0.0001 |
| Energy Intake (kcal), mean (SD) | 2165.39 (772.06) | 2327.86 (766.33) | 2002.92 (744.26) | <0.0001 |
| Time of FFQ and blood draw, n (%) | ||||
| FFQ and blood draw on same day | 354 (60.31) | 88 (29.33) | 266 (92.68) | <0.0001 |
| FFQ before blood draw c | 221 (37.65) | 206 (68.67) | 15 (5.23) | |
| FFQ after blood draw c | 12 (2.04) | 6 (2.00) | 6 (2.09) | |
| Food items (servings/day), median (IQR) | ||||
| Citrus food | 0.57 (0.95) | 0.64 (0.99) | 0.43 (0.89) | <0.0001 |
| Fruits and vegetables | 6.28 (5.74) | 5.12 (4.26) | 7.85 (6.06) | <0.0001 |
| Tea | 0.43 (0.98) | 0.14 (0.57) | 1.0 (1.36) | <0.0001 |
| Coffee | 0 (0.14) | 0.02 (0.64) | 0 (0.00) | <0.0001 |
| Canned fish | 0 (0.03) | 0.03 (0.07) | 0 (0.00) | <0.0001 |
| Fried fish | 0 (0.03) | 0.01 (0.03) | 0 (0.02) | <0.0001 |
| Seafood | 0 (0.01) | 0.01 (0.02) | 0 (0.00) | <0.0001 |
| Chicken | 0.10 (0.29) | 0.14 (0.21) | 0 (0.14) | <0.0001 |
| Eggs | 0.21 (0.40) | 0.20 (0.32) | 0.29 (0.57) | 0.9927 |
| Red meat | 0.20 (0.44) | 0.41 (0.35) | 0.01 (0.15) | <0.0001 |
| Nuts and legumes | 0.71 (0.92) | 0.62 (0.83) | 0.85 (0.97) | <0.0001 |
| Full-fat dairy | - | 1.05 (1.11) | - | - |
| Fish/fish oil | - | 0.08 (0.15) | - | - |
| Metabolite concentration, median (IQR) | ||||
| Proline betaine | 1.81 (3.82) | 2.33 (5.52) | 1.40 (2.47) | <0.0001 |
| Hippuric acid | 10.01 (9.87) | 9.68 (9.03) | 10.07 (10.36) | 0.8848 |
| TMAO | 2.53 (1.95) | 2.68 (1.96) | 2.24 (1.99) | <0.0001 |
| 3-methylhistidine | 7.17 (4.12) | 8.64 (4.90) | 6.14 (2.24) | <0.0001 |
| Carnitine | 15.61 (3.82) | 15.35 (3.69) | 15.89 (3.98) | 0.0117 |
| Tryptophan betaine | 1.27 (0.37) | 1.19 (0.14) | 1.47 (0.37) | <0.0001 |
| Fatty acids, median (IQR) d | ||||
| Myristic acid (14:0) | - | 2.19 (0.74) | - | - |
| Pentadecanoic acid (15:0) | - | 0.24 (0.08) | - | - |
| Heptadecanoic acid (17:0) | - | 0.69 (0.23) | - | - |
| Eicosapentaenoic acid (EPA or 20:5n-3) | - | 0.51 (0.26) | - | - |
| Docosahexaenoic acid (DHA or 22:6n-3) | - | 0.67 (0.29) | - | - |
| Proline Betaine | Hippuric Acid | 3-Methyl Histidine | Carnitine | Tryptophan Betaine | TMAO | |
|---|---|---|---|---|---|---|
| Factor | b (95% CI) | b (95% CI) | b (95% CI) | b (95% CI) | b (95% CI) | b (95% CI) |
| Age (years) | 0.04 * (0.01, 0.07) | 0.01 (0.00, 0.03) | 0.00 (−0.01, 0.01) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.03) | 0.02 * (0.00, 0.04) |
| Gestational age (weeks) | 0.02 (−0.03, 0.06) | 0.01 (−0.01, 0.03) | 0.01 * (0.00, 0.02) | −0.01 *** (−0.02, −0.01) | 0.00 (0.00, 0.00) | 0.01 (−0.01, 0.03) |
| Parity | −0.10 (−0.25, 0.06) | 0.03 (−0.05, 0.11) | −0.01 (−0.05, 0.03) | 0.01 (−0.01, 0.02) | −0.01 (−0.02, 0.01) | 0.01 (−0.07, 0.09) |
| Gestational diabetes (GDM) | 0.05 (−0.24, 0.35) | 0.06 (−0.10, 0.21) | 0.02 (−0.05, 0.10) | 0.02 (−0.02, 0.05) | 0.02 (−0.01, 0.05) | 0.03 (−0.13, 0.19) |
| Pre-pregnancy BMI (kg/m2) | −0.02 (−0.05, 0.00) | −0.01 (−0.02, 0.00) | −0.01 (−0.01, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | −0.01 (−0.02, 0.01) |
| Smoking history (ever vs. never smoked) | −0.60 *** (−0.95, −0.25) | −0.12 (−0.30, 0.06) | 0.04 (−0.06, 0.13) | 0.06 ** (0.02, 0.10) | 0.00 (−0.03, 0.03) | −0.01 (−0.20, 0.17) |
| Physical activity (low vs. high) | −0.13 (−0.42, 0.17) | 0.02 (−0.14, 0.18) | −0.03 (−0.10, 0.05) | −0.01 (−0.04, 0.02) | 0.00 (−0.03, 0.03) | −0.04 (−0.21, 0.12) |
| Social disadvantage index | −0.05 (−0.15, 0.06) | −0.02 (−0.08, 0.03) | 0.00 (−0.03, 0.02) | 0.00 (−0.01, 0.01) | 0.00 (−0.01, 0.01) | −0.01 (−0.06, 0.05) |
| Fiber intake (g/day) | 0.01 (−0.01, 0.02) | 0.01 (−0.01, 0.02) | 0.00 (−0.01, 0.00) | 0.00 (0.00, 0.00) | 2.68 × 10−3 ** (0.00, 0.00) | 0.00 (−0.01, 0.01) |
| Energy intake (kcal) | 0.00 (0.00, 0.00) | −1.6 × 10−4 ** (−0.00, −0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | −3 × 10−5 * (0.00, 0.00) | 0.00 (0.00, 0.00) |
| FFQ before blood draw vs. FFQ at the same time as blood draw | 0.02 (−0.30, 0.35) | 0.11 (−0.05, 0.27) | −0.05 (−0.13, 0.04) | 0.00 (−0.03, 0.04) | −0.01 (−0.04, 0.02) | 0.09 (−0.08, 0.26) |
| FFQ after blood draw vs. FFQ at the same time as blood draw | 0.50 (−0.34, 1.34) | 0.08 (−0.37, 0.54) | 0.04 (−0.18, 0.26) | 0.06 (−0.04, 0.16) | −0.04 (−0.12, 0.04) | −0.11 (−0.56, 0.35) |
| Citrus food (servings/day) | 0.27 *** (0.20, 0.34) | |||||
| Fruits and vegetables (servings/day) | 0.22 ** (0.08, 0.36) | |||||
| Tea (servings/day) | 0.01 (−0.01, 0.04) | |||||
| Coffee (servings/day) | 0.02 (0.00, 0.04) | |||||
| Chicken (servings/day) | 0.02 * (0.00, 0.04) | |||||
| Red meat (servings/day) | 0.03 * (0.01, 0.06) | 0.00 (0.00, 0.01) | 0.00 (−0.04, 0.04) | |||
| Eggs (servings/day) | 0.01 (−0.01, 0.02) | 0.00 (−0.03, 0.04) | ||||
| Nuts and legumes (servings/day) | 0.02 (−0.02, 0.06) | 0.02 * (0.00, 0.03) | ||||
| Canned fish (servings/day) | 0.01 (−0.03, 0.04) | |||||
| Fried fish (servings/day) | 0.01 (−0.03, 0.05) | |||||
| Seafood (servings/day) | 0.08 *** (0.04, 0.12) |
| Even-Chain SFA | Odd-Chain SFA | ω-3 PUFA | ||||
|---|---|---|---|---|---|---|
| 14:0 | 15:0 | 17:0 | EPA | DHA | EPA + DHA | |
| Variable | b (95% CI) | b (95% CI) | b (95% CI) | b (95% CI) | b (95% CI) | b (95% CI) |
| Age (years) | 4.24 × 10−3 (−0.00, 0.01) | −3.54 × 10−4 (−0.01, 0.01) | −0.01 (−0.01, 0.00) | −0.01 (−0.03, 0.00) | −4.77 × 10−3 (−0.01, 0.00) | −0.01 (−0.02, 0.00) |
| Gestational age (weeks) | −0.01 (−0.02, 0.00) | −0.02 *** (−0.03, −0.01) | −0.01 ** (−0.02, −0.00) | −0.01 (−0.03, 0.01) | −0.01 (−0.02, 0.00) | −0.01 (−0.02, 0.00) |
| Parity | −0.01 (−0.04, 0.03) | 2.105 × 10−4 (−0.03, 0.03) | 2.21 × 10−3 (−0.03, 0.03) | −2.07 × 10−5 (−0.06, 0.06) | −0.03 (−0.08, 0.01) | −0.02 (−0.07, 0.03) |
| Gestational diabetes (GDM) | 0.02 (−0.07, 0.10) | −0.01 (−0.09, 0.07) | −0.06 (−0.14, 0.03) | −0.06 (−0.22, 0.10) | −0.07 (−0.18, 0.04) | −0.07 (−0.19, 0.05) |
| Pre-pregnancy BMI (kg/m2) | −0.01 * (−0.01, −0.00) | −0.01 ** (−0.01, −0.00) | −0.01 * (−0.01, −0.00) | −2.86 × 10−3 (−0.01, 0.01) | −0.01 * (−0.02, −0.00) | −0.01 (−0.01, 0.00) |
| Smoking history (ever vs. never smoked) | −0.02 (−0.08, 0.05) | −0.04 (−0.10, 0.03) | −0.05 (−0.12, 0.01) | −0.01 (−0.13, 0.10) | −0.05 (−0.14, 0.03) | −0.04 (−0.12, 0.05) |
| Physical activity (low vs. high) | −0.01 (−0.09, 0.08) | −0.01 (−0.09, 0.06) | −0.10 ** (−0.17, −0.02) | −0.03 (−0.18, 0.11) | −0.03 (−0.12, 0.06) | −0.03 (−0.13, 0.08) |
| Social disadvantage index | −0.02 (−0.04, 0.01) | −1.79 × 10−3 (−0.03, 0.03) | 0.02 (−0.01, 0.05) | 0.04 (−0.03, 0.10) | 0.04 (−0.00, 0.08) | 0.04 (−0.01, 0.08) |
| Fiber intake (g/day) | −1.12 × 10−3 (−0.01, 0.01) | 2.84 × 10−3 (−0.00, 0.01) | 1.45 × 10−3 (−0.00, 0.01) | 4.51 × 10−3 (−0.00, 0.01) | 2.01 × 10−3 (−0.00, 0.01) | 3.48 × 10−3 (−0.00, 0.01) |
| Energy intake (kcal) | −1.05 × 10−5 (−0.00, −0.00) | −4.76 × 10−5 (−0.00, 0.00) | −2.42 × 10−5 (−0.00, 0.00) | −8.23 × 10−5 (−0.00, 0.00) | −4.77 × 10−5 (−0.00, 0.00) | −6.21 × 10−5 (−0.00, 0.00) |
| FFQ before blood draw vs. FFQ at the same time as blood draw | −0.03 (−0.10, 0.04) | 0.06 (−0.01, 0.13) | 0.01 (−0.06, 0.08) | −3.01 × 10−3 (−0.14, 0.14) | 0.05 (−0.04, 0.15) | 0.02 (−0.07, 0.12) |
| FFQ after blood draw vs. FFQ at the same time as blood draw | −0.05 (−0.26, 0.16) | 0.02 (−0.09, 0.13) | 0.04 (−0.10, 0.19) | 0.06 (−0.26, 0.38) | 0.24 * (0.02, 0.46) | 0.16 (−0.07, 0.40) |
| Full-fat dairy (servings/day) | 0.02 (−0.02, 0.06) | 0.06 *** (0.03, 0.10) | 0.04 ** (0.01, 0.07) | |||
| Fish/Fish oil (servings/day) | 0.05 (−0.00, 0.11) | 0.11 *** (0.07, 0.14) | 0.08 *** (0.04, 0.12) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, T.; Azab, S.M.; Anand, S.S.; Thabane, L.; Shanmuganathan, M.; Morrison, K.M.; Atkinson, S.A.; Stearns, J.C.; Teo, K.K.; Britz-McKibbin, P.; et al. Sources of Variation in Food-Related Metabolites during Pregnancy. Nutrients 2022, 14, 2503. https://doi.org/10.3390/nu14122503
Rafiq T, Azab SM, Anand SS, Thabane L, Shanmuganathan M, Morrison KM, Atkinson SA, Stearns JC, Teo KK, Britz-McKibbin P, et al. Sources of Variation in Food-Related Metabolites during Pregnancy. Nutrients. 2022; 14(12):2503. https://doi.org/10.3390/nu14122503
Chicago/Turabian StyleRafiq, Talha, Sandi M. Azab, Sonia S. Anand, Lehana Thabane, Meera Shanmuganathan, Katherine M. Morrison, Stephanie A. Atkinson, Jennifer C. Stearns, Koon K. Teo, Philip Britz-McKibbin, and et al. 2022. "Sources of Variation in Food-Related Metabolites during Pregnancy" Nutrients 14, no. 12: 2503. https://doi.org/10.3390/nu14122503
APA StyleRafiq, T., Azab, S. M., Anand, S. S., Thabane, L., Shanmuganathan, M., Morrison, K. M., Atkinson, S. A., Stearns, J. C., Teo, K. K., Britz-McKibbin, P., & de Souza, R. J. (2022). Sources of Variation in Food-Related Metabolites during Pregnancy. Nutrients, 14(12), 2503. https://doi.org/10.3390/nu14122503







