Vitamin A Nutritional Status of Urban Lactating Chinese Women and Its Associated Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Data Collection
2.2.1. Dietary Data
2.2.2. Blood and Breast Milk Sample Collection and Laboratory Analysis
2.2.3. Anthropometric Measurements and Assessments
2.2.4. Other Covariates
2.3. Statistics
3. Results
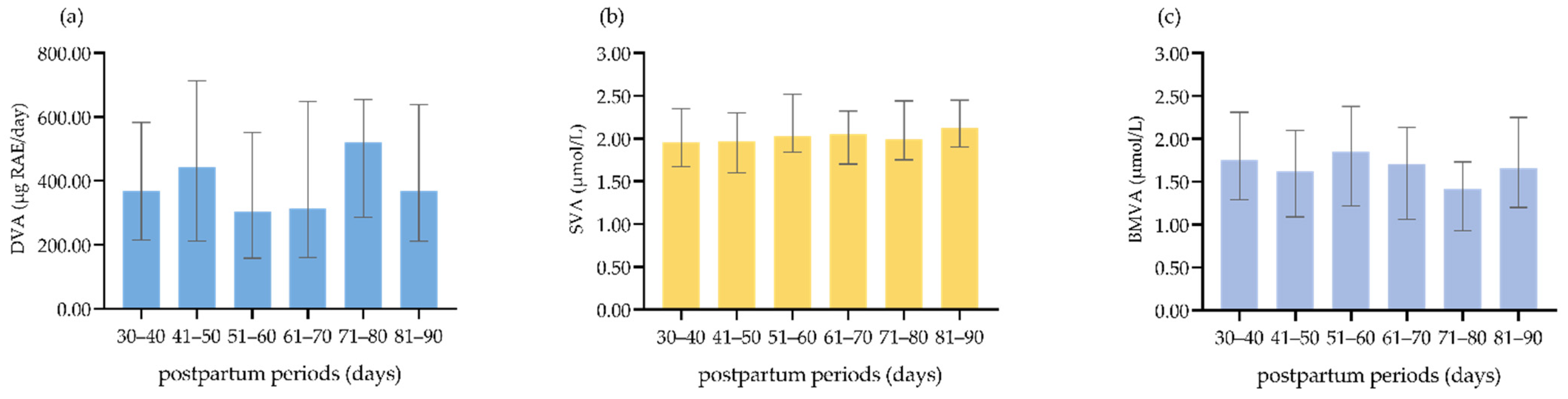
3.1. DVA, SVA, and BMVA Status
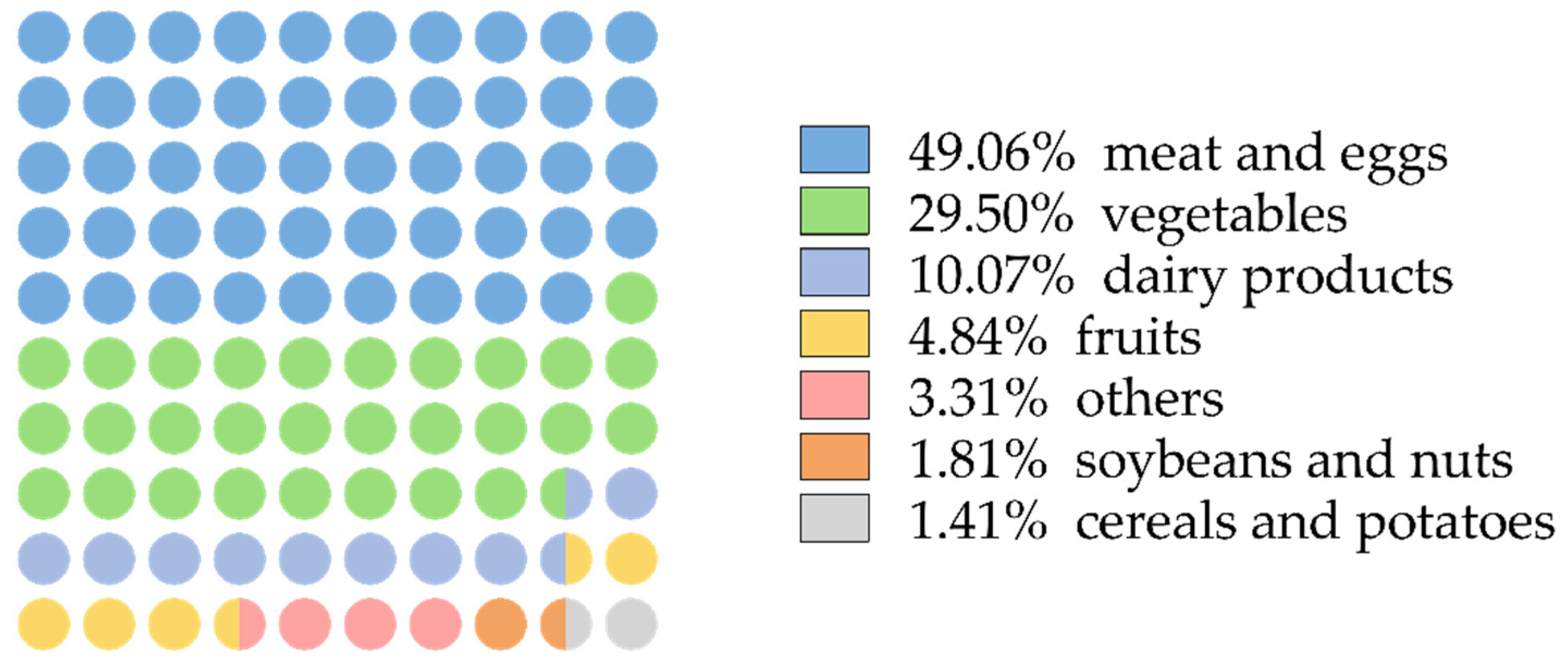
3.2. Dietary Sources of VA
3.3. DVA, SVA, and BMVA among Lactating Women with Different Characteristics
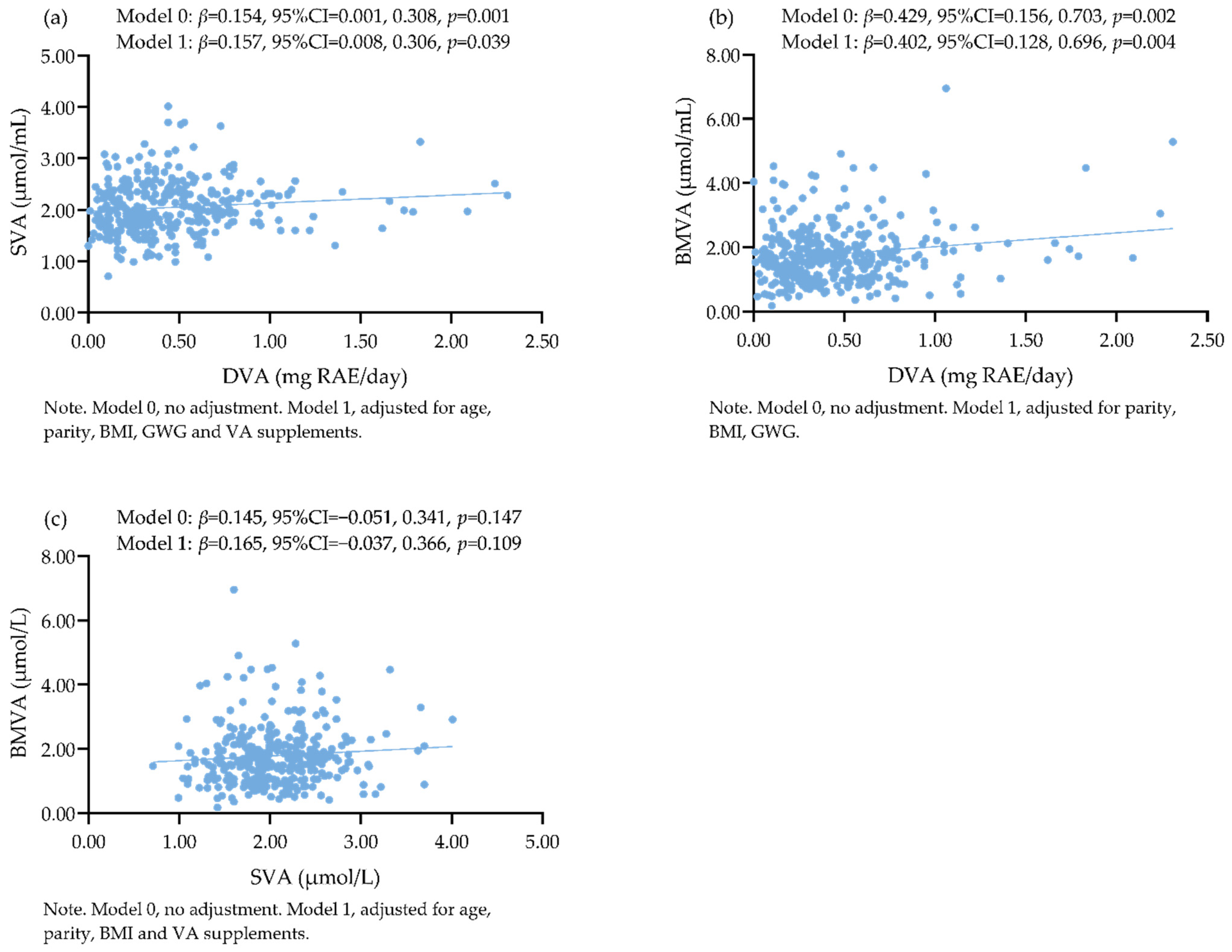
3.4. Associations among DVA, SVA, and BMVA
4. Discussion
4.1. VA Intake of Urban Lactating Chinese Women
4.2. SVA and BMVA of Urban Lactating Chinese Women
4.3. Related Factors of SVA and BMVA of Urban Lactating Chinese Women
4.4. Associations among DVA, SVA, and BMVA of Urban Lactating Chinese Women
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jouanne, M.; Oddoux, S.; Noël, A.; Voisin-Chiret, A.S. Nutrient requirements during pregnancy and lactation. Nutrients 2021, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Aserese, A.D.; Atenafu, A.; Sisay, M.; Sorrie, M.B.; Yirdaw, B.W.; Zegeye, M.K. Adequate vitamin A rich food consumption and associated factors among lactating mothers visiting child immunization and post-natal clinic at health institutions in Gondar Town, Northwest Ethiopia. PLoS ONE 2020, 15, e0239308. [Google Scholar] [CrossRef] [PubMed]
- Bastos Maia, S.; Rolland Souza, A.S.; Costa Caminha, M.F.; Lins da Silva, S.; Callou Cruz, R.; Carvalho Dos Santos, C.; Batista Filho, M. Vitamin A and pregnancy: A narrative review. Nutrients 2019, 11, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries, J.Y.; Pundir, S.; Mckenzie, E.; Keijer, J.; Kussmann, M. Maternal circulating vitamin status and colostrum vitamin composition in healthy lactating women-a systematic approach. Nutrients 2018, 10, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.T.T.; Kim, J.; Lee, H.; Won, S.; Kim, Y.; Jung, J.A.; Li, D.; To, X.H.M.; Huynh, K.T.N.; Le, T.V.; et al. A comparison of vitamin and lutein concentrations in breast milk from four asian countries. Nutrients 2020, 12, 1794. [Google Scholar] [CrossRef]
- Kasalová, E.; Aufartová, J.; Krčmová, L.K.; Solichová, D.; Solich, P. Recent trends in the analysis of vitamin D and its metabolites in milk-a review. Food Chem. 2015, 171, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Caminha Mde, F.; Batista Filho, M.; Fernandes, T.F.; Arruda, I.K.; Diniz Ada, S. Vitamin A supplementation during puerperium: Systematic review. Rev. Saude Publica 2009, 43, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Society of Child Health, Chinese Preventive Medicine Association. Expert consensus on clinical application of vitamin A and vitamin D in Children of China. Chin. J. Child Health Care 2021, 29, 110–116. (In Chinese) [Google Scholar]
- Deminice, T.M.M.; Ferraz, I.S.; Monteiro, J.P.; Jordão, A.A.; Ambrósio, L.; Nogueira-de-Almeida, C.A. Vitamin A intake of Brazilian mothers and retinol concentrations in maternal blood, human milk, and the umbilical cord. J. Int. Med. Res. 2018, 46, 1555–1569. [Google Scholar] [CrossRef]
- Atalhi, N.; El Hamdouchi, A.; Barkat, A.; Elkari, K.; Hamrani, A.; El Mzibri, M.; Haskell, M.J.; Mokhtar, N.; Aguenaou, H. Combined consumption of a single high-dose vitamin A supplement with provision of vitamin A fortified oil to households maintains adequate milk retinol concentrations for 6 months in lactating Moroccan women. Appl. Physiol. Nutr. Metab. 2020, 45, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.R.; Kamp, F.; Nunes, J.C.; El-Bacha, T.; Torres, A.G. Breast milk content of vitamin A and E from early- to mid-lactation is affected by inadequate dietary intake in Brazilian adult women. Nutrients 2019, 11, 2025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, A.; de Sousa Rebouças, A.; Mendonça, B.M.A.; Silva, D.; Dimenstein, R.; Ribeiro, K. Relationship between the dietary intake, serum, and breast milk concentrations of vitamin A and vitamin E in a cohort of women over the course of lactation. Matern. Child Nutr. 2019, 15, e12772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Indayati, W.; Basnet, T.B.; Li, F.; Luo, H.; Pan, H.; Wang, Z. Dietary intake in lactating mothers in China 2018: Report of a survey. Nutr. J. 2020, 19, 72. [Google Scholar] [CrossRef]
- Yang, Y. China Food Composition (Book 1), 2nd ed.; Beijing Medical University Press: Beijing, China, 2016. [Google Scholar]
- Chinese Nutrition Society. Chinese Dietary Guidelines; People’s Medical Publishing House: Beijing, China, 2016. [Google Scholar]
- Harari, A.; Coster, A.C.F.; Jenkins, A.; Xu, A.; Greenfield, J.R.; Harats, D.; Shaish, A.; Samocha-Bonet, D. Obesity and insulin resistance are inversely associated with serum and adipose tissue carotenoid concentrations in adults. J. Nutr. 2020, 150, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.S.; da Silva Ribeiro, K.D.; Pires, J.F.; Bezerra, D.F.; Bellot, P.E.; de Oliveira Weigert, L.P.; Dimenstein, R. Breast milk retinol concentration in mothers of preterm newborns. Early Hum. Dev. 2017, 106, 41–45. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of the People’s Republic of China. WS/T 428-2013 Criteria of Weight for Adults; Standards Press of China: Beijing, China, 2013. [Google Scholar]
- Chinese Nutrition Society. Weight Monitoring and Evaluation during Pregancy Period of Chinese Women. Available online: https://www.cnsoc.org/otherNotice/392100200.html (accessed on 16 June 2022).
- Fan, M.; Lyu, J.; He, P. Chinese guidelines for data processing and analysis concerning the International Physical Activity Questionnaire. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi 2014, 35, 961–964. (In Chinese) [Google Scholar]
- Moyersoen, I.; Lachat, C.; Cuypers, K.; Ridder, K.; Devleesschauwer, B.; Tafforeau, J.; Vandevijvere, S.; Vansteenland, M.; De Meulenaer, B.; Van Camp, J.; et al. Do current fortification and supplementation programs assure adequate intake of fat-soluble vitamins in Belgian infants, toddlers, pregnant women, and lactating women? Nutrients 2018, 10, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayaraghavan, K. National control programme against nutritional blindness due to vitamin A deficiency: Current status & future strategy. Indian J. Med. Res. 2018, 148, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.P.; Turck, D.; Tavoularis, G.; Ferry, C.; Dupont, C. The role of young child formula in ensuring a balanced diet in young children (1–3 years old). Nutrients 2019, 11, 2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maina, E.G.; Madivoli, E.S.; Ouma, J.A.; Ogilo, J.K.; Kenya, J.M. Evaluation of nutritional value of Asystasia mysorensis and Sesamum angustifolia and their potential contribution to human health. Food Sci. Nutr. 2019, 7, 2176–2185. [Google Scholar] [CrossRef] [Green Version]
- Lyu, J.; Fan, Y.; Zhang, J.; Zhao, A.; Yang, F.; Zhang, Y. Dietary evaluation of 52 lactating mothers in Beijing in 2018 and its relationship with breast milk composition. Wei Sheng Yan Jiu = J. Hyg. Res. 2020, 49, 392–396. (In Chinese) [Google Scholar]
- Hu, M.; Qin, R.; Lin, X.; Ding, Y.; Li, F.; Wang, Z. Dietary status of lactating women from five cities of China in 2015–2016. Wei Sheng Yan Jiu = J. Hyg. Res. 2019, 48, 220–225. (In Chinese) [Google Scholar]
- Cui, Z.; Hou, Z.; Sun, F. Effects of nutritional intervention on puerperal dietary behavior and postpartum recovery in puerperae. Chin. J. Mod. Nurs. 2019, 25, 4790–4793. (In Chinese) [Google Scholar] [CrossRef]
- Dong, C.; Yin, S. A 10-year review of the nutritional status of Chinese lactating mothers. Zhonghua Yu Fang Yi Xue Za Zhi Chin. J. Prev. Med. 2016, 50, 1108–1113. (In Chinese) [Google Scholar]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of nutrition for development (BOND)-Vitamin A review. J. Nutr. 2016, 146, 1816s–1848s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panpanich, R.; Vitsupakorn, K.; Harper, G.; Brabin, B. Serum and breast-milk vitamin A in women during lactation in rural Chiang Mai, Thailand. Ann. Trop. Paediatr. 2002, 22, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Sámano, R.; Martínez-Rojano, H.; Hernández, R.M.; Ramírez, C.; Flores Quijano, M.E.; Espíndola-Polis, J.M.; Veruete, D. Retinol and α-Tocopherol in the breast milk of women after a high-risk pregnancy. Nutrients 2017, 9, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Zhang, R.; Huang, L.; Zhao, D.; Su, D.; Meng, J.; Fang, Y. Serum levels of vitamin D, retinol, zinc, and CRP in relation to obesity among children and adolescents. Eur. J. Med. Res. 2022, 27, 51. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Guessous, I. Socio-demographic and lifestyle determinants of dietary patterns in French-speaking Switzerland, 2009–2012. BMC Public Health 2018, 18, 131. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Chen, J.; Liu, Z.; Yun, C.; Piao, J.; Yang, X. Prevalence and influence factors of vitamin A deficiency of Chinese pregnant women. Nutr. J. 2016, 15, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezerra, D.S.; Ribeiro, K.D.S.; Lima, M.S.R.; Pires Medeiros, J.F.; da Silva, A.; Dimenstein, R.; Osório, M.M. Retinol status and associated factors in mother-newborn pairs. J. Hum. Nutr. Diet. 2020, 33, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M.; Allert, R.; East, C.E. Vitamin A supplementation for postpartum women. Cochrane Database Syst. Rev. 2016, 3, CD005944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klevor, M.K.; Haskell, M.J.; Lartey, A.; Adu-Afarwuah, S.; Zeilani, M.; Dewey, K.G. Lipid-based nutrient supplements providing approximately the recommended daily intake of vitamin A do not increase breast milk retinol concentrations among Ghanaian women. J. Nutr. 2016, 146, 335–342. [Google Scholar] [CrossRef]
- Sânzio Gurgel, C.S.; Alves de Araújo Pereira, L.; de Assis Costa, A.; Adja da Silva Souza, M.; Araújo de Brito, P.; Miranda de Melo, L.R.; Dimenstein, R. Effect of routine prenatal supplementation on vitamin concentrations in maternal serum and breast milk. Nutrition 2017, 33, 261–265. [Google Scholar] [CrossRef]
- Soares, M.M.; Silva, M.A.; Garcia, P.P.C.; Silva, L.S.D.; Costa, G.D.D.; Araújo, R.M.A.; Cotta, R.M.M. Efect of vitamin A suplementation: A systematic review. Cienc. Saude Coletiva 2019, 24, 827–838. [Google Scholar] [CrossRef]
- Hotz, C.; Chileshe, J.; Siamusantu, W.; Palaniappan, U.; Kafwembe, E. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr. 2012, 15, 1688–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, T.; Burri, B.J.; Jamil, K.M.; Jamil, M. The effects of daily consumption of β-cryptoxanthin-rich tangerines and β-carotene-rich sweet potatoes on vitamin A and carotenoid concentrations in plasma and breast milk of Bangladeshi women with low vitamin A status in a randomized controlled trial. Am. J. Clin. Nutr. 2013, 98, 1200–1208. [Google Scholar] [CrossRef] [Green Version]
- Kolasa, K.M.; Firnhaber, G.; Haven, K. Diet for a healthy lactating woman. Clin. Obstet. Gynecol. 2015, 58, 893–901. [Google Scholar] [CrossRef]
- Pinkaew, S.; Udomkesmalee, E.; Davis, C.R.; Tanumihardjo, S.A. Vitamin A-fortified rice increases total body vitamin A stores in lactating Thai women measured by retinol isotope dilution: A double-blind, randomized, controlled trial. Am. J. Clin. Nutr. 2021, 113, 1372–1380. [Google Scholar] [CrossRef]
- de Azeredo, V.B.; Trugo, N.M. Retinol, carotenoids, and tocopherols in the milk of lactating adolescents and relationships with plasma concentrations. Nutrition 2008, 24, 133–139. [Google Scholar] [CrossRef]
- Cabezuelo, M.T.; Zaragozá, R.; Barber, T.; Viña, J.R. Role of Vitamin A in mammary gland development and lactation. Nutrients 2019, 12, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.C.; Hsu, C.C.; Wang, H.S.; Shin, S.J. Prospective randomized controlled trial to evaluate effectiveness of registered dietitian-led diabetes management on glycemic and diet control in a primary care setting in Taiwan. Diabetes Care 2010, 33, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | n (%) | DVA | pa | SVA | pa | BMVA | pa |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| ≤30 | 177 (54.3) | 373.69 (208.89, 614.95) | 0.781 | 1.94 (1.66, 2.32) | 0.090 | 1.62 (1.07, 2.09) | 0.109 |
| >30 | 149 (45.7) | 366.73 (212.45, 629.67) | 2.04 (1.76, 2.40) | 1.68 (1.23, 2.36) | |||
| College or university | |||||||
| No | 74 (22.7) | 350.63 (188.89, 597.49) | 0.538 | 2.00 (1.75, 2.40) | 0.405 | 1.68 (1.31, 2.27) | 0.471 |
| Yes | 252 (77.3) | 381.75 (210.50, 629.96) | 1.99 (1.70, 2.34) | 1.67 (1.08, 2.13) | |||
| Family monthly per capita income (Chinese yuan) | |||||||
| <5000 | 129 (39.6) | 363.10 (198.61, 644.87) | 0.955 | 2.01 (1.77, 2.35) | 0.736 | 1.54 (1.08, 2.06) | 0.288 |
| 5000~9999 | 137 (42.0) | 391.56 (219.68, 603.55) | 1.98 (1.70, 2.35) | 1.72 (1.14, 2.25) | |||
| ≥10,000 | 60 (18.4) | 365.11 (217.56, 603.99) | 1.95 (1.69, 2.35) | 1.68 (1.16, 2.35) | |||
| Parity | |||||||
| 1 | 227 (69.6) | 398.40 (213.20, 606.15) | 0.964 | 1.98 (1.70, 2.32) | 0.068 | 1.72 (1.18, 2.13) | 0.177 |
| ≥2 | 99 (30.4) | 351.08 (196.90, 650.04) | 2.05 (1.76, 2.44) | 1.53 (0.97, 2.20) | |||
| Delivery mode | |||||||
| Cesarean delivery | 127 (39.0) | 391.56 (175.60, 630.35) | 0.872 | 1.99 (1.69, 2.35) | 0.452 | 1.60 (1.06, 2.15) | 0.613 |
| Vaginal delivery | 199 (61.0) | 364.32 (222.96, 611.58) | 1.99 (1.73, 2.35) | 1.71 (1.25, 2.20) | |||
| BMI | |||||||
| Underweight or normal weight | 206 (63.2) | 409.39 (248.07, 644.65) | 0.022 | 1.97 (1.70, 2.32) | 0.030 | 1.73 (1.29, 2.27) | 0.003 |
| Overweight or obese | 120 (36.8) | 334.92 (163.73, 586.56) | 2.10 (1.77, 2.47) | 1.51 (1.03, 1.89) | |||
| GWG (kg) | |||||||
| Insufficient or appropriate | 169 (52.2) | 437.19 (244.42, 649.19) | 0.072 | 1.99 (1.73, 2.35) | 0.738 | 1.70 (1.16, 2.21) | 0.362 |
| Excessive | 155 (47.8) | 336.35 (189.80, 600.09) | 1.99 (1.70, 2.35) | 1.58 (1.08, 2.13) | |||
| Physical activities | |||||||
| Low | 141 (43.3) | 353.02 (207.41, 582.37) | 0.515 | 2.02 (1.76, 2.38) | 0.334 | 1.70 (1.07, 2.17) | 0.868 |
| Medium | 92 (28.2) | 409.39 (218.99, 649.59) | 1.95 (1.67, 2.31) | 1.60 (1.09, 2.11) | |||
| High | 93 (28.5) | 411.49 (193.40, 635.16) | 1.99 (1.68, 2.36) | 1.69 (1.24, 2.18) | |||
| VA supplements | |||||||
| No | 287 (88.0) | 391.56 (209.41, 620.18) | 0.272 | 1.98 (1.70, 2.35) | 0.090 | 1.66 (1.13, 2.13) | 0.602 |
| Yes | 39 (12.0) | 311.05 (196.90, 619.65) | 2.13 (1.82, 2.60) | 1.77 (1.08, 2.36) | |||
| Infant sex | |||||||
| Boy | 165 (50.6) | 364.32 (181.70, 600.66) | 0.336 | 1.98 (1.74, 2.33) | 0.411 | 1.72 (1.28, 2.13) | 0.187 |
| Girl | 161 (49.4) | 398.40 (222.23, 631.16) | 2.01 (1.69, 2.39) | 1.54 (1.05, 2.23) | |||
| Infant age (days) | |||||||
| 30~60 | 226 (69.3) | 373.40 (207.88, 610.58) | 0.984 | 1.98 (1.67, 2.35) | 0.210 | 1.68 (1.16, 2.21) | 0.189 |
| 60~90 | 100 (30.7) | 365.11 (215.64, 647.15) | 2.03 (1.77, 2.37) | 1.57 (1.07, 2.04) |
| Characteristics | BMVA | pa | |
|---|---|---|---|
| <1.05 μmol/L, n (%) | ≥1.05 μmol/L, n (%) | ||
| n | 67 | 259 | |
| Age (years) | |||
| ≤30 | 41 (23.2) | 136 (76.8) | 0.203 |
| >30 | 26 (17.4) | 123 (82.6) | |
| College or university | |||
| No | 13 (17.6) | 61 (82.4) | 0.470 |
| Yes | 54 (21.4) | 198 (78.6) | |
| Family monthly per capita income (Chinese yuan) | |||
| <5000 | 28 (21.7) | 101 (78.3) | 0.367 |
| 5000~9999 | 30 (21.9) | 107 (78.1) | |
| ≥10,000 | 9 (15.0) | 51 (85.0) | |
| Parity | |||
| 1 | 39 (17.2) | 188 (82.8) | 0.023 |
| ≥2 | 28 (28.3) | 71 (71.7) | |
| Delivery mode | |||
| Cesarean delivery | 28 (22.0) | 99 (78.0) | 0.594 |
| Vaginal delivery | 39 (19.6) | 160 (80.4) | |
| BMI | |||
| Underweight or normal weight | 35 (17.0) | 171 (83.0) | 0.037 |
| Overweight or obese | 32 (26.7) | 88 (73.3) | |
| GWG (kg) | |||
| Insufficient or appropriate | 33 (19.5) | 136 (80.5) | 0.694 |
| Excessive | 33 (21.3) | 122 (78.7) | |
| Physical activities | |||
| Low | 33 (23.4) | 108 (76.6) | 0.182 |
| Medium | 19 (20.7) | 73 (79.3) | |
| High | 15 (16.1) | 78 (83.9) | |
| VA supplements | |||
| No | 59 (20.6) | 228 (79.4) | 0.995 |
| Yes | 8 (20.5) | 31 (79.5) | |
| Infant sex | |||
| Boy | 29 (17.6) | 136 (82.4) | 0.178 |
| Girl | 38 (23.6) | 123 (76.4) | |
| Infant age (days) | |||
| 30~60 | 46 (20.4) | 180 (79.6) | 0.894 |
| 60~90 | 21 (21.0) | 79 (79.0) | |
| β | SE | p | 95%CI | |
|---|---|---|---|---|
| With VA supplements | ||||
| DVA | 0.034 | 0.341 | 0.921 | (−0.660, 0.728) |
| Without VA supplements | ||||
| DVA | 0.174 | 0.076 | 0.022 | (0.025, 0.324) |
| β | SE | p | 95%CI | |
|---|---|---|---|---|
| With VA supplements | ||||
| DVA a | −0.432 | 0.398 | 0.285 | (−1.241, 0.376) |
| SVA b | 0.026 | 0.036 | 0.469 | (−0.046, 0.098) |
| Without VA supplements | ||||
| DVA a | 0.501 | 0.149 | 0.001 | (0.208, 0.795) |
| SVA b | 0.136 | 0.116 | 0.244 | (−0.093, 0.364) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zhao, A.; Ren, Z.; Zhang, J.; Wang, P.; Zhang, Y. Vitamin A Nutritional Status of Urban Lactating Chinese Women and Its Associated Factors. Nutrients 2022, 14, 3184. https://doi.org/10.3390/nu14153184
Yang C, Zhao A, Ren Z, Zhang J, Wang P, Zhang Y. Vitamin A Nutritional Status of Urban Lactating Chinese Women and Its Associated Factors. Nutrients. 2022; 14(15):3184. https://doi.org/10.3390/nu14153184
Chicago/Turabian StyleYang, Chenlu, Ai Zhao, Zhongxia Ren, Jian Zhang, Peiyu Wang, and Yumei Zhang. 2022. "Vitamin A Nutritional Status of Urban Lactating Chinese Women and Its Associated Factors" Nutrients 14, no. 15: 3184. https://doi.org/10.3390/nu14153184
APA StyleYang, C., Zhao, A., Ren, Z., Zhang, J., Wang, P., & Zhang, Y. (2022). Vitamin A Nutritional Status of Urban Lactating Chinese Women and Its Associated Factors. Nutrients, 14(15), 3184. https://doi.org/10.3390/nu14153184






