Dried Wild-Grown Mushrooms Can Be Considered a Source of Selected Minerals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation Procedure
2.3. Determination of Concentrations of Mineral Components
2.4. Accuracy Check of the Methods
2.5. Statistical Analysis
2.6. Assessment of Mineral Content in the Dried Mushrooms in Relation to Nutrition Standards
3. Results
3.1. The Concentrations of the Assessed Trace Elements in Dried Mushrooms
3.2. Assessment of Daily Reference Intake (DRI) Coverage for the Tested Elements after the Consumption of a Standard Portion (2.7 g) of Dried Mushrooms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borowska, A. Changes in the value and volume of purchase of non-timber forest raw materials on the example of fresh forest mushrooms in Poland in the years 2008–1018. In Selected Market Aspects Agricultural Food in Poland; SGGW: Warsaw, Poland, 2020. [Google Scholar]
- Statistics Poland. Statistical Yearbook of Forestry; Statistics Poland: Warsaw, Poland, 2020. [Google Scholar]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium intake in bone health: A focus on calcium-rich mineral waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [Green Version]
- Palacios, C.; Hofmeyr, G.J.; Cormick, G.; Garcia-Casal, M.N.; Peña-Rosas, J.P.; Betrán, A.P. Current calcium fortification experiences: A review. Ann. N. Y. Acad. Sci. 2020, 1484, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in aging, health and diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in infectious diseases in older people. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Bermudez-Pena, C.; Rodriguez-Moran, M. Severe hypomagnesemia and low-grade inflammation in metabolic syndrome. Magnes. Res. 2011, 24, 45–53. [Google Scholar] [CrossRef]
- Lal, A. Iron in health and disease: An update. Indian J Pediatr 2020, 87, 58–65. [Google Scholar] [CrossRef]
- Pan, Z.; Choi, S.; Ouadid-Ahidouch, H.; Yang, J.M.; Beattie, J.H.; Korichneva, I. Zinc transporters and dysregulated channels in cancers. Front. Biosci. 2017, 22, 623–643. [Google Scholar] [CrossRef] [Green Version]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [Green Version]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambling, L.; McArdle, H.J. Iron, copper and fetal development. Proc. Nutr. Soc. 2004, 63, 553–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bang, E.S.; Lee, J.H.; Lee, J.D.; Kang, D.R.; Hong, J. Serum concentrations of trace elements zinc, copper, selenium, and manganese in critically ill patients. Biol. Trace Elem. Res. 2019, 188, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese is essential for neuronal health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, E.; Bermejo, L.M.; Lopez-Sobaler, A.M.; Ortega, R.M. An inadequate intake of manganese may favour insulin resistance in girls. Nutr. Hosp. 2011, 26, 965–970. [Google Scholar] [CrossRef]
- Kieliszek, M.; Blazejak, S. Selenium: Significance, and outlook for supplementation. Nutrition 2013, 29, 713–718. [Google Scholar] [CrossRef]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [Green Version]
- Braunstein, M.; Kusmenkov, T.; Zuck, C.; Angstwurm, M.; Becker, N.P.; Bocker, W.; Schomburg, L.; Bogner-Flatz, V. Selenium and selenoprotein p deficiency correlates with complications and adverse outcome after major trauma. Shock 2020, 53, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smina, T.P.; Nitha, B.; Devasagayam, T.P.; Janardhanan, K.K. Ganoderma lucidum total triterpenes induce apoptosis in MCF-7 cells and attenuate DMBA induced mammary and skin carcinomas in experimental animals. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2017, 813, 45–51. [Google Scholar] [CrossRef]
- Wu, Y.S.; Ho, S.Y.; Nan, F.H. Ganoderma lucidum β1,3/1,6 glucan as an immunomodulator in inflammation induced by a high-cholesterol diet. BMC Complement Altern. Med. 2016, 16, 500. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament and The Council of the European Union. Regulation (EU) of the European Parliament and of the Council (EU) No. 1169/2011 of 25 October 2011 on the provision of food information to consumers, amending Regulations of the European Parliament and of the Council (EC) No 1924/2006 and (EC) No 1925/2006 and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off. J. Eur. Union 2011, 304, 18–63. [Google Scholar]
- Nikkarinen, M.; Mertanen, E. Impact of geological origin on trace element composition of edible mushrooms. J. Food Compos. Anal. 2004, 16, 301–310. [Google Scholar] [CrossRef]
- Wojciechowska-Mazurek, M.; Mania, M.; Starska, K.; Rebeniak, M.; Karłowski, K. Noxious elements in edible mushrooms in Poland. Bromatol. Chem. Toksykol. 2011, 44, 143–149. [Google Scholar]
- Falandysz, J.; Drewnowska, M.; Jarzyńska, G.; Zhang, D.; Zhang, Y.; Wang, J. Mineral constituents in common chanterelles and soils collected from a high mountain and lowland sites in Poland. J. Mt. Sci. 2012, 9, 697–705. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. Mineral composition of three popular wild mushrooms from Poland. Molecules 2020, 25, 3588. [Google Scholar] [CrossRef]
- Brzezicha-Cirocka, J.; Mędyk, M.; Falandysz, J.; Szefer, P. Bio- and toxic elements in edible wild mushrooms from two regions of potentially different environmental conditions in eastern Poland. Environ. Sci. Pollut. Res. 2016, 23, 21517–21522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, D.; You, Y.; Zeng, S.; Li, Y.; Tang, Q.; Han, G.; Liu, A.; Feng, C.; Li, C.; et al. Nutritional composition of boletus mushrooms from Southwest China and their antihyperglycemic and antioxidant activities. Food Chem. 2016, 211, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Rasalanavhoa, M.; Moodley, R.; Jonnalagadda, S.B. Elemental bioaccumulation and nutritional value of five species of wild growing mushrooms from South Africa. Food Chem. 2020, 319, 126596. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, F.; Isiloglu, M.; Merdivan, M. Heavy metal levels in some macrofungi. Turk. J. Bot. 2003, 27, 45–56. [Google Scholar]
- Kuziemska, B.; Wysokiński, A.; Jaremko, D.; Popek, M.; Kożuchowska, M. The content of some heavy metals in edible mushrooms. Ecol. Eng. 2018, 19, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Świsłowski, P.; Dołhańczuk-Śródka, A.; Rajfur, M. Bibliometric analysis of European publications between 2001 and 2016 on concentrations of selected elements in mushrooms. Environ. Sci. Pollut. Res. Int. 2020, 27, 22235–22250. [Google Scholar] [CrossRef] [Green Version]
- Giannaccini, G.; Betti, L.; Palego, L.; Mascia, G.; Schmid, L.; Lanza, M.; Mela, A.; Fabbrini, L.; Biondi, L.; Lucacchini, A. The trace element content of top-soil and wild edible mushroom samples collected in Tuscany, Italy. Environ. Moni. Assess 2012, 184, 7579–7585. [Google Scholar] [CrossRef]
- Mleczek, M.; Magdziak, Z.; Gąsecka, M.; Niedzielski, P.; Kalač, P.; Siwulski, M.; Rzymski, P.; Zalicka, S.; Sobieralski, K. Content of selected elements and low-molecular-weight organic acids in fruiting bodies of edible mushroom Boletus badius (Fr.) Fr. from unpolluted and polluted areas. Environ. Sci. Pollut. Res. 2016, 23, 20609–20618. [Google Scholar] [CrossRef] [Green Version]
- Leonhardt, T.; Sácký, J.; Šimek, P.; Šantrůček, J.; Kotrba, P. Metallothionein-like peptides involved in sequestration of Zn in the Zn-accumulating ectomycorrhizal fungus Russula atropurpurea. Metallomics 2014, 6, 1693. [Google Scholar] [CrossRef] [Green Version]
- Mirończuk-Chodakowska, I.; Socha, K.; Zujko, M.E.; Terlikowska, K.M.; Borawska, M.H.; Witkowska, A.M. Copper, manganese, selenium and zinc in wild-growing edible mushrooms from the eastern territory of “Green Lungs of Poland”: Nutritional and toxicological implications. Int. J. Environ. Res. Public Health 2019, 16, 3614. [Google Scholar] [CrossRef] [Green Version]
- Karmańska, A.; Wędzisz, A. Content of selected macro- and microelements in various species of large fruiting body mushrooms collected in the province of Łódź. Bromat. Chem. Toksykol. 2010, 43, 124–129. [Google Scholar]
- Collin-Hansen, C.; Yttri, K.E.; Andersen, R.A.; Berthelsen, B.O.; Steinnes, E. Mushrooms from two metal-contaminated areas in Norway: Occurrence of metals and metallothionein-like proteins. Geoch. Explor. Env. A 2002, 2, 121–130. [Google Scholar] [CrossRef]
- Sensi, S.L.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and zinc dysregulation in Alzheimer’s disease. Trends Pharmacol. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J.; Sustar, N. Copper and zinc, biological role and significance of copper/zinc imbalance. J. Clinic. Toxicol. 2011, S3, 2161. [Google Scholar] [CrossRef] [Green Version]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.J.; Reboul, E. Nutritional composition and bioactive content of legumes: Characterization of pulses frequently consumed in France and effect of the cooking method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef] [Green Version]
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and dairy products and their nutritional contribution to the average Polish diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jâkobsone, I.; Kantâne, I.; Zute, S.; Jansone, I.; Bartkeviès, V. Macro-elements and trace elements in cereal grains cultivated in Latvia. Proc. Latv. Acad. Sci. Sect. B 2015, 69, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Falandysz, J. Selenium in edible mushrooms. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 256–299. [Google Scholar] [CrossRef] [Green Version]
- Wąsowicz, W.; Gromadzińska, J.; Rydzynński, K.; Tomczak, J. Selenium status of low-selenium area residents: Polish experience. Toxicol. Lett. 2003, 137, 95–101. [Google Scholar] [CrossRef]
- The European Comission. Commission Regulation (Eu) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 43, L136/1–L136/40. [Google Scholar]
- Court of Justice of the European Union. Judgement of the Court (Third Chamber) of 1 October 2020 in Case C-485/18: Request for a preliminary ruling under Article 267 TFEU from the Conseil d’État (Council of State, France), made by decision of 27 June 2018, received at the Court on 24 July 2018, in the proceedings—Groupe Lactalis v Premier ministre and Other, ECLI:EU:C:2020:763. Off. J. Eur. Union 2020, 63, 1–56. [Google Scholar]

| Supermarket/City | Company | X. badius No. of Batches | B. edulis No. of Batches |
|---|---|---|---|
| Auchan | PolGrzyb | 5 | 4 |
| Auchan | Tagros Polska | 5 | 6 |
| Kaufland | JamPol | 10 | 10 |
| Lidl | Nasza Chata | 10 | 10 |
| Carrefour | RunoPol | 5 | 5 |
| Tesco | RunoPol | 5 | 5 |
| Element | Wavelength (nm) | Lamp Current (mA) | Graphite Cuvette—Temperature Conditions |
|---|---|---|---|
| Ca | 422.7 | 7.5 | - |
| Mg | 285.2 | 7.5 | - |
| Fe | 248.3 | 12.5 | - |
| Zn | 213.9 | 6.5 | - |
| Cu | 324.8 | 7.5 | - |
| Mn | 279.5 | 7.5 | - |
| Se | 196.0 | 14.5 | Drying 70/100 °C Ashing 600/600 °C Atomization 2700/2700 °C Cuvette cleaning 2800/2800 °C |
| Element | Reference Material | |
|---|---|---|
| Dried Mushroom Powder | Simulated Diet D | |
| Certified Levels of Elements ± Expanded Uncertainty (mg/kg Dry Mass) | ||
| Ca | - | 510 ± 37 |
| Fe | - | 199 ± 16 |
| Mg | - | 676 ± 65 |
| Zn | 113.30 ± 3.28 | - |
| Cu | 18.73 ± 0.70 | - |
| Mn | - | 10.2 ± 0.6 |
| Se | 17.43 ± 1.36 | - |
| Element | |||||||
|---|---|---|---|---|---|---|---|
| Mushroom Species | Mean Content | Median | Min. | Max. | SD | Q1 | Q3 |
| Ca | |||||||
| B. edulis (n = 40) | 82.1 | 79.9 | 18.7 | 154.8 | 31.0 | 63.5 | 106.6 |
| X. badius (n = 40) | 67.5 | 56.8 | 21.7 | 131.0 | 27.6 | 48.5 | 76.7 |
| B. edulis vs. X. badius | p < 0.01 | ||||||
| Mg | |||||||
| B. edulis (n = 40) | 964.1 | 981.5 | 791.3 | 1143.3 | 86.2 | 894.7 | 1031.0 |
| X. badius (n = 40) | 1060.2 | 1024.6 | 868.6 | 1422.3 | 128.2 | 951.1 | 1158.6 |
| B. edulis vs. X. badius | p < 0.001 | ||||||
| Fe | |||||||
| B. edulis (n = 40) | 233.4 | 218.5 | 56.3 | 707.1 | 163.2 | 78.1 | 339.5 |
| X. badius (n = 40) | 87.8 | 80.9 | 42.9 | 219.9 | 33.3 | 67.0 | 97.2 |
| B. edulis vs. X. badius | p < 0.00001 | ||||||
| Zn | |||||||
| B. edulis (n = 40) | 97.9 | 93.5 | 63.8 | 163.6 | 25.7 | 77.5 | 104.4 |
| X. badius (n = 40) | 197.2 | 174.2 | 136.6 | 365.6 | 55.7 | 161.1 | 224.0 |
| B. edulis vs. X. badius | p < 0.000001 | ||||||
| Cu | |||||||
| B. edulis (n = 40) | 25.8 | 22.2 | 14.6 | 72.2 | 11.9 | 18.1 | 25.6 |
| X. badius (n = 40) | 33.9 | 33.1 | 22.4 | 42.6 | 4.5 | 31.6 | 36.6 |
| B. edulis vs. X. badius | p < 0.000001 | ||||||
| Zn:Cu ratio | |||||||
| B. edulis (n = 40) | 4.2 | 4.1 | 2.0 | 6.7 | 1.1 | 3.6 | 4.5 |
| X. badius (n = 40) | 5.9 | 5.4 | 3.6 | 10.4 | 1.7 | 4.7 | 7.0 |
| B. edulis vs. X. badius | p < 0.000001 | ||||||
| Mn | |||||||
| B. edulis (n = 40) | 22.1 | 22.5 | 9.0 | 36.6 | 6.0 | 17.9 | 24.8 |
| X. badius (n = 40) | 19.8 | 20.6 | 10.7 | 31.5 | 4.6 | 15.9 | 22.9 |
| B. edulis vs. X. badius | p < 0.05 | ||||||
| Se | |||||||
| B. edulis (n = 40) | 6501.6 | 6013.1 | 3391.1 | 17,265.8 | 2297.7 | 5138.6 | 7295.0 |
| X. badius (n = 40) | 282.4 | 156.2 | 41.1 | 1262.7 | 308.7 | 68.8 | 405.1 |
| B. edulis vs. X. badius | p < 0.000001 | ||||||
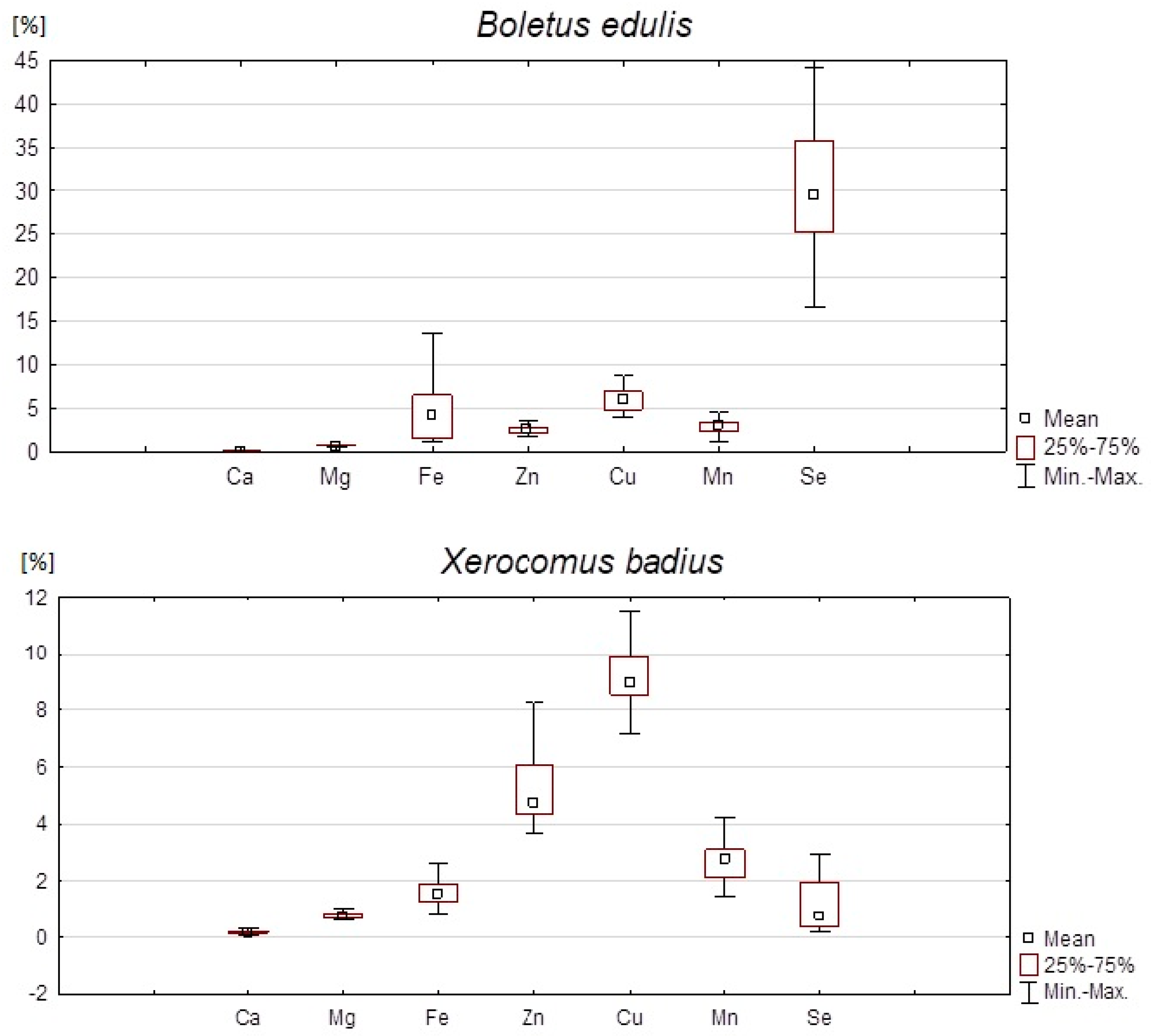
| Element | ||||
|---|---|---|---|---|
| Mushroom Species | Mean Content | Min. | Max. | SD |
| Ca | ||||
| B. edulis | 0.22 | 0.05 | 0.42 | 0.08 |
| X. badius | 0.18 | 0.06 | 0.35 | 0.07 |
| Mg | ||||
| B. edulis | 2.60 | 2.14 | 3.09 | 0.23 |
| X. badius | 2.86 | 2.35 | 3.84 | 0.35 |
| Fe | ||||
| B. edulis | 0.63 | 0.15 | 1.91 | 0.44 |
| X. badius | 0.24 | 0.12 | 0.59 | 0.09 |
| Zn | ||||
| B. edulis | 0.26 | 0.17 | 0.44 | 0.07 |
| X. badius | 0.53 | 0.37 | 0.99 | 0.15 |
| Cu | ||||
| B. edulis | 0.07 | 0.04 | 0.20 | 0.03 |
| X. badius | 0.09 | 0.06 | 0.12 | 0.01 |
| Mn | ||||
| B. edulis | 0.06 | 0.02 | 0.10 | 0.02 |
| X. badius | 0.05 | 0.03 | 0.08 | 0.01 |
| Se | ||||
| B. edulis | 17.55 | 9.16 | 46.62 | 6.20 |
| X. badius | 0.76 | 0.11 | 3.41 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orywal, K.; Socha, K.; Nowakowski, P.; Zoń, W.; Mroczko, B.; Perkowski, M. Dried Wild-Grown Mushrooms Can Be Considered a Source of Selected Minerals. Nutrients 2022, 14, 2750. https://doi.org/10.3390/nu14132750
Orywal K, Socha K, Nowakowski P, Zoń W, Mroczko B, Perkowski M. Dried Wild-Grown Mushrooms Can Be Considered a Source of Selected Minerals. Nutrients. 2022; 14(13):2750. https://doi.org/10.3390/nu14132750
Chicago/Turabian StyleOrywal, Karolina, Katarzyna Socha, Patryk Nowakowski, Wojciech Zoń, Barbara Mroczko, and Maciej Perkowski. 2022. "Dried Wild-Grown Mushrooms Can Be Considered a Source of Selected Minerals" Nutrients 14, no. 13: 2750. https://doi.org/10.3390/nu14132750