Dyslipidaemia Is Associated with Severe Disease Activity and Poor Prognosis in Ulcerative Colitis: A Retrospective Cohort Study in China
Abstract
:1. Introduction
2. Materials and Methods
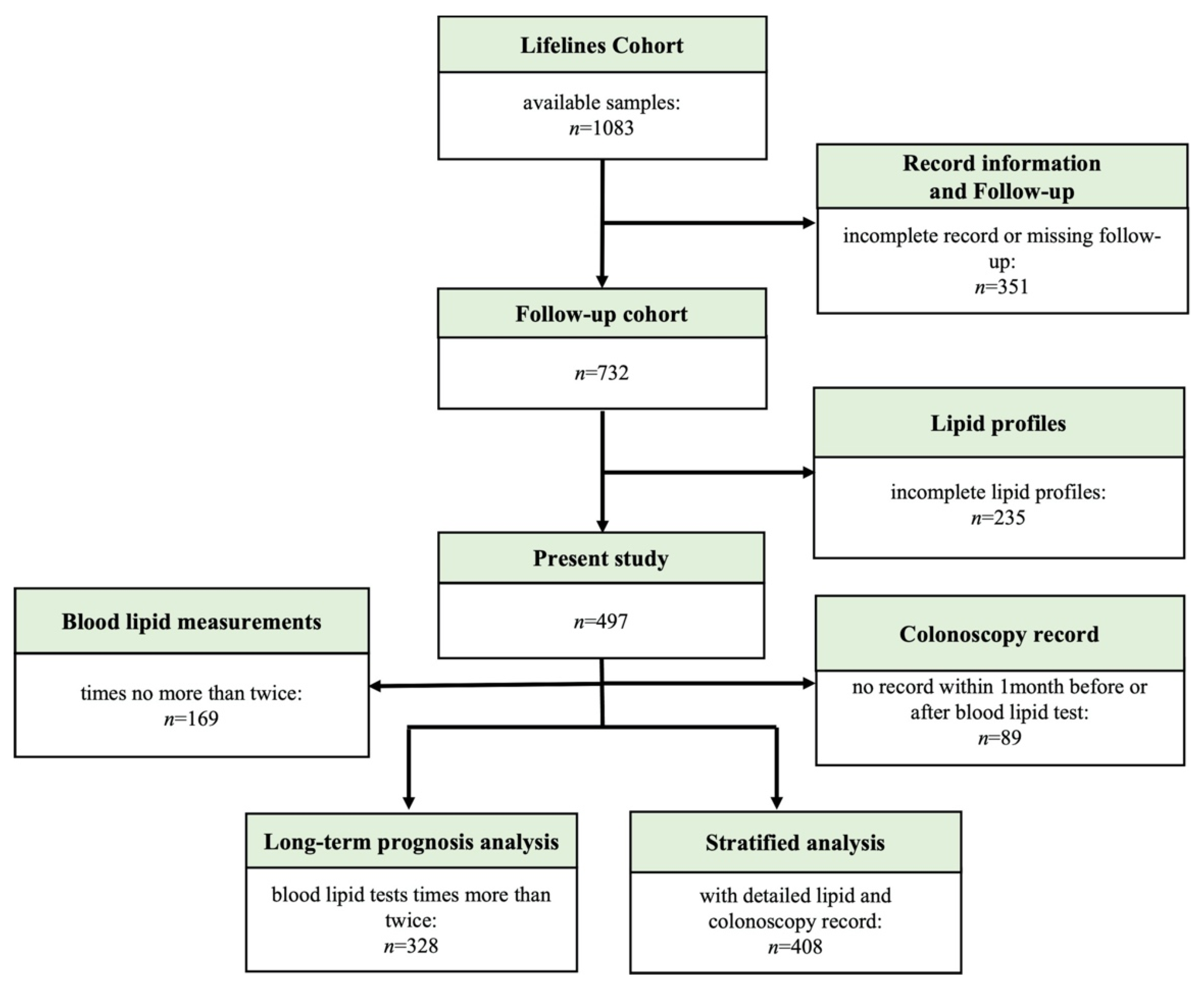
2.1. Patients
2.2. Outcome Measures
2.3. Study Variables
2.4. Definitions
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
3.2. Correlation between Lipid Levels and Disease Activity
3.3. Correlation between Lipid Levels and Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beaugerie, L.; Itzkowitz, S.H. Cancers complicating inflammatory bowel disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shen, X.; Li, Y.; Guo, Z.; Zhu, W.; Zuo, L.; Zhao, J.; Gu, L.; Gong, J.; Li, J. Therapeutic Potential to Modify the Mucus Barrier in Inflammatory Bowel Disease. Nutrients 2016, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyengar, N.M.; Hudis, C.A.; Dannenberg, A.J. Obesity and cancer: Local and systemic mechanisms. Annu. Rev. Med. 2015, 66, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 2013, 19, 6074–6083. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- van Kruijsdijk, R.C.; van der Wall, E.; Visseren, F.L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Zhang, H.; Lu, J.; Ding, Q.; Li, X.; Wang, X.; Sun, D.; Tan, L.; Mu, L.; Liu, J.; et al. Prevalence of Dyslipidemia and Availability of Lipid-Lowering Medications Among Primary Health Care Settings in China. JAMA Netw. Open. 2021, 4, e2127573. [Google Scholar] [CrossRef]
- Primatesta, P.; Poulter, N.R. Levels of dyslipidaemia and improvement in its management in England: Results from the Health Survey for England 2003. Clin. Endocrinol. 2006, 64, 292–298. [Google Scholar] [CrossRef]
- Guallar-Castillón, P.; Gil-Montero, M.; León-Muñoz, L.M.; Graciani, A.; Bayán-Bravo, A.; Taboada, J.M.; Banegas, J.R.; Rodrigues-Artalejo, F. Magnitude and management of hypercholesterolemia in the adult population of Spain, 2008–2010: The ENRICA Study. Rev. Esp. Cardiol. 2012, 65, 551–558. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, P.; Zhou, T.; Lu, J.; Spatz, E.S.; Nasir, K.; Jiang, L.; Krumholz, H.M. Comparison of Prevalence, Awareness, Treatment, and Control of Cardiovascular Risk Factors in China and the United States. J. Am. Heart Assoc. 2018, 7, e007462. [Google Scholar] [CrossRef] [Green Version]
- Sappati Biyyani, R.S.; Putka, B.S.; Mullen, K.D. Dyslipidemia and lipoprotein profiles in patients with inflammatory bowel disease. J. Clin. Lipidol. 2010, 4, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.; Im, J.P.; Han, K.; Park, S.; Hong, S.W.; Moon, J.M.; Kang, E.A.; Chun, J.; Lee, H.J.; Kim, J.S. Crohn’s disease and ulcerative colitis are associated with different lipid profile disorders: A nationwide population-based study. Aliment. Pharmacol. Ther. 2020, 51, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, S.; Iijima, H.; Otake, Y.; Amano, T.; Tani, M.; Yoshihara, T.; Tashiro, T.; Tsujii, Y.; Inoue, T.; Hayashi, Y.; et al. Novel mass spectrometry-based comprehensive lipidomic analysis of plasma from patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2020, 35, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpakis, E.; Ramos-Rivers, C.; Regueiro, M.; Hashash, J.G.; Barrie, A.; Swoger, J.; Baidoo, L.; Schwartz, M.; Dunn, M.A.; Koutroubakis, I.E.; et al. Association Between Long-Term Lipid Profiles and Disease Severity in a Large Cohort of Patients with Inflammatory Bowel Disease. Dig. Dis. Sci. 2016, 61, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Guo, J.; Zhang, T.; Gu, J.; Li, H.; Wang, J. The Role of Dyslipidemia in Colitis-Associated Colorectal Cancer. J. Oncol. 2021, 2021, 6640384. [Google Scholar] [CrossRef] [PubMed]
- Dragasevic, S.; Stankovic, B.; Kotur, N.; Sokic-Milutinovic, A.; Milovanovic, T.; Lukic, S.; Milosavljevic, T.; Srzentic Drazilov, S.; Klaassen, K.; Pavlovic, S.; et al. Metabolic Syndrome in Inflammatory Bowel Disease: Association with Genetic Markers of Obesity and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 31–38. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2021, 16, 2–17. [Google Scholar] [CrossRef]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Travis, S.P.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.F.; Feagan, B.G.; Hanauer, S.B.; Lichtenstein, G.R.; Marteau, P.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Verstockt, B.; Mertens, E.; Dreesen, E.; Outtier, A.; Noman, M.; Tops, S.; Schops, G.; Van Assche, G.; Vermeire, S.; Gils, A.; et al. Influence of Drug Exposure on Vedolizumab-Induced Endoscopic Remission in Anti-Tumour Necrosis Factor [TNF] Naïve and Anti-TNF Exposed IBD Patients. J. Crohns Colitis 2020, 14, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripollés Piquer, B.; Nazih, H.; Bourreille, A.; Segain, J.P.; Huvelin, J.M.; Galmiche, J.P.; Bard, J.-M. Altered lipid, apolipoprotein, and lipoprotein profiles in inflammatory bowel disease: Consequences on the cholesterol efflux capacity of serum using Fu5AH cell system. Metabolism 2006, 55, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Rizwan, Y.; Thibault, L.; Lepage, G.; Brunet, S.; Bouthillier, L.; Seidman, E. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am. J. Clin. Nutr. 2000, 71, 807–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motobayashi, M.; Matsuoka, K.; Takenaka, K.; Fujii, T.; Nagahori, M.; Ohtsuka, K.; Iwamoto, F.; Tsuchiya, K.; Negi, M.; Eishi, Y.; et al. Predictors of mucosal healing during induction therapy in patients with acute moderate-to-severe ulcerative colitis. J. Gastroenterol. Hepatol. 2019, 34, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Romanato, G.; Scarpa, M.; Angriman, I.; Faggian, D.; Ruffolo, C.; Marin, R.; Zambon, S.; Basato, S.; Zanoni, S.; Filosa, T.; et al. Plasma lipids and inflammation in active inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2009, 29, 298–307. [Google Scholar] [CrossRef]
- Yu, B.L.; Wang, S.H.; Peng, D.Q.; Zhao, S.P. HDL and immunomodulation: An emerging role of HDL against atherosclerosis. Immunol. Cell Biol. 2010, 88, 285–290. [Google Scholar] [CrossRef]
- Becker, S.A.; McClave, S.A. Lipid profiles in Crohn’s disease patients with and without ileal resection. Am. J. Gastroenterol. 1996, 91, 2452. [Google Scholar]
- Romanato, G.; Scarpa, M.; Ruffolo, C.; Marin, R.; Zambon, S.; Zanoni, S.; Basato, S.; Filosa, T.; Pilon, F.; Angriman, I.; et al. Lipid and phospholipid profile after bowel resection for Crohn’s disease. Int. J. Colorectal Dis. 2008, 23, 931–938. [Google Scholar] [CrossRef]
- Tanaka, M.; Iwao, Y.; Sasaki, S.; Okamoto, S.; Ogata, H.; Hibi, T.; Kazuma, K. Moderate dietary temperance effectively prevents relapse of Crohn disease: A prospective study of patients in remission. Gastroenterol. Nurs. 2007, 30, 202–210. [Google Scholar] [CrossRef]
- Li, T.; Qian, Y.; Li, H.; Deng, J. Combination of serum lipids and cancer antigens as a novel marker for colon cancer diagnosis. Lipids Health Dis. 2018, 17, 261. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Lei, X.; Pan, X.; Zeng, X.; Li, W. Association between serum lipids and breast cancer risk in premenopausal women: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211061033. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, M.; Hutri-Kähönen, N.; Tossavainen, P.; Sipponen, T.; Pitkänen, N.; Laitinen, T.; Jokinen, E.; Viikari, J.S.A.; Raitakari, O.T.; Juonala, M.; et al. Low childhood high density lipoprotein cholesterol levels and subsequent risk for chronic inflammatory bowel disease. Dig. Liver Dis. 2018, 50, 348–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, D.; Dayer, J.M. High-density lipoprotein-associated apolipoprotein A-I: The missing link between infection and chronic inflammation? Autoimmun. Rev. 2002, 1, 111–117. [Google Scholar] [CrossRef]
- Ossoli, A.; Wolska, A.; Remaley, A.T.; Gomaraschi, M. High-density lipoproteins: A promising tool against cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159068. [Google Scholar] [CrossRef]
- Catapano, A.L.; Pirillo, A.; Bonacina, F.; Norata, G.D. HDL in innate and adaptive immunity. Cardiovasc. Res. 2014, 103, 372–383. [Google Scholar] [CrossRef] [Green Version]
- Berberich, A.J.; Hegele, R.A. A modern approach to dyslipidemia. Endocr. Rev. 2021, 43, 611–653. [Google Scholar] [CrossRef]


| Variables n (%) | All (n = 497) | Dyslipidaemia (n = 278) | Normal Lipid (n = 219) | p-Value # |
|---|---|---|---|---|
| Time to follow-up, median years (IQR) | 7.5 (3.8–13.3) | 7.4 (3.7–13.7) | 7.6 (3.8–13.1) | 0.27 |
| Age, median years (IQR) | 35.4 (27.3–46.4) | 37.2 (28.1–47.6) | 34.1 (27.2–44.8) | 0.91 |
| Male | 274 (55.1) | 173 (62.2) | 101 (46.1) | <0.001 *** |
| a Disease duration ≥ 10 years | 246 (49.5) | 141 (50.7) | 105 (47.9) | 0.54 |
| BMI ≥ 23 (kg/m2) | 228 (48.9) | 161 (57.9) | 67 (30.6) | <0.001 *** |
| Smoke | 129 (25.9) | 87 (31.3) | 42 (19.2) | |
| Never | 368 (74.0) | 191 (68.7) | 177 (80.8) | <0.01 ** |
| Former | 100 (20.1) | 65 (23.4) | 35 (16.0) | |
| Current | 29 (5.9) | 22 (7.9) | 7 (3.2) | |
| Maximum extent diagnosed | ||||
| E1 | 10 (2.0) | 4 (1.4) | 6 (2.7) | 0.482 b |
| E2 | 60 (12.1) | 27 (9.7) | 33 (15.1) | 0.069 |
| E3 | 427 (85.9) | 247 (88.9) | 180 (82.2) | 0.03 * |
| Comorbidities | ||||
| Hypertension | 60 (12.1) | 45 (16.2) | 15 (6.8) | <0.01 ** |
| Hyperuricemia | 113 (22.7) | 73 (26.3) | 40 (18.3) | 0.04 * |
| Diabetes | 55 (11.1) | 44 (15.8) | 11 (5.0) | <0.001 *** |
| NAFLD | 34 (6.8) | 25 (9.0) | 9 (4.1) | 0.03 * |
| Appendectomy | 40 (8.0) | 31 (6.1) | 9 (4.1) | <0.01 ** |
| Severe complications | 72 (14.5) | 43 (15.5) | 29 (13.2) | 0.48 |
| EIMs | 75 (15.1) | 47 (16.9) | 28 (12.8) | 0.20 |
| Medications | ||||
| Glucocorticoid | 402 (80.9) | 232 (83.5) | 170 (77.6) | 0.10 |
| IM | 165 (33.2) | 98 (35.3) | 67 (30.6) | 0.27 |
| Biological therapy | 75 (15.1) | 39 (14.0) | 36 (16.4) | 0.46 |
| 5-ASA | 474 (95.4) | 267 (96.0) | 207 (94.5) | 0.76 |
| Aspirin | 19 (3.8) | 13 (4.7) | 6 (2.7) | <0.001 *** |
| DAI (n = 408) | ||||
| MS | ||||
| Remission | 43 (8.7) | 2 (0.9) | 41 (22.9) | <0.001 *** |
| Mild | 101 (20.3) | 29 (12.7) | 72 (40.2) | |
| Moderate | 170 (34.2) | 107 (46.7) | 63 (35.2) | |
| Severe | 94 (18.9) | 91 (39.7) | 3 (1.7) | |
| MES | ||||
| Remission | 51 (10.3) | 9 (3.9) | 42 (23.5) | <0.001 *** |
| Mild | 66 (13.3) | 19 (8.3) | 47 (26.3) | |
| Moderate | 108 (21.7) | 63 (27.5) | 45 (25.1) | |
| Severe | 183 (36.8) | 138 (60.3) | 45 (25.1) | |
| UCEIS | ||||
| Remission | 45 (9.1) | 3 (1.3) | 42 (23.5) | <0.001 *** |
| Mild | 105 (21.1) | 26 (11.3) | 79 (44.1) | |
| Moderate | 168 (33.8) | 111 (48.5) | 57 (31.8) | |
| Severe | 90 (18.1) | 89 (38.9) | 1 (0.6) | |
| hsCRP, median mg/L (IQR) (n = 408) | 5.28 (1.42–28.79) | 14.05 (3.75–48.44) | 2.28 (0.70–6.43) | <0.001 *** |
| Laboratory Tests | Dyslipidaemia (n = 229) | p-Value | Normal Lipid (n = 179) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Remission (n = 2) | Mild (n = 29) | Moderate (n = 107) | Severe (n = 91) | Remission (n = 41) | Mild (n = 72) | Moderate (n = 63) | Severe (n = 3) | ||
| TC (mmol/L) | 4.1 (3.2-) | 5.7 (4.0–6.4) | 4.5 (3.6–5.9) | 3.6 (3.0–4.5) | <0.001 *** | 4.7 (4.1–5.2) | 4.6 (4.0–5.2) | 4.4 (4.0–5.1) | 4.6 (3.4-) | 0.69 |
| TG (mmol/L) | 1.5 (1.2-) | 1.4 (1.1–1.8) | 1.4 (1.1–2.4) | 1.2 (0.8–1.8) | 0.06 | 1.2 (0.9–1.6) | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.1 (0.8-) | 0.85 |
| HDL-C (mmol/L) | 1.0 (0.7-) | 1.1 (0.9–1.4) | 0.9 (0.8–1.1) | 0.8 (0.7–0.9) | <0.001 *** | 1.4 (1.1–1.5) | 1.3 (1.1–1.6) | 1.2 (1.1–1.5) | 0.6 (0.5-) | 0.02 * |
| LDL-C (mmol/L) | 3.0 (1.7-) | 3.6 (3.0–4.3) | 2.6 (2.1–3.1) | 2.1 (1.6–2.6) | <0.001 *** | 2.8 (2.3–3.1) | 2.6 (2.2–3.2) | 2.5 (2.2–3.1) | 2.8 (1.5-) | 0.77 |
| ApoA1 (g/L) | 1.3 (1.2-) | 1.2 (1.1–1.4) | 1.2 (1.0–1.5) | 1.0 (0.8–1.2) | 0.09 | 1.2 (1.2–1.8) | 1.5 (1.2–1.8) | 1.3 (1.2–1.6) | 1.2 (1.1-) | 0.30 |
| ApoB (mg/L) | 1.0 (0.8-) | 1.1 (1.0–1.4) | 1.0 (0.8–1.2) | 0.9 (0.7–1.0) | 0.07 | 0.9 (0.6–0.9) | 0.8 (0.7–1.0) | 0.8 (0.7–1.0) | 1.0 (0.7-) | 0.94 |
| Lp α (mg/L) | 96.0 (82.0-) | 82.0 (70.0–277.0) | 205.0 (71.5–428.0) | 102.5 (32.0–188.3) | 0.12 | 136.0 (25.0–211.8) | 120.0 (53.0–248.0) | 101.0 (70.8–144.5) | 171.0 (101.0-) | 0.96 |
| FFA (umol/L) | 453.5 (402.0-) | 551.0 (463.0–560.0) | 551.0 (338.8–656.5) | 481.5 (322.5–618.3) | 0.69 | 551.0 (222.0–698.8) | 400.0 (312.5–802.5) | 470.5 (322.8–722.5) | 634.0 (567.0-) | 0.99 |
| hsCRP (mg/L) | 2.1(1.9-) | 4.6 (1.2–36.5) | 9.4 (1.8–44.0) | 20.5 (11.6–55.4) | <0.01 ** | 2.8 (1.4–4.5) | 1.6 (0.5–6.8) | 2.2 (0.8–5.7) | 35.2 (1.3-) | 0.22 |
| Variables n (%) | All (n = 497) | Dyslipidaemia (n = 278) | Normal Lipid (n = 219) | p-Value # |
|---|---|---|---|---|
| UC-related surgery | 94 (18.9) | 64 (23.0) | 30 (13.7) | < 0.01 ** |
| Tumorigenesis | 53 (10.7) | 37 (13.3) | 16 (7.3) | 0.03 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Tang, H.; Liang, H.; Bai, X.; Zhang, H.; Yang, H.; Wang, H.; Wang, L.; Qian, J. Dyslipidaemia Is Associated with Severe Disease Activity and Poor Prognosis in Ulcerative Colitis: A Retrospective Cohort Study in China. Nutrients 2022, 14, 3040. https://doi.org/10.3390/nu14153040
Liu Z, Tang H, Liang H, Bai X, Zhang H, Yang H, Wang H, Wang L, Qian J. Dyslipidaemia Is Associated with Severe Disease Activity and Poor Prognosis in Ulcerative Colitis: A Retrospective Cohort Study in China. Nutrients. 2022; 14(15):3040. https://doi.org/10.3390/nu14153040
Chicago/Turabian StyleLiu, Zhaoshi, Hao Tang, Haozheng Liang, Xiaoyin Bai, Huimin Zhang, Hong Yang, Hongying Wang, Li Wang, and Jiaming Qian. 2022. "Dyslipidaemia Is Associated with Severe Disease Activity and Poor Prognosis in Ulcerative Colitis: A Retrospective Cohort Study in China" Nutrients 14, no. 15: 3040. https://doi.org/10.3390/nu14153040
APA StyleLiu, Z., Tang, H., Liang, H., Bai, X., Zhang, H., Yang, H., Wang, H., Wang, L., & Qian, J. (2022). Dyslipidaemia Is Associated with Severe Disease Activity and Poor Prognosis in Ulcerative Colitis: A Retrospective Cohort Study in China. Nutrients, 14(15), 3040. https://doi.org/10.3390/nu14153040






