The Effects of Eggs in a Plant-Based Diet on Oxidative Stress and Inflammation in Metabolic Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Blood Sample Collection
2.3. Liver Enzymes and C-Reactive Protein (CRP)
2.4. Plasma Cytokines
2.5. Total Antioxidant Capacity (TAC)
2.6. Glycoprotein A (GlycA)
2.7. Malondialdehyde (MDA), 8-Isoprostanes, and Oxidized LDL (oxLDL)
2.8. HDL Components—Paraoxonase-1 (PON-1) Activity and Serum Amyloid A (SAA)
2.9. Metabolic Syndrome Evaluation
2.10. Statistical Analysis
3. Results
3.1. Plasma CRP, Liver Enzymes, and Inflammatory Cytokines
3.2. HDL Components—PON-1 and SAA
3.3. Biomarkers of Oxidative Stress—TAC, MDA, 8-Isoprostanes, GlycA, and Oxidized LDL
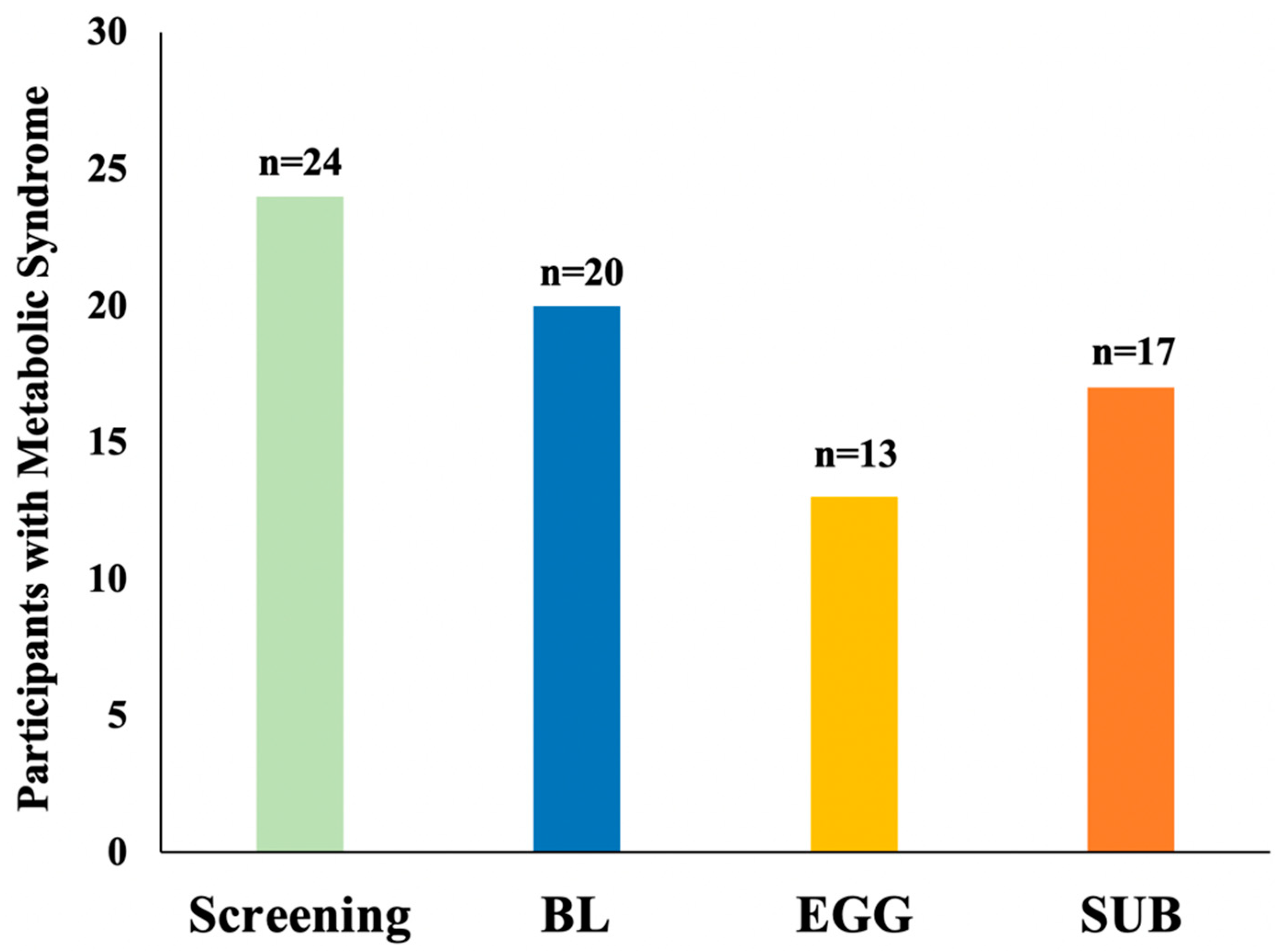
3.4. Changes in MetS Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yang, X.; Huang, Y.; Zhu, D.; Wang, Y.; Wen, Y.; Zhao, J.; Liu, Y.; Yang, C.-X.; Pan, J.; et al. Association between Baseline and Changes in High-Sensitive C-Reactive Protein and Metabolic Syndrome: A Nationwide Cohort Study and Meta-Analysis. Nutr. Metab. 2022, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Gozashti, M.H.; Aghadavood, M.; Mehdizadeh, M.R.; Hayatbakhsh, M.M. Clinical Significance of Serum IL-6 and TNF-α Levels in Patients with Metabolic Syndrome. Rep. Biochem. Mol. Biol. 2017, 6, 74–79. [Google Scholar] [PubMed]
- Fernandez, M.L.; Thomas, M.S.; Lemos, B.S.; DiMarco, D.M.; Missimer, A.; Melough, M.; Chun, O.K.; Murillo, A.G.; Alyousef, H.M.; Medina-Vera, I. TA-65, A Telomerase Activator Improves Cardiovascular Markers in Patients with Metabolic Syndrome. Curr. Pharm. Des. 2018, 24, 1905–1911. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schwingshackl, L.; Fedirko, V.; Aleksandrova, K. Effect of Plant-Based Diets on Obesity-Related Inflammatory Profiles: A Systematic Review and Meta-Analysis of Intervention Trials. Obes. Rev. 2016, 17, 1067–1079. [Google Scholar] [CrossRef]
- Menzel, J.; Biemann, R.; Longree, A.; Isermann, B.; Mai, K.; Schulze, M.B.; Abraham, K.; Weikert, C. Associations of a Vegan Diet with Inflammatory Biomarkers. Sci. Rep. 2020, 10, 1933. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, G.; Li, Y.; Shi, G.; Li, M. Potential Application of Plant-Based Functional Foods in the Development of Immune Boosters. Front. Pharmacol. 2021, 12, 637782. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; de Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of Nutritional Quality of the Vegan, Vegetarian, Semi-Vegetarian, Pesco-Vegetarian and Omnivorous Diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef]
- Thomas, M.; Blesso, C.N.; Calle, M.; Puglisi, M.; Fernandez, M.-L. Dietary Influences on Gut Microbiota with a Focus on Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Craddock, J.C.; Neale, E.P.; Peoples, G.E.; Probst, Y.C. Vegetarian-Based Dietary Patterns and Their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 433–451. [Google Scholar] [CrossRef] [PubMed]
- Nimalaratne, C.; Wu, J. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [Green Version]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volk, B.; Volek, J.S.; Fernandez, M.L. Effects of Carbohydrate Restriction and Dietary Cholesterol Provided by Eggs on Clinical Risk Factors in Metabolic Syndrome. J. Clin. Lipidol. 2013, 7, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Dibella, M.; Thomas, M.S.; Alyousef, H.; Millar, C.; Blesso, C.; Malysheva, O.; Caudill, M.A.; Fernandez, M.L. Choline Intake as Supplement or as a Component of Eggs Increases Plasma Choline and Reduces Interleukin-6 without Modifying Plasma Cholesterol in Participants with Metabolic Syndrome. Nutrients 2020, 12, 3120. [Google Scholar] [CrossRef]
- Thomas, M.S.; Puglisi, M.; Malysheva, O.; Caudill, M.A.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Eggs Improve Plasma Biomarkers in Patients with Metabolic Syndrome Following a Plant-Based Diet—A Randomized Crossover Study. Nutrients 2022, 14, 2138. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.S.; Fernandez, M.L. Whole Egg Consumption Improves Lipoprotein Profiles and Insulin Sensitivity to a Greater Extent than Yolk-Free Egg Substitute in Individuals with Metabolic Syndrome. Metab. Clin. Exp. 2013, 62, 400–410. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Lee, S.-G.; Davis, C.G.; Koo, S.I.; Chun, O.K. Dietary Total Antioxidant Capacity Is Associated with Diet and Plasma Antioxidant Status in Healthy Young Adults. J. Acad. Nutr. Diet. 2012, 112, 1626–1635. [Google Scholar] [CrossRef]
- Connelly, M.A.; Otvos, J.D.; Shalaurova, I.; Playford, M.P.; Mehta, N.N. GlycA, a Novel Biomarker of Systemic Inflammation and Cardiovascular Disease Risk. J. Transl. Med. 2017, 15, 219. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.G.; Wang, T.; Vance, T.M.; Hubert, P.; Kim, D.-O.; Koo, S.I.; Chun, O.K. Validation of Analytical Methods for Plasma Total Antioxidant Capacity by Comparing with Urinary 8-Isoprostane Level. J. Microbiol. Biotechnol. 2017, 27, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Alyousuf, H.; Lemos, B.; Thomas, M.S.; Melough, M.; Chun, O.K.; Fernandez, M.L. Low Plasma HDL Cholesterol Is Associated with Greater Risk for Cardiovascular Disease in Subjects with Metabolic Syndrome. Curr. Top. Biochem. Res. 2018, 19, 25–36. [Google Scholar]
- Millar, C.L.; Duclos, Q.; Garcia, C.; Norris, G.H.; Lemos, B.S.; DiMarco, D.M.; Fernandez, M.L.; Blesso, C.N. Effects of Freeze-Dried Grape Powder on High-Density Lipoprotein Function in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Study. Metab. Syndr. Relat. Disord. 2018, 16, 464–469. [Google Scholar] [CrossRef]
- Millar, C.L.; Norris, G.H.; Jiang, C.; Kry, J.; Vitols, A.; Garcia, C.; Park, Y.-K.; Lee, J.-Y.; Blesso, C.N. Long-Term Supplementation of Black Elderberries Promotes Hyperlipidemia, but Reduces Liver Inflammation and Improves HDL Function and Atherosclerotic Plaque Stability in Apolipoprotein E-Knockout Mice. Mol. Nutr. Food Res. 2018, 62, e1800404. [Google Scholar] [CrossRef]
- Khersonsky, O.; Tawfik, D.S. Structure-Reactivity Studies of Serum Paraoxonase PON1 Suggest That Its Native Activity Is Lactonase. Biochemistry 2005, 44, 6371–6382. [Google Scholar] [CrossRef]
- DiBella, M.; Thomas, M.; Alyousef, H.; Millar, C.; Blesso, C.; Fernandez, M.-L. Intake of 3 Eggs Per Day for 4 Weeks Did Not Raise Plasma Cholesterol in Individuals with Metabolic Syndrome. Curr. Dev. Nutr. 2020, 4, 389. [Google Scholar] [CrossRef]
- Thomas, M.S.; DiBella, M.; Blesso, C.N.; Malysheva, O.; Caudill, M.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Comparison between Egg Intake versus Choline Supplementation on Gut Microbiota and Plasma Carotenoids in Subjects with Metabolic Syndrome. Nutrients 2022, 14, 1179. [Google Scholar] [CrossRef]
- Hutcheson, R.; Rocic, P. The Metabolic Syndrome, Oxidative Stress, Environment, and Cardiovascular Disease: The Great Exploration. Exp. Diabetes Res. 2012, 2012, 271028. [Google Scholar] [CrossRef] [Green Version]
- Hildrum, B.; Mykletun, A.; Hole, T.; Midthjell, K.; Dahl, A.A. Age-Specific Prevalence of the Metabolic Syndrome Defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 Study. BMC Public Health 2007, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Gómez, C.; Mateos, D.; Ripoll-Vera, T.; Tur, J.A.; Sureda, A. Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants 2022, 11, 901. [Google Scholar] [CrossRef]
- Rosa, E.M.d.; Scatola, R.P.; Possa, R. Cardiovascular Risk in Vegetarians and Omnivores: A Comparative Study. Arq. Bras. Cardiol. 2008, 91, 214–221. [Google Scholar]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, Vegan Diets and Multiple Health Outcomes: A Systematic Review with Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and Adequacy of the Vegan Diet. A Systematic Review of the Evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Bellissimo, N.; Totosy de Zepetnek, J.O.; Rouhani, M.H. Association of Vegetarian Diet with Inflammatory Biomarkers: A Systematic Review and Meta-Analysis of Observational Studies. Public Health Nutr. 2017, 20, 2713–2721. [Google Scholar] [CrossRef]
- Nettleton, J.A.; Steffen, L.M.; Mayer-Davis, E.J.; Jenny, N.S.; Jiang, R.; Herrington, D.M.; Jacobs, D.R. Dietary Patterns Are Associated with Biochemical Markers of Inflammation and Endothelial Activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Clin. Nutr. 2006, 83, 1369–1379. [Google Scholar] [CrossRef]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Legarrea, P.; de la Iglesia, R.; Abete, I.; Navas-Carretero, S.; Martinez, J.A.; Zulet, M.A. The Protein Type within a Hypocaloric Diet Affects Obesity-Related Inflammation: The RESMENA Project. Nutrition 2014, 30, 424–429. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and Atherosclerotic Cardiovascular Disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- Kotani, K.; Yamada, T.; Gugliucci, A. Paired Measurements of Paraoxonase 1 and Serum Amyloid A as Useful Disease Markers. BioMed Res. Int. 2013, 2013, 481437. [Google Scholar] [CrossRef] [Green Version]
- Bakhtiari, A.; Hajian-Tilaki, K.; Omidvar, S.; Nasiri Amiri, F. Association of Lipid Peroxidation and Antioxidant Status with Metabolic Syndrome in Iranian Healthy Elderly Women. Biomed. Rep. 2017, 7, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Moreto, F.; de Oliveira, E.P.; Manda, R.M.; Burini, R.C. The Higher Plasma Malondialdehyde Concentrations Are Determined by Metabolic Syndrome-Related Glucolipotoxicity. Oxidative Med. Cell. Longev. 2014, 2014, 505368. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Karimi Galougahi, K.; Liu, C.-C.; Bhindi, R.; Figtree, G.A. Biological Markers of Oxidative Stress: Applications to Cardiovascular Research and Practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, J.M.; Girman, C.; Rhodes, T.; Nijpels, G.; Stehouwer, C.D.A.; Bouter, L.M.; Heine, R.J. Metabolic Syndrome and 10-Year Cardiovascular Disease Risk in the Hoorn Study. Circulation 2005, 112, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Ballout, R.A.; Remaley, A.T. GlycA: A New Biomarker for Systemic Inflammation and Cardiovascular Disease (CVD) Risk Assessment. J. Lab. Precis. Med. 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Cho, S.W.; Park, Y.K. Long-Term Vegetarians Have Low Oxidative Stress, Body Fat, and Cholesterol Levels. Nutr. Res. Pract. 2012, 6, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameter | BL | EGG | SUB | p Value |
|---|---|---|---|---|
| CRP (mg/dL) | 0.25 ± 0.24 | 0.40 ± 0.57 | 0.27 ± 0.26 | 0.448 |
| ALT (U/L) | 28.3 ± 17.0 | 28.7 ± 13.8 | 29.4 ± 21.3 | 0.679 |
| AST (U/L) | 23.0 ± 7.7 | 23.9 ± 8.2 | 22.4 ± 7.6 | 0.494 |
| IL-6 (pg/mL) | 3.5 ± 1.0 | 3.8 ± 1.2 | 3.7 ± 1.0 | 0.429 |
| MCP-1 (pg/mL) | 177.1 ± 65.4 | 174.1 ± 84.6 | 171.1 ± 73.8 | 0.632 |
| TNF-α (pg/mL) | 7.4 ± 1.5 | 7.3 ± 1.4 | 7.4 ± 1.7 | 0.855 |
| Parameter | BL | EGG | SUB | p Value |
|---|---|---|---|---|
| TAC (mg VCE/L) | 184.0 ± 36.1 | 185.9 ± 40.4 | 172.3 ± 35.4 | 0.377 |
| MDA (ng/mL) 2 | 397.1 ± 88.7 a | 389.2 ± 96.8 a | 426.2 ± 129.3 b | 0.049 |
| 8-Isoprostanes (pg/mL) | 55.3 ± 15.2 | 58.4 ± 16.4 | 61.8 ± 11.8 | 0.277 |
| GlycA (μm/L) | 432.3 ± 66.5 | 434.4 ± 74.2 | 430.8 ± 66.3 | 0.908 |
| OxLDL (ng/mL) | 153.9 ± 119.1 | 151.1 ± 125.4 | 175.6 ± 117.5 | 0.057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.S.; Huang, L.; Garcia, C.; Sakaki, J.R.; Blesso, C.N.; Chun, O.K.; Fernandez, M.L. The Effects of Eggs in a Plant-Based Diet on Oxidative Stress and Inflammation in Metabolic Syndrome. Nutrients 2022, 14, 2548. https://doi.org/10.3390/nu14122548
Thomas MS, Huang L, Garcia C, Sakaki JR, Blesso CN, Chun OK, Fernandez ML. The Effects of Eggs in a Plant-Based Diet on Oxidative Stress and Inflammation in Metabolic Syndrome. Nutrients. 2022; 14(12):2548. https://doi.org/10.3390/nu14122548
Chicago/Turabian StyleThomas, Minu S., Lindsey Huang, Chelsea Garcia, Junichi R. Sakaki, Christopher N. Blesso, Ock K. Chun, and Maria Luz Fernandez. 2022. "The Effects of Eggs in a Plant-Based Diet on Oxidative Stress and Inflammation in Metabolic Syndrome" Nutrients 14, no. 12: 2548. https://doi.org/10.3390/nu14122548
APA StyleThomas, M. S., Huang, L., Garcia, C., Sakaki, J. R., Blesso, C. N., Chun, O. K., & Fernandez, M. L. (2022). The Effects of Eggs in a Plant-Based Diet on Oxidative Stress and Inflammation in Metabolic Syndrome. Nutrients, 14(12), 2548. https://doi.org/10.3390/nu14122548









