Impact of Time-Restricted Feeding on Adaptation to a 6-Hour Delay Phase Shift or a 12-Hour Phase Shift in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Schedules
2.3. Wheel Running Activity
2.4. Sepsis Model
2.5. Statistical Analysis
3. Results
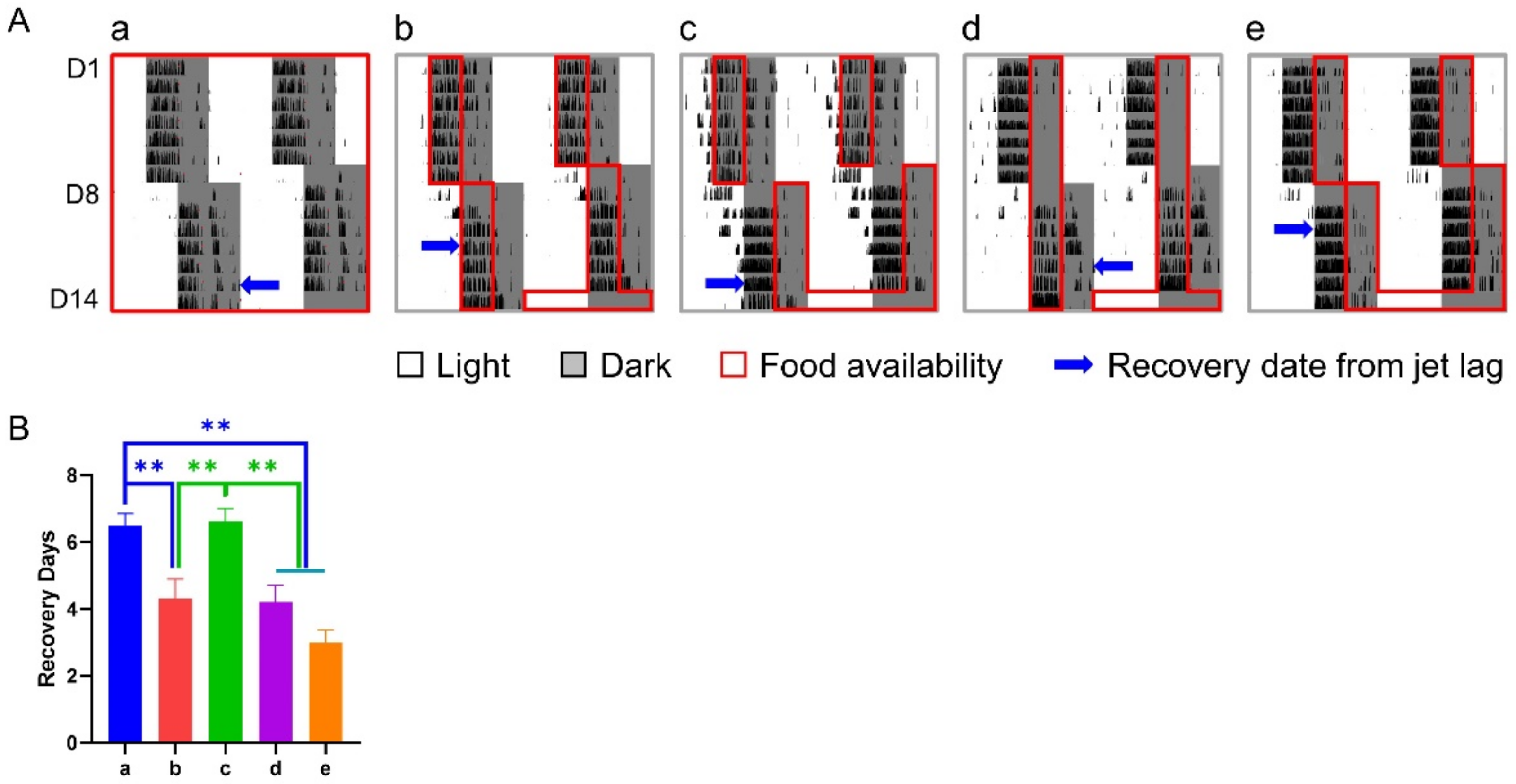
3.1. Effect of 6-h TRFs on Mice Treated with a 6-h Delay Shift
3.2. Effect of 6-h TRFs on Mice Treated with a 12-h Shift
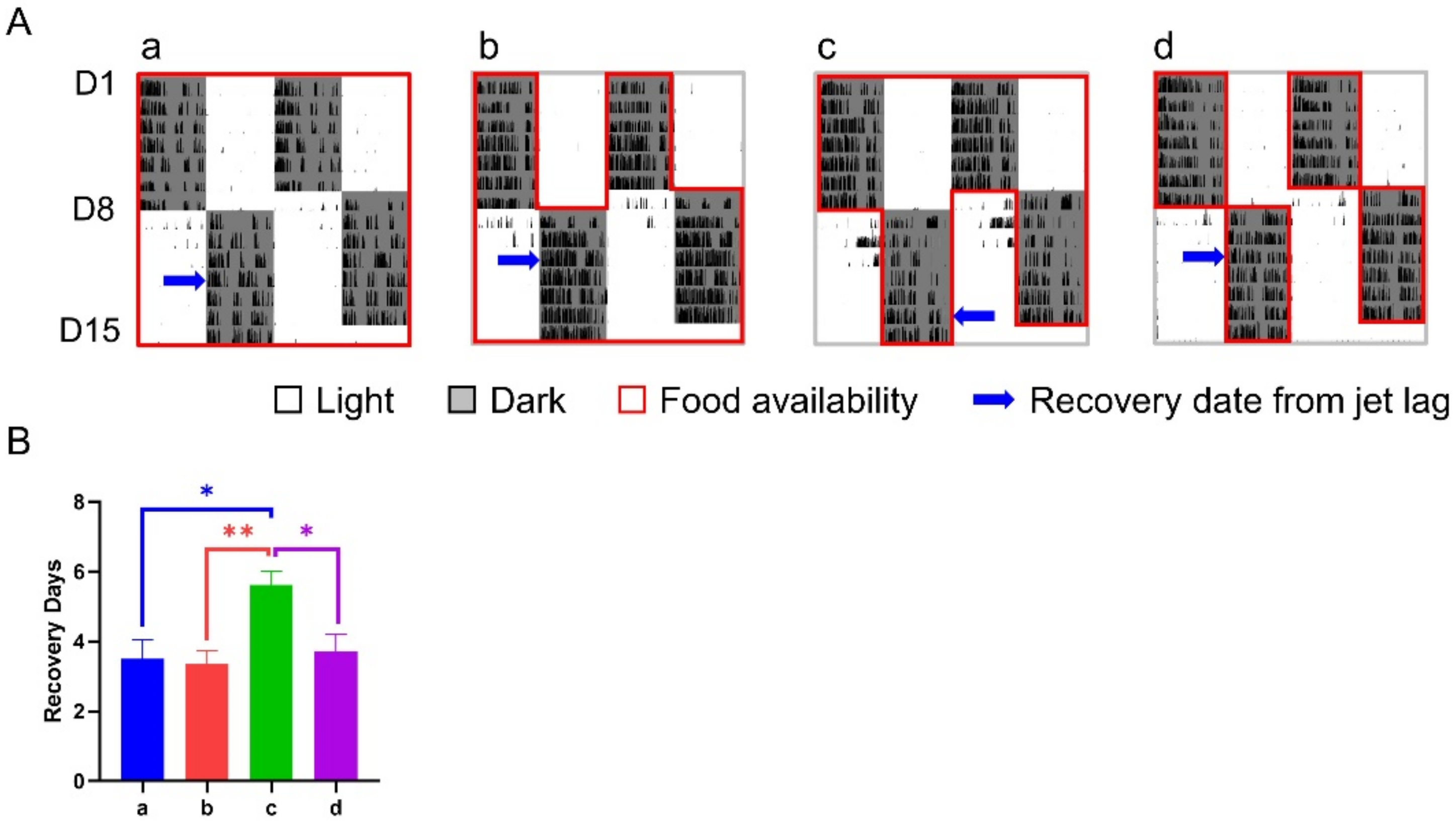
3.3. Effect of 12-h TRFs on Mice Treated with a 12-h Shift
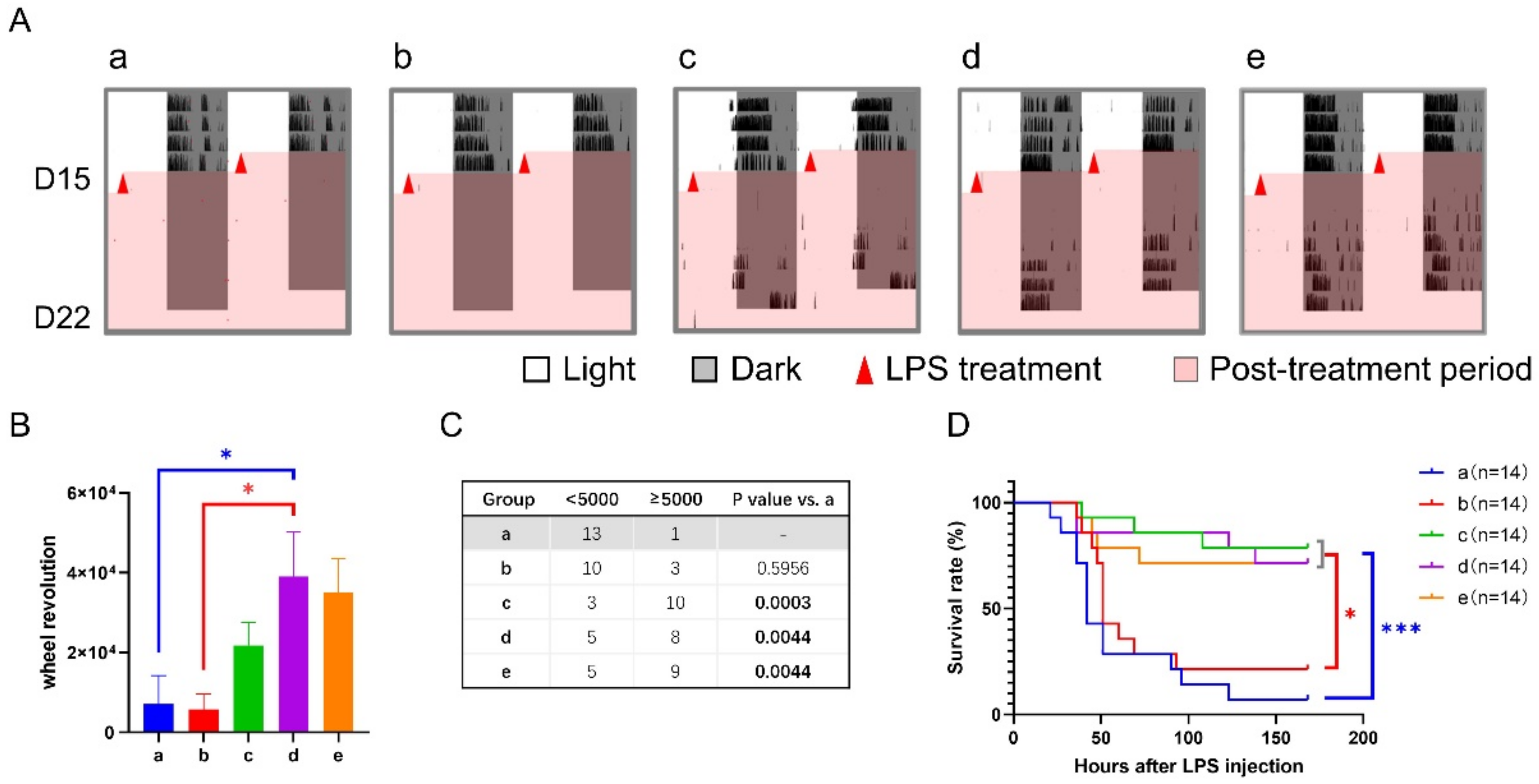
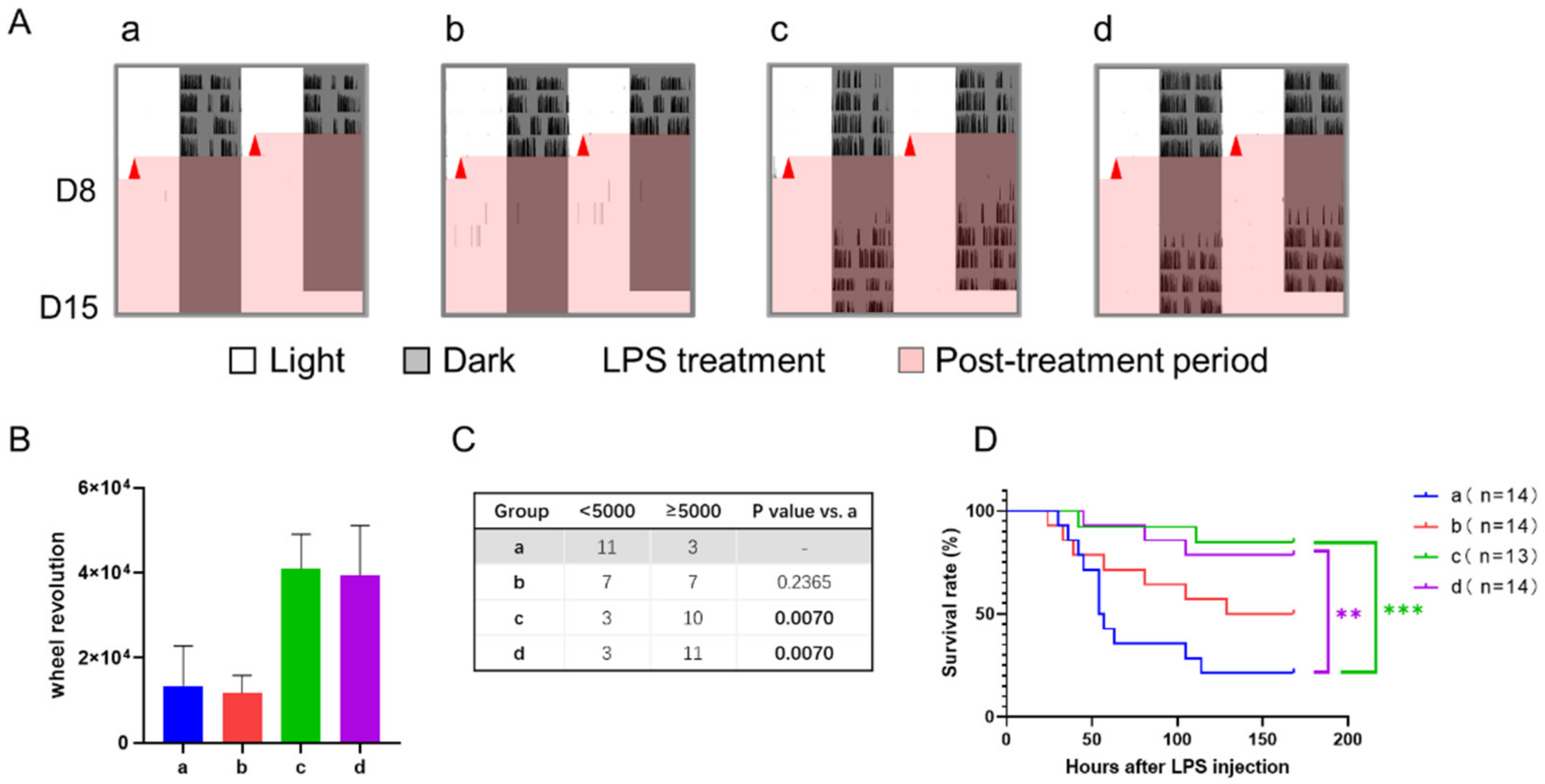
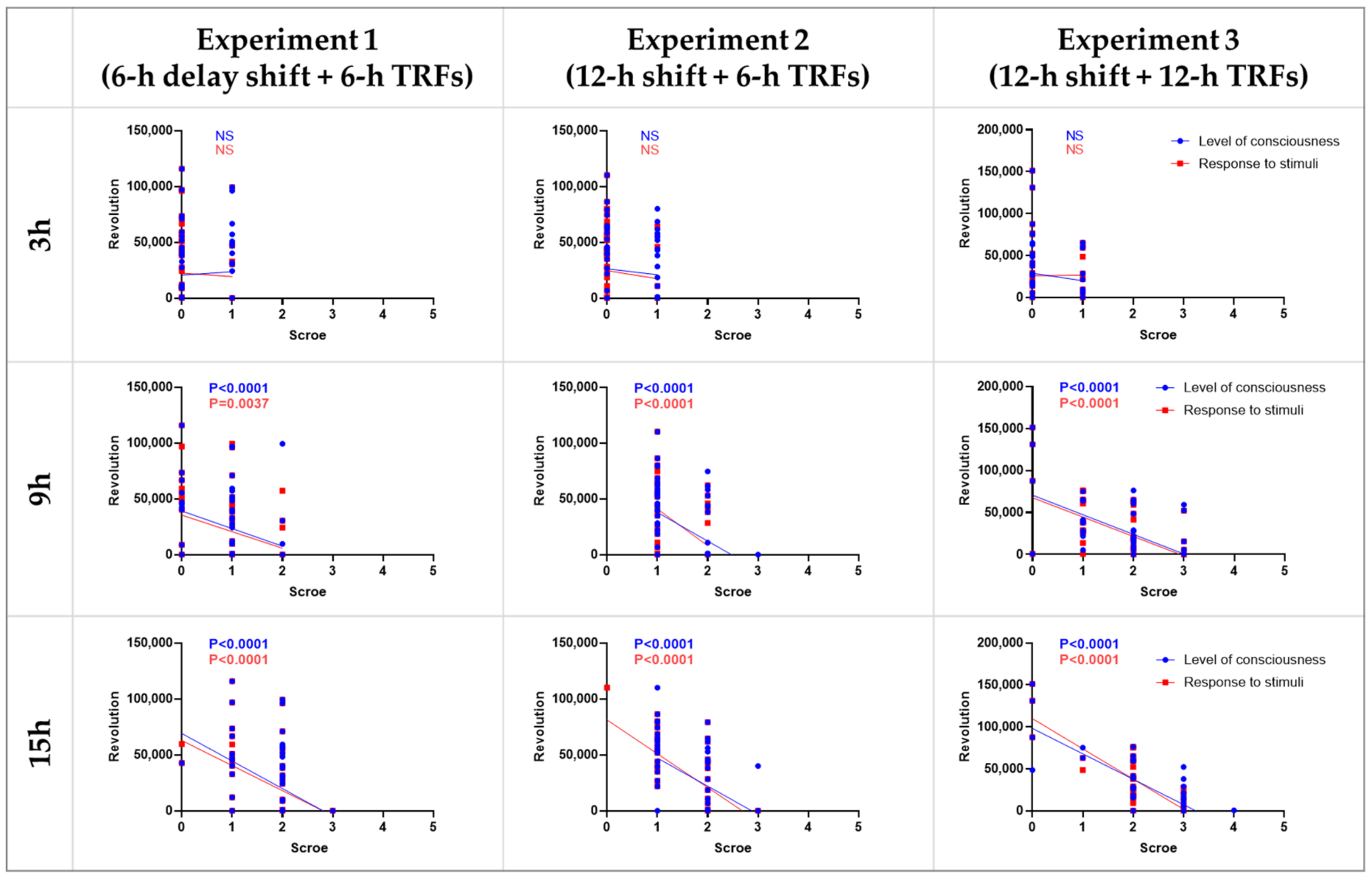
3.4. Correlation between Septic Score and Wheel-Running Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallach, T.; Kramer, A. Chemical chronobiology: Toward drugs manipulating time. FEBS Lett. 2015, 589, 1530–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, B.; Ma, C.; Chen, L.; FitzGerald, G.A.; Yang, G. Impact of Time-Restricted Feeding to Late Night on Adaptation to a 6 h Phase Advance of the Light-Dark Cycle in Mice. Front. Physiol. 2021, 12, 634187. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [Green Version]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [Green Version]
- Ángeles-Castellanos, M.; Amaya, J.M.; Salgado-Delgado, R.; Buijs, R.M.; Escobar, C. Scheduled food hastens re-entrainment more than melatonin does after a 6-h phase advance of the light-dark cycle in rats. J. Biol. Rhythms 2011, 26, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Ubaldo-Reyes, L.M.; Buijs, R.M.; Escobar, C.; Ángeles-Castellanos, M. Scheduled meal accelerates entrainment to a 6-h phase advance by shifting central and peripheral oscillations in rats. Eur. J. Neurosci. 2017, 46, 1875–1886. [Google Scholar] [CrossRef]
- Schilperoort, M.; van den Berg, R.; Dolle, M.E.T.; van Oostrom, C.T.M.; Wagner, K.; Tambyrajah, L.L.; Wackers, P.; Deboer, T.; Hulsegge, G.; Proper, K.I.; et al. Time-restricted feeding improves adaptation to chronically alternating light-dark cycles. Sci. Rep. 2019, 9, 7874. [Google Scholar] [CrossRef]
- Pfeffer, M.; Korf, H.W.; von Gall, C. Chronotype and stability of spontaneous locomotor activity rhythm in BMAL1-deficient mice. Chronobiol. Int. 2014, 32, 81–91. [Google Scholar] [CrossRef]
- Yang, G.; Paschos, G.; Curtis, A.M.; Musiek, E.S.; McLoughlin, S.C.; Fitzgerald, G.A. Knitting Up the Raveled Sleave of Care. Sci. Transl. Med. 2013, 5, 212–213. [Google Scholar] [CrossRef]
- Kriebs, A. The benefits of time-restricted eating. Nat. Rev. Endocrinol. 2020, 16, 68. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Manoogian, E.N.C.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.; Rijo-Ferreira, F.; Izumo, M.; Xu, P.; Wight-Carter, M.; Green, C.B.; Takahashi, J.S. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 2022, 376, 1192–1202. [Google Scholar] [CrossRef]
- Castanon-Cervantes, O.; Wu, M.; Ehlen, J.C.; Paul, K.; Gamble, K.L.; Johnson, R.L.; Besing, R.C.; Menaker, M.; Gewirtz, A.T.; Davidson, A.J. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010, 185, 5796–5805. [Google Scholar] [CrossRef] [Green Version]
- Manoogian, E.N.C.; Chaix, A.; Panda, S. When to Eat: The Importance of Eating Patterns in Health and Disease. J. Biol. Rhythms 2019, 34, 579–581. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, L.P.; Alzamendi, A.; Giovambattista, A.; Chiesa, J.J.; Golombek, D.A. Effects of chronic forced circadian desynchronization on body weight and metabolism in male mice. Physiol. Rep. 2016, 4, e12743. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Saderi, N.; Buijs, R.M.; Escobar, C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 2010, 151, 1019–1029. [Google Scholar] [CrossRef]
- Oike, H.; Sakurai, M.; Ippoushi, K.; Kobori, M. Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. Biochem. Biophys. Res. Commun. 2015, 465, 556–561. [Google Scholar] [CrossRef]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Heilbronn, L.K. Time-Restricted Eating: Benefits, Mechanisms, and Challenges in Translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Chen, L.; Zhang, J.; Ren, B.; FitzGerald, G.A. Bmal1 deletion in mice facilitates adaptation to disrupted light/dark conditions. JCI Insight 2019, 4, e125133. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. Recent advances in circadian rhythms in cardiovascular system. Front. Pharmacol. 2015, 6, 71. [Google Scholar] [CrossRef] [Green Version]



| Schedule | Hours after LPS | Type of Sepsis Score | Group o | Group a | Group b | Group c | Group d | Group e | Other Comparisons (Low vs. High, p < 0.05) |
|---|---|---|---|---|---|---|---|---|---|
| Experiment 1 (6-h delay shift + 6-h TRFs) | 3 | Level of consciousness | - | 0.43 ± 0.14 | 0.50 ± 0.14 | 0.50 ± 0.13 | 0.43 ± 0.14 | 0.07 ± 0.07 | - |
| Response to stimuli | - | 0.50 ± 0.14 | 0.21 ± 0.11 | 0.21 ± 0.11 | 0.07 ± 0.07 | 0.14 ± 0.10 | - | ||
| 9 | Level of consciousness | - | 1.79 ± 0.11 | 1.21 ± 0.15 | 1.21 ± 0.19 | 0.57 ± 0.14 *** | 0.79 ± 0.15 *** | d vs. b, c | |
| Response to stimuli | - | 1.43 ± 0.14 | 1.00 ± 0.15 | 1.00 ± 0.18 | 0.50 ± 0.17 ** | 0.64 ± 0.17 ** | - | ||
| 15 | Level of consciousness | - | 2.36 ± 0.13 | 2.14 ± 0.14 | 1.79 ± 0.11 * | 1.64 ± 0.13 ** | 1.64 ± 0.17 ** | - | |
| Response to stimuli | - | 2.36 ± 0.13 | 2.14 ± 0.14 | 1.79 ± 0.11 * | 1.64 ± 0.13 ** | 1.21 ± 0.19 *** | e vs. b, c | ||
| Experiment 2 (12-h shift + 6-h TRFs) | 3 | Level of consciousness | 0.29 ± 0.13 | 0.50 ± 0.14 | 0.69 ± 0.13 | 0.36 ± 0.13 | 0.71 ± 0.13 | 0.43 ± 0.14 | - |
| Response to stimuli | 0.14 ± 0.10 | 0.14 ± 0.10 | 0.08 ± 0.08 | 0.07 ± 0.07 | 0.14 ± 0.10 | 0.07 ± 0.07 | - | ||
| 9 | Level of consciousness | 2.07 ± 0.13 | 1.79 ± 0.11 | 1.39 ± 0.14 | 1.21 ± 0.11 * | 1.64 ± 0.13 | 1.21 ± 0.11 * | b, c, e vs. o | |
| Response to stimuli | 1.93 ± 0.07 | 1.93 ± 0.07 | 1.39 ± 0.14 * | 1.29 ± 0.13 ** | 1.29 ± 0.13 ** | 1.21 ± 0.11 *** | b, c, d, e vs. o | ||
| 15 | Level of consciousness | 2.21 ± 0.19 * | 2.79 ± 0.11 | 1.92 ± 0.14 *** | 1.21 ± 0.11 *** | 1.86 ± 0.10 *** | 1.50 ± 0.14 *** | b, c, d, e vs. o | |
| Response to stimuli | 2.36 ± 0.13 | 2.79 ± 0.11 | 1.46 ± 0.18 *** | 1.29 ± 0.12 *** | 1.86 ± 0.10 *** | 1.43 ± 0.14 *** | b, c, d, e vs. o | ||
| Experiment 3 (12-h shift + 12-h TRFs) | 3 | Level of consciousness | - | 0.36 ± 0.13 | 0.29 ± 0.13 | 0.38 ± 0.14 | 0.21 ± 0.11 | - | - |
| Response to stimuli | - | 0.36 ± 0.13 | 0.21 ± 0.11 | 0.54 ± 0.14 | 0.43 ± 0.14 | - | - | ||
| 9 | Level of consciousness | - | 2.21 ± 0.24 | 2.29 ± 0.16 | 1.62 ± 0.18 | 1.50 ± 0.27 | - | - | |
| Response to stimuli | - | 2.07 ± 0.25 | 2.07 ± 0.22 | 1.39 ± 0.18 | 1.64 ± 0.27 | - | - | ||
| 15 | Level of consciousness | - | 2.71 ± 0.24 | 2.86 ± 0.10 | 2.15 ± 0.15 | 1.79 ± 0.32 * | - | d vs. b | |
| Response to stimuli | - | 2.71 ± 0.22 | 2.71 ± 0.13 | 2.15 ± 0.10 | 1.71 ± 0.27 ** | - | d vs. b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.; Huang, Y.; Zhang, J.; Li, J.; Liu, Z.; Guan, Y.; Chen, L.; Yang, G. Impact of Time-Restricted Feeding on Adaptation to a 6-Hour Delay Phase Shift or a 12-Hour Phase Shift in Mice. Nutrients 2022, 14, 3025. https://doi.org/10.3390/nu14153025
Ren B, Huang Y, Zhang J, Li J, Liu Z, Guan Y, Chen L, Yang G. Impact of Time-Restricted Feeding on Adaptation to a 6-Hour Delay Phase Shift or a 12-Hour Phase Shift in Mice. Nutrients. 2022; 14(15):3025. https://doi.org/10.3390/nu14153025
Chicago/Turabian StyleRen, Baoyin, Yingzhi Huang, Jiayang Zhang, Jiazhi Li, Zhaiyi Liu, Youfei Guan, Lihong Chen, and Guangrui Yang. 2022. "Impact of Time-Restricted Feeding on Adaptation to a 6-Hour Delay Phase Shift or a 12-Hour Phase Shift in Mice" Nutrients 14, no. 15: 3025. https://doi.org/10.3390/nu14153025






