Hesperidin Functions as an Ergogenic Aid by Increasing Endothelial Function and Decreasing Exercise-Induced Oxidative Stress and Inflammation, Thereby Contributing to Improved Exercise Performance
Abstract
:1. Introduction
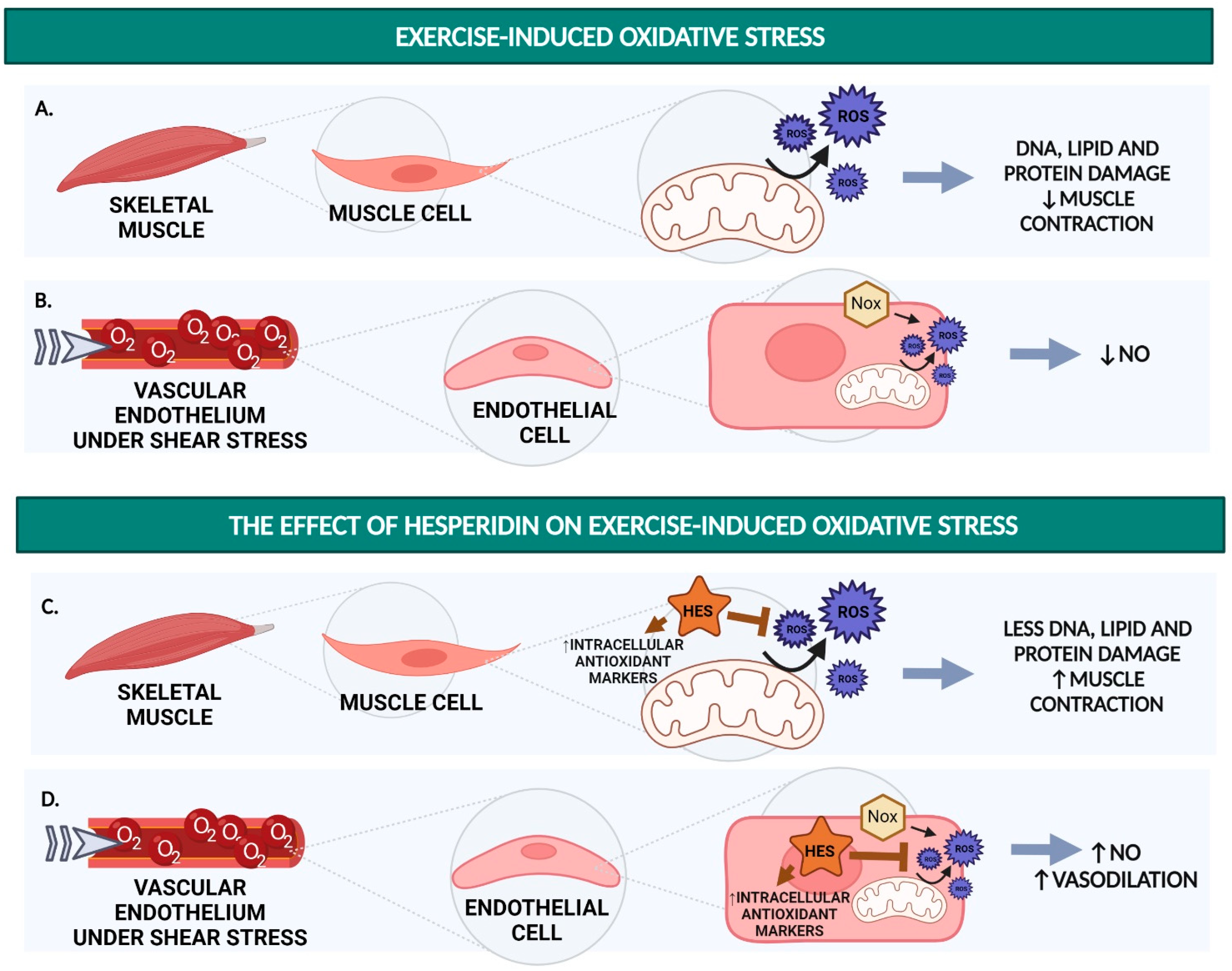
1.1. Excessive Production of ROS Results in Decreased Force Output and Decreased NO Availability
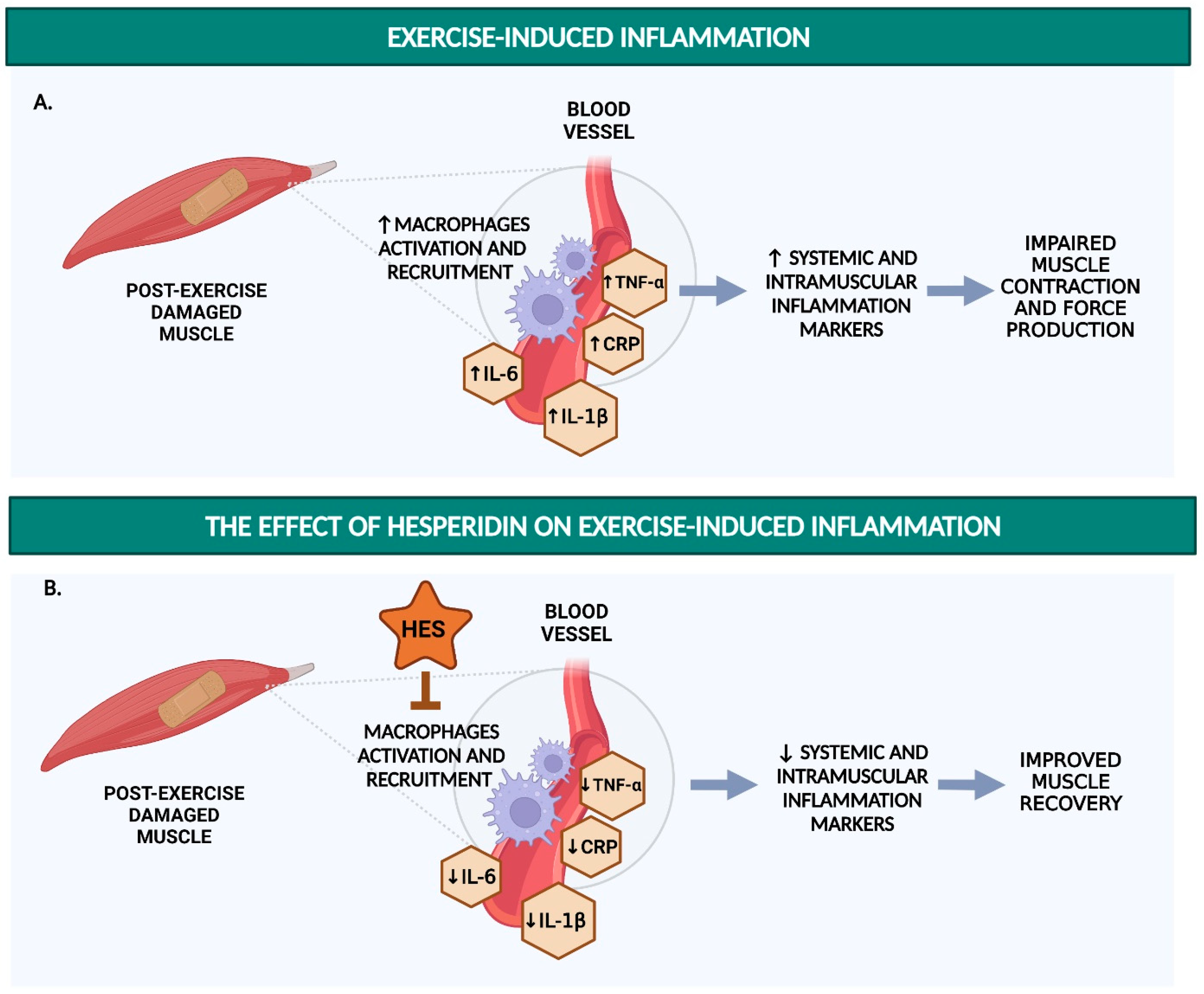
1.2. Detrimental Effects of Post-Exercise Inflammation on Endurance Performance and Endothelial Function
1.3. Hesperidin Supplementation: A Potential Ergogenic Aid
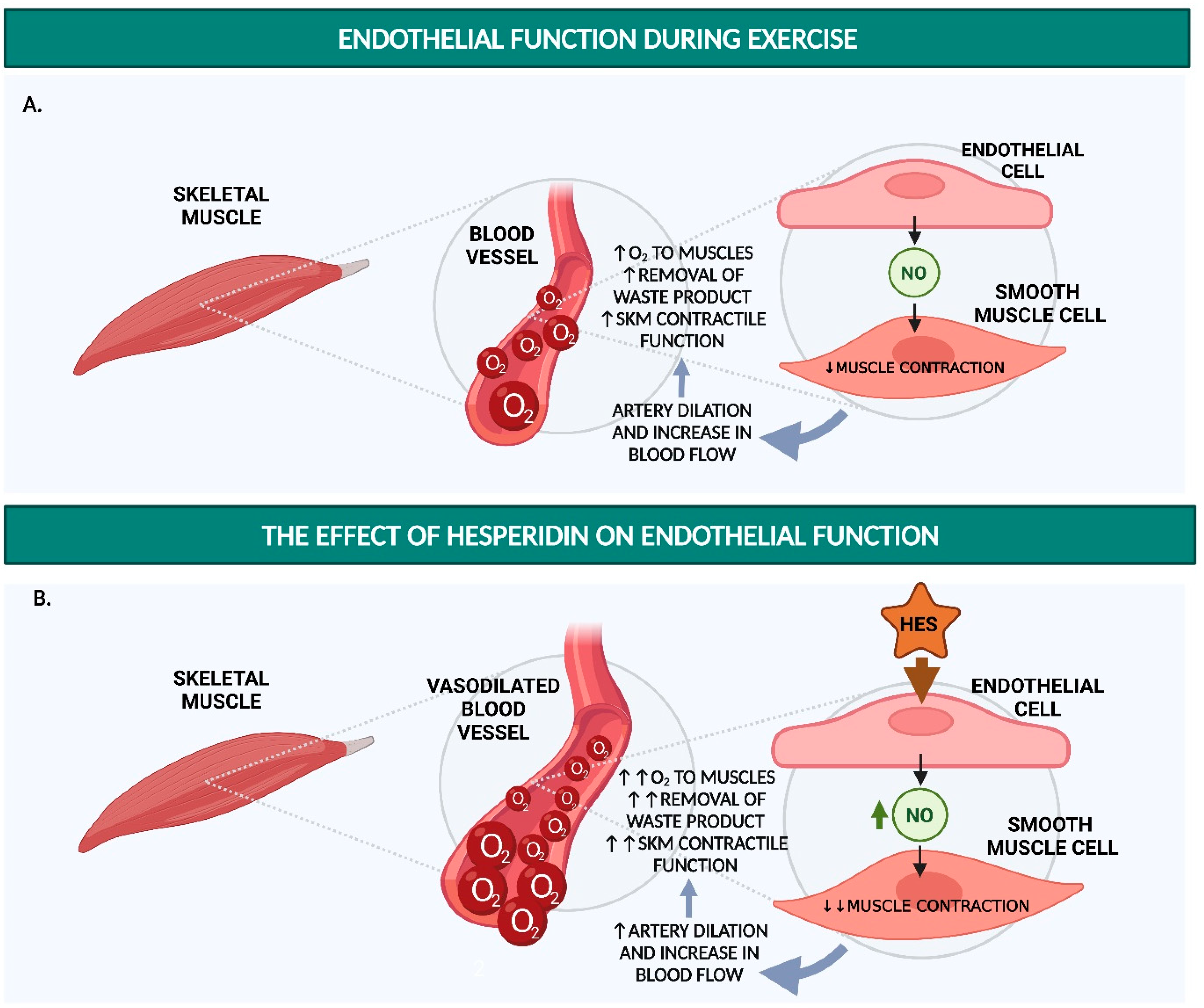
2. Hesperidin Increases Endothelial Function
2.1. Hesperidin and Hesperetin Increase NO Production and Decrease Monocyte Adhesion in Endothelial Cells
2.2. Hesperidin and Hesperetin Decrease Blood Pressure and Increase Endothelium-Dependent Vasodilation in Hypertensive Rats
2.3. Hesperidin Increases Flow-Mediated Vasodilation and Decreases sVCAM-1 and sICAM-1 in Humans
2.4. Acute Supplementation
2.5. Chronic Supplementation
3. Hesperidin Reduces Exercise-Induced Oxidative Stress
3.1. Hesperidin and Hesperetin Function as an Antioxidant In Vitro
3.2. Hesperidin Decreases ROS and Increases Antioxidant Markers in Rats
3.3. Hesperidin Supplementation Increases CAT and Decreases MDA after Strenuous Exercise Performance in Humans
4. Hesperidin Reduces Inflammatory Markers
4.1. Hesperidin and Hesperetin Decrease Pro-Inflammatory Responses in LPS-Stimulated Macrophages
4.2. Hesperidin Decreases Renal and Plasma Levels of TNF-α in Rat and Mouse Models
4.3. Hesperidin Decreases CRP, TNF-α, and IL-6 in Humans
5. Hesperidin Improves Exercise Performance
5.1. Hesperidin Supplementation Increases Maximum Running Performance in Rats
5.2. Hesperidin Improves Anaerobic Exercise Performance Outcomes in Human
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barclay, C.J. Energy demand and supply in human skeletal muscle. J. Muscle Res. Cell Motil. 2017, 38, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poole, D.C.; Copp, S.W.; Hirai, D.M.; Musch, T.I. Dynamics of muscle microcirculatory and blood-myocyte O2 flux during contractions. Acta Physiol. 2011, 202, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Segal, S.S.; Kurjiaka, D.T. Coordination of blood flow control in the resistance vasculature of skeletal muscle. Med. Sci. Sports Exerc. 1995, 27, 1158–1164. [Google Scholar] [CrossRef]
- Brodal, P.; Ingjer, F.; Hermansen, L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am. J. Physiol. 1977, 232, H705–H712. [Google Scholar] [CrossRef]
- Kabbach, E.Z.; Heubel, A.D.; da Luz Goulart, C.; Di Lorenzo, V.A.P.; Phillips, S.A.; Borghi-Silva, A.; Mendes, R.G. Association of exercise capacity and endothelial function in patients with severe exacerbations of chronic obstructive pulmonary disease. Sci. Rep. 2021, 11, 461. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Sturtzel, C. Endothelial Cells. In The Immunology of Cardiovascular Homeostasis and Pathology; Sattler, S., Kennedy-Lydon, T., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 71–91. [Google Scholar] [CrossRef]
- Lambiase, M.J.; Dorn, J.; Thurston, R.C.; Roemmich, J.N. Flow-mediated dilation and exercise blood pressure in healthy adolescents. J. Sci. Med. Sport 2014, 17, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Duncker, D.J.; Bache, R.J. Regulation of coronary blood flow during exercise. Physiol. Rev. 2008, 88, 1009–1086. [Google Scholar] [CrossRef] [Green Version]
- Marasciulo, F.L.; Montagnani, M.; Potenza, M.A. Endothelin-1: The yin and yang on vascular function. Curr. Med. Chem. 2006, 13, 1655–1665. [Google Scholar] [CrossRef]
- Garry, A.; Edwards, D.H.; Fallis, I.F.; Jenkins, R.L.; Griffith, T.M. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc. Res. 2009, 84, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef] [Green Version]
- Hendrickse, P.; Degens, H. The role of the microcirculation in muscle function and plasticity. J. Muscle Res. Cell Motil. 2019, 40, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Bassett, D.R., Jr.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef]
- Powers, S.K.; Duarte, J.; Kavazis, A.N.; Talbert, E.E. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp. Physiol. 2010, 95, 1–9. [Google Scholar] [CrossRef]
- Westerblad, H.; Allen, D.G. Emerging roles of ROS/RNS in muscle function and fatigue. Antioxid. Redox Signal. 2011, 15, 2487–2499. [Google Scholar] [CrossRef]
- Mastaloudis, A.; Leonard, S.W.; Traber, M.G. Oxidative stress in athletes during extreme endurance exercise. Free Radic. Biol. Med. 2001, 31, 911–922. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [Green Version]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef]
- Hsieh, H.-J.; Liu, C.-A.; Huang, B.; Tseng, A.H.H.; Wang, D.L. Shear-induced endothelial mechanotransduction: The interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef] [Green Version]
- Minuz, P.; Patrignani, P.; Gaino, S.; Degan, M.; Menapace, L.; Tommasoli, R.; Seta, F.; Capone, M.L.; Tacconelli, S.; Palatresi, S.; et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation 2002, 106, 2800–2805. [Google Scholar] [CrossRef] [Green Version]
- Hajjar, D.P.; Gotto, A.M., Jr. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef] [Green Version]
- Heymes, C.; Bendall, J.K.; Ratajczak, P.; Cave, A.C.; Samuel, J.L.; Hasenfuss, G.; Shah, A.M. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 2003, 41, 2164–2171. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Liu, H.B.; Li, P.S.; Yuan, W.X.; Liu, B.; Liu, S.T.; Qin, K.R. ROS and NO Dynamics in Endothelial Cells Exposed to Exercise-Induced Wall Shear Stress. Cell. Mol. Bioeng. 2019, 12, 107–120. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Kratofil, R.M.; Kubes, P.; Deniset, J.F. Monocyte Conversion During Inflammation and Injury. Arter. Thromb. Vasc. Biol. 2017, 37, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Chazaud, B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef]
- Cheng, A.J.; Jude, B.; Lanner, J.T. Intramuscular mechanisms of overtraining. Redox Biol. 2020, 35, 101480. [Google Scholar] [CrossRef]
- Paulsen, G.; Crameri, R.; Benestad, H.B.; Fjeld, J.G.; Mørkrid, L.; Hallén, J.; Raastad, T. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 75–85. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, G.; Ramer Mikkelsen, U.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Immunol. Rev. 2012, 18, 42–79. [Google Scholar]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [Green Version]
- Gorski, T.; De Bock, K. Metabolic regulation of exercise-induced angiogenesis. Vasc. Biol. 2019, 1, H1–H8. [Google Scholar] [CrossRef] [Green Version]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 2014, 49, 155–170. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Sampson, J.N.B.; Pennel, K.; Vingren, J.L.; Hill, D.W. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin. BBA Clin. 2016, 5, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [Green Version]
- Buford, T.W.; Cooke, M.B.; Willoughby, D.S. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur. J. Appl. Physiol. 2009, 107, 463–471. [Google Scholar] [CrossRef]
- Vella, L.; Markworth, J.F.; Peake, J.M.; Snow, R.J.; Cameron-Smith, D.; Russell, A.P. Ibuprofen supplementation and its effects on NF-κB activation in skeletal muscle following resistance exercise. Physiol. Rep. 2014, 2, e12172. [Google Scholar] [CrossRef]
- Place, N.; Ivarsson, N.; Venckunas, T.; Neyroud, D.; Brazaitis, M.; Cheng, A.J.; Ochala, J.; Kamandulis, S.; Girard, S.; Volungevičius, G.; et al. Ryanodine receptor fragmentation and sarcoplasmic reticulum Ca2+ leak after one session of high-intensity interval exercise. Proc. Natl. Acad. Sci. USA 2015, 112, 15492–15497. [Google Scholar] [CrossRef] [Green Version]
- Peake, J.M.; Markworth, J.F.; Nosaka, K.; Raastad, T.; Wadley, G.D.; Coffey, V.G. Modulating exercise-induced hormesis: Does less equal more? J. Appl. Physiol. 2015, 119, 172–189. [Google Scholar] [CrossRef] [Green Version]
- Pizza, F.X.; Peterson, J.M.; Baas, J.H.; Koh, T.J. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J. Physiol. 2005, 562, 899–913. [Google Scholar] [CrossRef]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A. Prevention, diagnosis, and treatment of the overtraining syndrome: Joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, M.; Foster, C.; Keul, J. Overtraining in endurance athletes: A brief review. Med. Sci. Sports Exerc. 1993, 25, 854–862. [Google Scholar] [CrossRef]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef]
- Froiland, K.; Koszewski, W.; Hingst, J.; Kopecky, L. Nutritional supplement use among college athletes and their sources of information. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 104–120. [Google Scholar] [CrossRef]
- Maughan, R.J.; Greenhaff, P.L.; Hespel, P. Dietary supplements for athletes: Emerging trends and recurring themes. J. Sports Sci. 2011, 29 (Suppl. 1), S57–S66. [Google Scholar] [CrossRef]
- Jenkinson, D.M.; Harbert, A.J. Supplements and sports. Am. Fam. Physician 2008, 78, 1039–1046. [Google Scholar]
- McGuine, T.A.; Sullivan, J.C.; Bernhardt, D.T. Creatine supplementation in high school football players. Clin. J. Sport Med 2001, 11, 247–253. [Google Scholar] [CrossRef]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN exercise & sport nutrition review: Research & recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Sastre, J.; Asensi, M.; Gascó, E.; Pallardó, F.V.; Ferrero, J.A.; Furukawa, T.; Viña, J. Exhaustive physical exercise causes oxidation of glutathione status in blood: Prevention by antioxidant administration. Am. J. Physiol. 1992, 263, R992–R995. [Google Scholar] [CrossRef]
- Romain, C.; Freitas, T.T.; Martínez-Noguera, F.J.; Laurent, C.; Gaillet, S.; Chung, L.H.; Alcaraz, P.E.; Cases, J. Supplementation with a Polyphenol-Rich Extract, TensLess®, Attenuates Delayed Onset Muscle Soreness and Improves Muscle Recovery from Damages After Eccentric Exercise. Phytotherapy Res. 2017, 31, 1739–1746. [Google Scholar] [CrossRef]
- Nicol, L.M.; Rowlands, D.S.; Fazakerly, R.; Kellett, J. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS). Eur. J. Appl. Physiol. 2015, 115, 1769–1777. [Google Scholar] [CrossRef]
- Trombold, J.R.; Barnes, J.N.; Critchley, L.; Coyle, E.F. Ellagitannin consumption improves strength recovery 2-3 d after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate Supplementation Accelerates Recovery of Muscle Damage and Soreness and Inflammatory Markers after a Weightlifting Training Session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef] [Green Version]
- Gulick, D.T.; Kimura, I.F. Delayed onset muscle soreness: What is it and how do we treat it? J. Sport Rehabil. 1996, 5, 234–243. [Google Scholar] [CrossRef]
- Murase, T.; Haramizu, S.; Shimotoyodome, A.; Nagasawa, A.; Tokimitsu, I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R708–R715. [Google Scholar] [CrossRef] [Green Version]
- Malaguti, M.; Angeloni, C.; Hrelia, S. Polyphenols in exercise performance and prevention of exercise-induced muscle damage. Oxid. Med. Cell. Longev. 2013, 2013, 825928. [Google Scholar] [CrossRef] [Green Version]
- Swamy, M.; Naveen, S.; Singsit, D.; Naika, M.; Khanum, F. Anti-fatigue effects of polyphenols extracted from pomegranate peel. Int. J. Integr. Biol. 2011, 11, 69–72. [Google Scholar]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Garg, S.; Zaneveld, L.J.; Singla, A.K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytotherapy Res. 2001, 15, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.J.; Kim, U.; Kim, I.S.; Kim, Y.; Kim, D.-H.; Han, S.B.; Kim, D.-H.; Kwon, O.-S.; Yoo, H.H. Effects of Gut Microflora on Pharmacokinetics of Hesperidin: A Study on Non-Antibiotic and Pseudo-Germ-Free Rats. J. Toxicol. Environ. Health Part A 2010, 73, 1441–1450. [Google Scholar] [CrossRef]
- Brett, G.M.; Hollands, W.; Needs, P.W.; Teucher, B.; Dainty, J.R.; Davis, B.D.; Brodbelt, J.S.; Kroon, P.A. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br. J. Nutr. 2009, 101, 664–675. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, I.L.; Chee, W.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.N.; Williamson, G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: A randomized, double-blind, crossover trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma Kinetics and Urinary Excretion of the Flavanones Naringenin and Hesperetin in Humans after Ingestion of Orange Juice and Grapefruit Juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Haidari, F.; Heybar, H.; Jalali, M.T.; Ahmadi Engali, K.; Helli, B.; Shirbeigi, E. Hesperidin supplementation modulates inflammatory responses following myocardial infarction. J. Am. Coll. Nutr. 2015, 34, 205–211. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Milenkovic, D.; Deval, C.; Dubray, C.; Mazur, A.; Morand, C. Hesperidin displays relevant role in the nutrigenomic effect of orange juice on blood leukocytes in human volunteers: A randomized controlled cross-over study. PLoS ONE 2011, 6, e26669. [Google Scholar] [CrossRef] [Green Version]
- Miwa, Y.; Yamada, M.; Sunayama, T.; Mitsuzumi, H.; Tsuzaki, Y.; Chaen, H.; Mishima, Y.; Kibata, M. Effects of glucosyl hesperidin on serum lipids in hyperlipidemic subjects: Preferential reduction in elevated serum triglyceride level. J. Nutr. Sci. Vitaminol. 2004, 50, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Miwa, Y.; Mitsuzumi, H.; Sunayama, T.; Yamada, M.; Okada, K.; Kubota, M.; Chaen, H.; Mishima, Y.; Kibata, M. Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality. J. Nutr. Sci. Vitaminol. 2005, 51, 460–470. [Google Scholar] [CrossRef] [Green Version]
- Yari, Z.; Movahedian, M.; Imani, H.; Alavian, S.M.; Hedayati, M.; Hekmatdoost, A. The effect of hesperidin supplementation on metabolic profiles in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Nutr. 2020, 59, 2569–2577. [Google Scholar] [CrossRef]
- Hong, Y.; An, Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch. Pharmacal. Res. 2018, 41, 655–663. [Google Scholar] [CrossRef]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]
- Papandreou, D.; Magriplis, E.; Abboud, M.; Taha, Z.; Karavolia, E.; Karavolias, C.; Zampelas, A. Consumption of Raw Orange, 100% Fresh Orange Juice, and Nectar- Sweetened Orange Juice-Effects on Blood Glucose and Insulin Levels on Healthy Subjects. Nutrients 2019, 11, 2171. [Google Scholar] [CrossRef] [Green Version]
- Sthijns, M.; van Blitterswijk, C.A.; LaPointe, V.L.S. Redox regulation in regenerative medicine and tissue engineering: The paradox of oxygen. J. Tissue Eng. Regen. Med. 2018, 12, 2013–2020. [Google Scholar] [CrossRef] [Green Version]
- Takumi, H.; Nakamura, H.; Simizu, T.; Harada, R.; Kometani, T.; Nadamoto, T.; Mukai, R.; Murota, K.; Kawai, Y.; Terao, J. Bioavailability of orally administered water-dispersible hesperetin and its effect on peripheral vasodilatation in human subjects: Implication of endothelial functions of plasma conjugated metabolites. Food Funct. 2012, 3, 389–398. [Google Scholar] [CrossRef]
- Liu, L.; Xu, D.-m.; Cheng, Y.-y. Distinct Effects of Naringenin and Hesperetin on Nitric Oxide Production from Endothelial Cells. J. Agric. Food Chem. 2008, 56, 824–829. [Google Scholar] [CrossRef]
- Chiou, C.-S.; Lin, J.-W.; Kao, P.-F.; Liu, J.-C.; Cheng, T.-H.; Chan, P. Effects of hesperidin on cyclic strain-induced endothelin-1 release in human umbilical vein endothelial cells. Clin. Exp. Pharmacol. Physiol. 2008, 35, 938–943. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Claude, S.; Maier, J.A.; Kamran Khan, M.; Rakotomanomana, N.; Shinkaruk, S.; Bérard, A.M.; Bennetau-Pelissero, C.; Mazur, A.; et al. Flavanone metabolites decrease monocyte adhesion to TNF-α-activated endothelial cells by modulating expression of atherosclerosis-related genes. Br. J. Nutr. 2013, 110, 587–598. [Google Scholar] [CrossRef] [Green Version]
- Nizamutdinova, I.T.; Jeong, J.J.; Xu, G.H.; Lee, S.H.; Kang, S.S.; Kim, Y.S.; Chang, K.C.; Kim, H.J. Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells. Int. Immunopharmacol. 2008, 8, 670–678. [Google Scholar] [CrossRef]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.C.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Investig. 2001, 107, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Maneesai, P.; Bunbupha, S.; Potue, P.; Berkban, T.; Kukongviriyapan, U.; Kukongviriyapan, V.; Prachaney, P.; Pakdeechote, P. Hesperidin Prevents Nitric Oxide Deficiency-Induced Cardiovascular Remodeling in Rats via Suppressing TGF-β1 and MMPs Protein Expression. Nutrients 2018, 10, 1549. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Jokura, H.; Hashizume, K.; Ominami, H.; Shibuya, Y.; Suzuki, A.; Hase, T.; Shimotoyodome, A. Hesperidin metabolite hesperetin-7-O-glucuronide, but not hesperetin-3’-O-glucuronide, exerts hypotensive, vasodilatory, and anti-inflammatory activities. Food Funct. 2013, 4, 1346–1351. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Ras, R.T.; Streppel, M.T.; Draijer, R.; Zock, P.L. Flow-mediated dilation and cardiovascular risk prediction: A systematic review with meta-analysis. Int. J. Cardiol. 2013, 168, 344–351. [Google Scholar] [CrossRef]
- Sultan, S.; Gosling, M.; Nagase, H.; Powell, J.T. Shear stress-induced shedding of soluble intercellular adhesion molecule-1 from saphenous vein endothelium. FEBS Lett. 2004, 564, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Videm, V.; Albrigtsen, M. Soluble ICAM-1 and VCAM-1 as markers of endothelial activation. Scand. J. Immunol. 2008, 67, 523–531. [Google Scholar] [CrossRef]
- Jublanc, C.; Beaudeux, J.L.; Aubart, F.; Raphael, M.; Chadarevian, R.; Chapman, M.J.; Bonnefont-Rousselot, D.; Bruckert, E. Serum levels of adhesion molecules ICAM-1 and VCAM-1 and tissue inhibitor of metalloproteinases, TIMP-1, are elevated in patients with autoimmune thyroid disorders: Relevance to vascular inflammation. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial Dysfunction and Hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Llauradó, E.; Pla-Pagà, L.; Companys, J.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Hesperidin in orange juice improves human endothelial function in subjects with elevated blood pressure and stage 1 hypertension: A randomized, controlled trial (Citrus study). J. Funct. Foods 2021, 85, 104646. [Google Scholar] [CrossRef]
- Schär, M.Y.; Curtis, P.J.; Hazim, S.; Ostertag, L.M.; Kay, C.D.; Potter, J.F.; Cassidy, A. Orange juice–derived flavanone and phenolic metabolites do not acutely affect cardiovascular risk biomarkers: A randomized, placebo-controlled, crossover trial in men at moderate risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 101, 931–938. [Google Scholar] [CrossRef] [Green Version]
- Buscemi, S.; Rosafio, G.; Arcoleo, G.; Mattina, A.; Canino, B.; Montana, M.; Verga, S.; Rini, G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am. J. Clin. Nutr. 2012, 95, 1089–1095. [Google Scholar] [CrossRef]
- Salden, B.N.; Troost, F.J.; de Groot, E.; Stevens, Y.R.; Garcés-Rimón, M.; Possemiers, S.; Winkens, B.; Masclee, A.A. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am. J. Clin. Nutr. 2016, 104, 1523–1533. [Google Scholar] [CrossRef] [Green Version]
- Kalpana, K.B.; Srinivasan, M.; Menon, V.P. Evaluation of antioxidant activity of hesperidin and its protective effect on H2O2 induced oxidative damage on pBR322 DNA and RBC cellular membrane. Mol. Cell. Biochem. 2009, 323, 21–29. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, K.J.; Choi, J.S.; Chung, H.Y. Hesperetin: A Potent Antioxidant Against Peroxynitrite. Free Radic. Res. 2004, 38, 761–769. [Google Scholar] [CrossRef]
- Chen, M.; Gu, H.; Ye, Y.; Lin, B.; Sun, L.; Deng, W.; Zhang, J.; Liu, J. Protective effects of hesperidin against oxidative stress of tert-butyl hydroperoxide in human hepatocytes. Food Chem. Toxicol. 2010, 48, 2980–2987. [Google Scholar] [CrossRef]
- Yang, H.L.; Chen, S.C.; Senthil Kumar, K.J.; Yu, K.N.; Lee Chao, P.D.; Tsai, S.Y.; Hou, Y.C.; Hseu, Y.C. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J. Agric. Food Chem. 2012, 60, 522–532. [Google Scholar] [CrossRef]
- Sthijns, M.; Schiffers, P.M.; Janssen, G.M.; Lemmens, K.J.A.; Ides, B.; Vangrieken, P.; Bouwman, F.G.; Mariman, E.C.; Pader, I.; Arnér, E.S.J.; et al. Rutin protects against H2O2-triggered impaired relaxation of placental arterioles and induces Nrf2-mediated adaptation in Human Umbilical Vein Endothelial Cells exposed to oxidative stress. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1177–1189. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Massot-Cladera, M.; Garcia-Cerdà, P.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Protective Effect of Hesperidin on the Oxidative Stress Induced by an Exhausting Exercise in Intensively Trained Rats. Nutrients 2019, 11, 783. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, E.-S.M.; Abo-Salem, O.M.; Abd-Ellah, M.F.; Abd-Alla, G.M. Hesperidin, an antioxidant flavonoid, prevents acrylonitrile-induced oxidative stress in rat brain. J. Biochem. Mol. Toxicol. 2008, 22, 268–273. [Google Scholar] [CrossRef]
- Sahu, B.D.; Kuncha, M.; Sindhura, G.J.; Sistla, R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine 2013, 20, 453–460. [Google Scholar] [CrossRef]
- Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Rubio-Arias, J.A.; Alcaraz, P.E. Acute Effects of Hesperidin in Oxidant/Antioxidant State Markers and Performance in Amateur Cyclists. Nutrients 2019, 11, 1898. [Google Scholar] [CrossRef] [Green Version]
- Boussetta, N.; Abedelmalek, S.; Khouloud, A.; Ben Anes, A.; Souissi, N. Does red orange juice supplementation has a protective effect on performance, cardiovascular parameters, muscle damage and oxidative stress markers following the Yo-Yo Intermittent Recovery Test Level-1 under polluted air? Int. J. Environ. Health Res. 2020, 30, 630–642. [Google Scholar] [CrossRef]
- Shen, C.-Y.; Lin, J.-J.; Jiang, J.-G.; Wang, T.-X.; Zhu, W. Potential roles of dietary flavonoids from Citrus aurantium L. var. amara Engl. in atherosclerosis development. Food Funct. 2020, 11, 561–571. [Google Scholar] [CrossRef]
- Sakata, K.; Hirose, Y.; Qiao, Z.; Tanaka, T.; Mori, H. Inhibition of inducible isoforms of cyclooxygenase and nitric oxide synthase by flavonoid hesperidin in mouse macrophage cell line. Cancer Lett. 2003, 199, 139–145. [Google Scholar] [CrossRef]
- Kazłowska, K.; Hsu, T.; Hou, C.-C.; Yang, W.-C.; Tsai, G.-J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Kikuchi, S.-i.; Hasunuma, R.; Maruyama, H.; Yoshikawa, T.; Kumazawa, Y. A Citrus Flavonoid Hesperidin Suppresses Infection-Induced Endotoxin Shock in Mice. Biol. Pharm. Bull. 2004, 27, 679–683. [Google Scholar] [CrossRef] [Green Version]
- Kometani, T.; Fukuda, T.; Kakuma, T.; Kawaguchi, K.; Tamura, W.; Kumazawa, Y.; Nagata, K. Effects of alpha-glucosylhesperidin, a bioactive food material, on collagen-induced arthritis in mice and rheumatoid arthritis in humans. Immunopharmacol. Immunotoxicol. 2008, 30, 117–134. [Google Scholar] [CrossRef]
- Hill, A.V.; Lupton, H. Muscular Exercise, Lactic Acid, and the Supply and Utilization of Oxygen. QJM Int. J. Med. 1923, os-16, 135–171. [Google Scholar] [CrossRef]
- Overdevest, E.; Wouters, J.A.; Wolfs, K.H.M.; van Leeuwen, J.J.M.; Possemiers, S. Citrus Flavonoid Supplementation Improves Exercise Performance in Trained Athletes. J. Sports Sci. Med. 2018, 17, 24–30. [Google Scholar]
- Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Alcaraz, P.E. Effects of 8 Weeks of 2S-Hesperidin Supplementation on Performance in Amateur Cyclists. Nutrients 2020, 12, 3911. [Google Scholar] [CrossRef]
- Van Iersel, L.E.; Stevens, Y.R.; Conchillo, J.M.; Troost, F.J. The effect of citrus flavonoid extract supplementation on anaerobic capacity in moderately trained athletes: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2021, 18, 2. [Google Scholar] [CrossRef]
- Baranauskas, M.N.; Coggan, A.R.; Gruber, A.H.; Altherr, C.A.; Raglin, J.S.; Carter, S.J. Dietary Nitrate Supplementation and Exercise-Related Performance. Nutr. Today 2020, 55, 211–217. [Google Scholar] [CrossRef]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Hart, J.D.; Dulhunty, A.F. Nitric oxide activates or inhibits skeletal muscle ryanodine receptors depending on its concentration, membrane potential and ligand binding. J. Membr. Biol. 2000, 173, 227–236. [Google Scholar] [CrossRef]
- Viner, R.I.; Williams, T.D.; Schöneich, C. Nitric oxide-dependent modification of the sarcoplasmic reticulum Ca-ATPase: Localization of cysteine target sites. Free Radic. Biol. Med. 2000, 29, 489–496. [Google Scholar] [CrossRef]
- Layec, G.; Blain, G.M.; Rossman, M.J.; Park, S.Y.; Hart, C.R.; Trinity, J.D.; Gifford, J.R.; Sidhu, S.K.; Weavil, J.C.; Hureau, T.J.; et al. Acute High-Intensity Exercise Impairs Skeletal Muscle Respiratory Capacity. Med. Sci. Sports Exerc. 2018, 50, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.T.; Blain, G.M.; Hart, C.R.; Layec, G.; Rossman, M.J.; Park, S.Y.; Trinity, J.D.; Gifford, J.R.; Sidhu, S.K.; Weavil, J.C.; et al. Acute high-intensity exercise and skeletal muscle mitochondrial respiratory function: Role of metabolic perturbation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R687–R698. [Google Scholar] [CrossRef] [PubMed]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br. J. Pharm. 2009, 157, 527–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytotherapy Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Jacobs, H.; Moalin, M.; Bast, A.; van der Vijgh, W.J.; Haenen, G.R. An essential difference between the flavonoids monoHER and quercetin in their interplay with the endogenous antioxidant network. PLoS ONE 2010, 5, e13880. [Google Scholar] [CrossRef] [Green Version]
| Author, Year, Country | Cell Type | Treatment Characteristics | Treatment Duration | Endothelial Function Outcomes (Hesperidin or Hesperetin vs. Control) |
|---|---|---|---|---|
| Rizza et al.[71] 2011 Italy | BAEC | Hesperetin 0.01 μM, 0.1 μM, 1 μM, 10 μM | 10 min | ↑pAMPK protein levels (1 μM, 10 μM) ↑pAkt protein levels (1 μM, 10 μM) ↑p-eNOS protein levels (1 μM, 10 μM) =pAMPK, pAkt and p-eNOS protein levels (0.01 μM, 0.1 μM) |
| 1 h | ↑NO production (10 μΜ) =NO production (0.01 μM, 0.1 μM, 1 μM) | |||
| ↓TNF-α-stimulated VCAM-1 protein levels (10 μM) ↓TNF-α-stimulated monocyte adhesion (10 μM) | ||||
| Takumi et al.[80] 2012 Japan | HUVECs | Hesperetin, HPT7G 25 µM, 50 µM | 24 h | ↑Release of NO, in a dose-dependent manner |
| Liu et al.[81] 2008 China | HUVECs | Hesperetin 12.5 μM, 25 μM, 50 μM, 100 μM | 24 h | ↑Release of NO in a dose-dependent manner |
| ↑eNOS mRNA expression (50 μM) ↑eNOS protein levels (50 μM) | ||||
| Chiou et al.[82] 2008 Taiwan | HUVECs | Hesperidin 1 µM, 10 µM, 100 µM | 30 min prior to strain treatment (computer-controlled application of sinusoidal negative pressure) | ↓strain-induced ET-1 secretion (10 µM, 100 µM) =strain-induced ET-1 secretion (1 µM) |
| 30 min | ↑NO production (100 µM) ↑eNOS phosphorylation (100 µM) ↑Akt phosphorylation (100 µM) | |||
| 60 min | ↑NO production (10 µM, 100 µM) =NO production (1 µM) ↑NOS activity (10 µM, 100 µM) =NOS activity (1 µM) ↑eNOS phosphorylation (100 µM) =Akt phosphorylation (100 µM) | |||
| Chanet et al.[83] 2013 France | HUVECs | Hesperetin, HPT3′G, HPT3′S, HPT7G 2 μM | 24 h | ↓TNF-α-stimulated monocyte adhesion |
| Nizamutdinova et al.[84] 2008 Korea | HUVECs | Hesperidin, hesperidin methyl chalone 1 µM, 5 µM, 10 µM, 50 µM | 24 h | ↓TNF-α-stimulated VCAM-1 protein expression (5 µM, 10 µM, 50 µM) =TNF-α-stimulated VCAM-1 protein expression (1 µM) =TNF-α-stimulated ICAM-1 protein expression (1 µM, 5 µM, 10 µM, 50 µM) |
| ↓TNF-α-stimulated monocyte adhesion (5 µM, 10 µM, 50 µM) ↓TNF-α-stimulated monocyte adhesion (1 µM) |
| Author, Year, Country | Sample Characteristics | Intervention Characteristics | Intervention Duration | Endothelial Function Outcomes (Hesperidin or Hesperetin vs. Control Groups) |
|---|---|---|---|---|
| Maneesai et al.[86] 2018 Thailand | Male Sprague–Dawley rats with hypertension (treated with L-NAME) | Hesperidin, 15 mg/kg/day and 30 mg/kg/day | 5 weeks | ↓SBP, DBP ↑plasma NOx |
| Yamamoto et al.[87] 2013 Japan | Male SHRs | Hesperetin, HPT7G, HPT3′G 5 mg/kg | 3 min | ↓SBP (hesperetin, HPT7G) =SBP (HPT3′G) =DBP (hesperetin, HPT7G, HPT3′G) |
| Yamamoto et al.[87] 2013 Japan | Thoracic aortic rings from SHRs and WKY rats | HPT7G HPT3′G 100 µM | 20 min | SHRs: ↑ACh-induced endothelium-dependent vasodilation (HPT7G) =Ach-induced endothelium-dependent vasodilation (HPT3′G) =SNP-induced endothelium-independent vasodilation (HPT7G, HPT3′G) WKY: =ACh-induced endothelium-dependent vasodilation (HPT7G, HPT3′G) =SNP-induced endothelium-independent vasodilation (HPT7G, HPT3′G) |
| Author, Year, Country | Sample Characteristics (Study Design) | Intervention Characteristics | Intervention Duration | Endothelial Function Outcomes (Hesperidin vs. Control Groups) |
|---|---|---|---|---|
| Morand et al.[94] 2011 France | n = 24 healthy males Age = 56 (1) y BMI = 27.4 (0.3) kg/m2 (RCT) | 292 mg hesperidin/day | Acute (6 h before test) | ↑microvascular reactivity |
| Chronic (4 weeks) | ↓DBP =sICAM-1 =sVCAM-1 =NOx, trend for improvement | |||
| Valls et al.[95] 2021 Spain | n = 159 subjects with pre- or stage 1 hypertension Age = 19–67 y BMI = 18.5–40.5 kg/m2 (RTC) | 600 mg hesperidin/day | Acute (6 h before test) | ↑IRH |
| Chronic (12 weeks) | ↑IRH | |||
| Takumi et al.[80] 2011 Japan | n = 10 healthy female subjects Age = 18–22 y (RTC) | 17 mg or 170 mg hesperidin | Acute (test within 70 min after intake) | ↓drop in blood flow Comment: while subjects stayed in an air-conditioned room; significant drop in both INT dosages |
| Schar et al.[96] 2015 UK | n = 16 men at moderate CVD riskAge = 60.6 (8.4) y BMI = 25.6 (0.8) kg/m2 (RCT) | 320 mg hesperidin | Acute (5 h before test) | =P-selectin expression = BP =Cardiac BRS |
| Buscemi et al.[97] 2012 Italy | n = 21 with increased cardiovascular risk Age = 19–67 y BMI = 18.5–40.5 kg/m2 (RCT) | 159.5 mg/day hesperidin | Chronic (7 days) | ↑FMD |
| Rizza et al.[71] 2011 Italy | n = 24 with MetS Age = 52 (2) BMI = 34.7 (1.5) kg/m2 (RCT) | 500 mg/day hesperidin | Chronic (3 weeks) | ↑FMD =VCAM-1 |
| Salden et al.[98] 2016 The Netherlands | n = 48 subjects with baseline FMD ≥3% Age = 53 (14) y BMI = 29 (2.6) kg/m2 (RTC) | 450 mg/day hesperidin | Chronic (6 weeks) | ↑FMD ↓sVCAM-1 ↓sICAM-1 |
| Yari et al.[75] 2020 Iran | n = 49 subjects with MetSAge = 45.1 (11.1) y BMI = 31.3 (4.9) kg/m2 (RCT) | 1 g/day hesperidin | Chronic (12 weeks) | ↓SBP |
| Author, Year, Country | Cell Type | Radical Scavenging Activity Assay | Treatment Characteristics | Treatment Duration | Oxidative Stress Outcomes (Hesperidin or Hesperetin vs. Control) |
|---|---|---|---|---|---|
| Kalpana et al. 2009 [99] India | Human erythrocytes | ·OH, ·O2, ·NO and ABTS•+ radical scavenging activity assay | Hesperidin, 0.5 mM, 1 mM, 1.5 mM, 2 mM, 2.5 mM Hesperidin 0.5 mM, 1 mM, 1.5 mM, 2 mM, 2.5 mM | Assay-dependent | =free radical scavenging activity compared to ascorbic acid and trolox, in a dose-dependent manner |
| 30 min | ↓H2O2-induced TBARS production, in a dose-dependent manner | ||||
| Kim et al.[100] 2004 South Korea | YPEN-1 prostatic endothelial cells | ONOO−, ·O2−, ·NO scavenging activity assay | Hesperetin 5 µM, 15 µM, 50 µM, 200 µM | 2 h | =ONOO− and ·O2− scavenging activity compared to penicillamine and Trolox, respectively ↓·NO scavenging activity compared to carboxy-PTIO ↓t-BHP-induced intracellular ROS generation in a dose-dependent manner |
| Chiou et al.[82] 2008 Taiwan | HUVECs | Hesperidin, 1 µM, 10 µM, 100 µM | 1 h exposure in the presence of strain treatment (computer-controlled application of sinusoidal negative pressure) | = strain-increased ROS formation (1 µM) ↓strain-increased ROS formation (10 µM, 100 µM) | |
| Chen et al.[101] 2010 China | L02 hepatic cells | Hesperidin 20 µM, 40 µM, 80 µM | 24 h | =t-BHP-induced intracellular ROS levels (20 µM) ↓t-BHP-induced intracellular ROS levels (40 µM, 80 µM) =t-BHP-induced MDA production (20 µM)↓t-BHP-induced MDA production (40 µM, 80 µM) | |
| Yang et al.[102] 2012 Taiwan | Macrophage RAW264.7 cells and fibroblast A7r5 cells | Hesperetin, Hesperetin metabolites extracted from rat serum 1 μM, 5 μM, 10 μM | 60 min for RAW264.7 cells 5 min for A7r5 cells | ↓LPS-induced intracellular ROS level (1 μM, 5 μM, 10 μM) Hesperetin metabolites showed greater antioxidant potential compared to hesperetin |
| Author, Year, Country | Sample Characteristics | Intervention Characteristics | Intervention Duration | Oxidative Stress Outcomes (Hesperidin vs. Control Groups) |
|---|---|---|---|---|
| Estruel-Amades et al.[104] 2019 Spain | Groups of Female Wister rats: Sedentary rats (SED) 5-week-trained rats (T) 5-week-trained rats undergoing an additional exhaustion test (TE) | 200 mg/kg of hesperidin three times per week | 5 weeks | ↓ROS production by peritoneal macrophages induced by the exhaustion test In thymus tissue: =CAT activity in all groupsHesperidin prevented the ↓ in SOD activity induced by the exhaustion test ↓SOD activity in SED group In spleen tissue: Hesperidin prevented the ↓ in CAT activity induced by the exhaustion test ↓SOD activity in SED and TE groups =SOD activity in T group In liver tissue: Hesperidin prevented the ↓ in CAT activity induced by the exhaustion test ↓CAT activity in SED group = CAT activity in T and TE groups ↓SOD activity all groups ↓GPx activity in SED and TE groups =GPx activity in T group |
| El-Sayed et al.[105] 2008 Egypt | Brain tissue from male Swiss albino rats | Hesperidin 200 mg/kg/day | 28 days | =MDA content ↓Acrylonitrile-induced increase in MDA content =GSH, GST content ↑SOD, GPx levels ↓CAT levels ↑Acrylonitrile-induced decrease in GSH, SOD, CAT, GPx, GST levels |
| Sahu et al.[106] 2013 India | Kidney tissue from male Wistar rats | Hesperidin 100 mg/kg/day, 200 mg/kg/day | 10 days | =ROS levels ↓cisplatin-induced increase in ROS (100, 200 mg/kg/day) =TBARS levels ↓cisplatin-induced increase in TBARS (100, 200 mg/kg/day) =SOD, GSH, CAT, GPx, GR, GST activity =cisplatin-induced decrease in GSH, CAT, GPx, GR activity (100 mg/kg/day) ↑cisplatin-induced decrease in SOD, GST activity (100 mg/kg/day) ↑cisplatin-induced decrease in SOD, GSH, CAT, GPx, GR, GST activity (200 mg/kg/day) |
| Maneesai et al.[86] 2018 Thailand | Male Sprague–Dawley rats with hypertension (treated with L-NAME) | Hesperidin 15 mg/kg/day, 30 mg/kg/day | 5 weeks | ↓vascular superoxide production (15, 30 mg/kg/day) ↓plasma MDA (15, 30 mg/kg/day) |
| Author, Year, Country | Sample Characteristics (Study Design) | Intervention Characteristics | Intervention Duration | Exercise Test | Exercise-Induced Oxidative Stress Outcomes (Hesperidin vs. Control Groups) |
|---|---|---|---|---|---|
| Martínez-Noguera et al.[107] 2019 Spain | n = 15 male amateur cyclists Age = 18–55 y, BMI = 19–25.5 kg/m2 (RCT) | 500 mg hesperidin | Acute (5 h before exercise) | Repeated sprints test (Wingate test) | =TBARS ↑CAT =SOD =GSH |
| Boussetta et al.[108] 2019 Tunisia | n = 11 healthy soccer players Age = 22.4 ± 0.5 BMI = 23.2 ± 0.4 kg/m2 (RCT) | INT: 217 mg hesperidin CON: placebo | Acute (2.5 h before the test) | Yo-Yo Intermittent Recovery Test (YYIRT) | =TAS ↓MDA |
| Author, Year, Country | Cell Type | Treatment Characteristics | Treatment Duration | Inflammatory Outcomes (Hesperidin or Hesperetin vs. Control) |
|---|---|---|---|---|
| Shen et al.[109] 2019 China | Macrophage RAW264.7 cells | HPT7G 3.13, 6.25, 12.5, 25, 50, 100 and 200 μg/mL | 24 h 12 h (for measurement of mRNA expression) | =LPS-induced NO production (3.13, 6.25 µg/mL) ↓LPS-induced NO production (12.5, 25, 50 µg/mL) ↓LPS-induced IL-6 production (50, 100, 200 µg/mL) =LPS-induced IL-6 mRNA expression (50 µg/mL) ↓LPS-induced IL-6 mRNA expression (100, 200 µg/mL) ↓LPS-induced IL-1β production (50, 100, 200 µg/mL) ↓LPS-induced IL-1β mRNA expression (50, 100, 200 µg/mL) =LPS-induced TNF-α production (50, 100, 200 µg/mL) =LPS-induced TNF-α mRNA expression (100, 200 µg/mL) ↓LPS-induced TNF-α mRNA expression (50 µg/mL) =LPS-induced COX-2 mRNA expression (50 µg/mL) ↓LPS-induced COX-2 mRNA expression (100, 200 µg/mL) |
| Yang et al.[102] 2012 Taiwan | Macrophage RAW264.7 cells and fibroblast A7r5 cells | Hesperetin, Hesperetin metabolites extracted from rat serum 1 μM, 5 μM, 10 μM | 18 h exposure for RAW264.7 cells 8 h exposure for A7r5 cells | ↓LPS-induced PGE2 production (1 μM, 5 μM, 10 μM in both cell types) ↓LPS-induced COX-2 protein levels (1 μM, 5 μM, 10 μM in both cell types) ↓LPS-induced NO production (1 μM, 5 μM, 10 μM in RAW264.7 cells) =LPS-induced NO production (1 μM, 5 μM, 10 μM in A7r5 cells) ↓iNOS protein levels ((1 μM, 5 μM, 10 μM in both cell types) ↓LPS-induced NF-κB transcriptional activation (1 μM, 5 μM, 10 μM in RAW264.7 cells) Hesperetin metabolites showed greater anti-inflammatory potential compared to hesperetin |
| Sakata et al. 2003 [110] Japan | Macrophage RAW264.7 cells | Hesperidin 10 μM, 20 μM, 30 μM | 30 min | =LPS-induced PGE2 production (10 μM) ↓LPS-induced PGE2 production (20 μM, 30 μM) =LPS-induced COX-2 protein level ((10 μM, 20 μM, 30 μM)) ↓LPS-induced NO2 production (10 μM, 20 μM, 30 μM) ↓LPS-induced iNOS protein level (10 μM, 20 μM, 30 μM) |
| Kazlowska et al.[111] 2010 Taiwan | Macrophage RAW264.7 cells | Hesperidin 5 μg/mL, 15 μg/mL, 80 μg/mL, 125 μg/mL, 150 μg/mL 250 μg/mL | 24 h | =LPS-induced NO production (5 μg/mL) ↓LPS-induced NO production (15 μg/mL, 125 μg/mL, 250 μg/mL) =LPS-induced iNOS promoter activity (80 μg/mL, 150 μg/mL, 250 μg/mL) =LPS-induced NF-κB activity (80 μg/mL, 150 μg/mL, 250 μg/mL) |
| Author, Year, Country | Sample Characteristics | Intervention Characteristics | Intervention Duration | Inflammatory Outcomes (Hesperidin vs. Control Groups) |
|---|---|---|---|---|
| Kawaguchi et al.[112] 2004 Japan | Female BALB/c and C57L/6 mice | Hesperidin, 0.1 mg, 0.3 mg, 1 mg, 3 mg/mouse | 3 h before LPS treatment | ↓LPS-induced increase in plasma TNF-α (0.3 mg, 1 mg, 3 mg/mouse) =LPS-induced increase in plasma TNF-α (0.1 mg/mouse) |
| Sahu et al.[106] 2013 India | Male Wistar rats | Hesperidin 100 mg/kg/day, 200 mg/kg/day | 10 days | =renal TNF-α (200 mg/kg/day) =cisplatin-induced increase in renal TNF-α (100 mg/kg/day) ↓cisplatin-induced increase in renal TNF-α (200 mg/kg/day) =renal myeloperoxidase (200 mg/kg/day) ↓cisplatin-induced increase in renal myeloperoxidase (100, 200 mg/kg/day) |
| Maneesai et al.[86] 2018 Thailand | Male Sprague–Dawley rats with hypertension (treated with L-NAME) | Hesperidin 15 mg/kg/day and 30 mg/kg/day | 5 weeks | ↓plasma TNF-α (15, 30 mg/kg/day) |
| Author, Year, Country | Subject Characteristics (Study Design) | Intervention Characteristics | Intervention Duration | Inflammatory Outcomes (Hesperidin vs. Control Groups) |
|---|---|---|---|---|
| Buscemi et al.[97] 2012 Italy | n = 21 subjects with increased cardiovascular risk Age = 19–67 y BMI = 18.5–40.5 kg/m2 (RCT) | 159.5 mg/day hesperidin | 7 days | ↓hs-CRP ↓IL-6 ↓TNF-α |
| Yari et al.[75] 2020 Iran | n = 49 subjects with MetS Age = 45.1 ± 11.1 y BMI = 31.3 ± 4.9 kg/m2 (RCT) | 1 g/day hesperidin | 12 weeks | ↓TNF-α =hs-CRP |
| Kometani et al.[113] 2008 Japan | n = 19 subjects with arthritis Age = 26–49 y (RCT) | 3 g/day hesperidin | 12 weeks | ↓CRP |
| Morand et al.[94] 2011 France | n = 24 healthy males Age = 56 ± 1 y BMI = 27.4 ± 0.3 kg/m2 (RCT) | 292 mg/day hesperidin | 4 weeks | =CRP =IL-6 |
| Author, Year, Country | Sample Characteristics (Study Design) | Intervention Characteristics | Intervention Duration | Exercise Test | Exercise Performance Outcomes (Hesperidin vs. Control Groups) |
|---|---|---|---|---|---|
| Estruel-Amades et al.[104] 2019 Spain | Female Wistar rats | 200 mg/kg of hesperidin three times per week | Chronic (5 weeks) | Maximum distance run until exhaustion test (2 times per week for 5 weeks) | ↑ maximum distance during all performed tests (week 1–5) |
| Author, Year, Country | Sample Characteristics (Study Design) | Intervention Characteristics | Intervention Duration | Exercise Test | Exercise Performance Outcomes (Hesperidin vs. Control Groups) |
|---|---|---|---|---|---|
| Martínez-Noguera et al.[107] 2019 Spain | n = 15 male amateur cyclists Age = 18–55 y, BMI = 19–25.5 kg/m2 (RCT) | 500 mg hesperidin | Acute (5 h before exercise) | Repeated sprints test (Wingate test) | ↑Average power ↑Maximal speed ↑Total energy |
| Boussetta et al.[108] 2019 Tunisia | n = 11 healthy soccer players Age = 22.4 ± 0.5 y BMI = 23.2 ± 0.4 kg/m2 (RCT) | 217 mg hesperidin | Acute 2.5 h before the test) | Yo-Yo intermittent recovery test (YYIRT) | =VO2max (increasing trend) =PRE |
| Overdevest et al.[115] 2018 The Netherlands | n = 39 trained males Age = 18–25 y BMI = 22.1 (0.30) kg/m2 (RCT) | 500 mg/day citrus fruit extract (450 mg hesperidin/day) | Chronic (4 weeks) | 10 min time-trial on a cycle ergometer | ↑Δ Power ↓VO2/Power ratio = Es VO2max |
| Martínez-Noguera et al.[116] 2020 Spain | n = 40 male amateur cyclists Age = 18–55 y, BMI = 19–25.5 kg/m2 (RCT) | 500 mg/day hesperidin | Chronic (8 weeks) | Repeated sprints test (Wingate test) | ↑Absolute peak power ↑Relative peak power |
| Incremental test until exhaustion | ↑ Maximum power ↑ Estimated FTP | ||||
| Van Iersel et al.[117] 2021 The Netherlands | n = 92 moderately trained healthy subjects Age = 24 ± 5 y BMI = 22.4 ± 2.2 kg/m2 (RCT) | 360 mg or 450 mg hesperidin | Chronic (4 and 8 weeks) | Wingate anaerobic test | ↑Average power (360 mg after 4 weeks) ↑Average power (360 mg after 8 weeks) ↑Average power (450 mg after 4 weeks) ↑5 s Peak power (360 mg after 4 weeks) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imperatrice, M.; Cuijpers, I.; Troost, F.J.; Sthijns, M.M.J.P.E. Hesperidin Functions as an Ergogenic Aid by Increasing Endothelial Function and Decreasing Exercise-Induced Oxidative Stress and Inflammation, Thereby Contributing to Improved Exercise Performance. Nutrients 2022, 14, 2955. https://doi.org/10.3390/nu14142955
Imperatrice M, Cuijpers I, Troost FJ, Sthijns MMJPE. Hesperidin Functions as an Ergogenic Aid by Increasing Endothelial Function and Decreasing Exercise-Induced Oxidative Stress and Inflammation, Thereby Contributing to Improved Exercise Performance. Nutrients. 2022; 14(14):2955. https://doi.org/10.3390/nu14142955
Chicago/Turabian StyleImperatrice, Maria, Iris Cuijpers, Freddy J. Troost, and Mireille M. J. P. E. Sthijns. 2022. "Hesperidin Functions as an Ergogenic Aid by Increasing Endothelial Function and Decreasing Exercise-Induced Oxidative Stress and Inflammation, Thereby Contributing to Improved Exercise Performance" Nutrients 14, no. 14: 2955. https://doi.org/10.3390/nu14142955
APA StyleImperatrice, M., Cuijpers, I., Troost, F. J., & Sthijns, M. M. J. P. E. (2022). Hesperidin Functions as an Ergogenic Aid by Increasing Endothelial Function and Decreasing Exercise-Induced Oxidative Stress and Inflammation, Thereby Contributing to Improved Exercise Performance. Nutrients, 14(14), 2955. https://doi.org/10.3390/nu14142955






