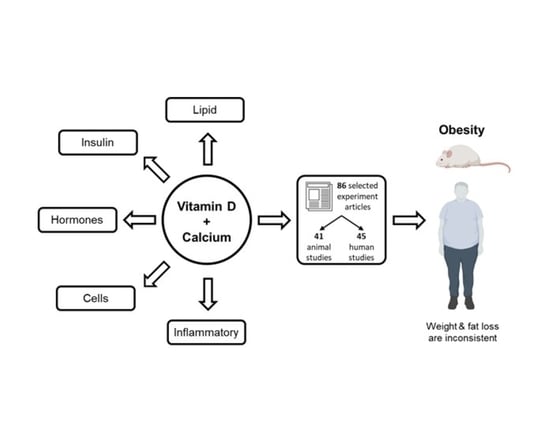
Interrelationship between Vitamin D and Calcium in Obesity and Its Comorbid Conditions
Abstract
1. Introduction
2. Search Strategy and Method
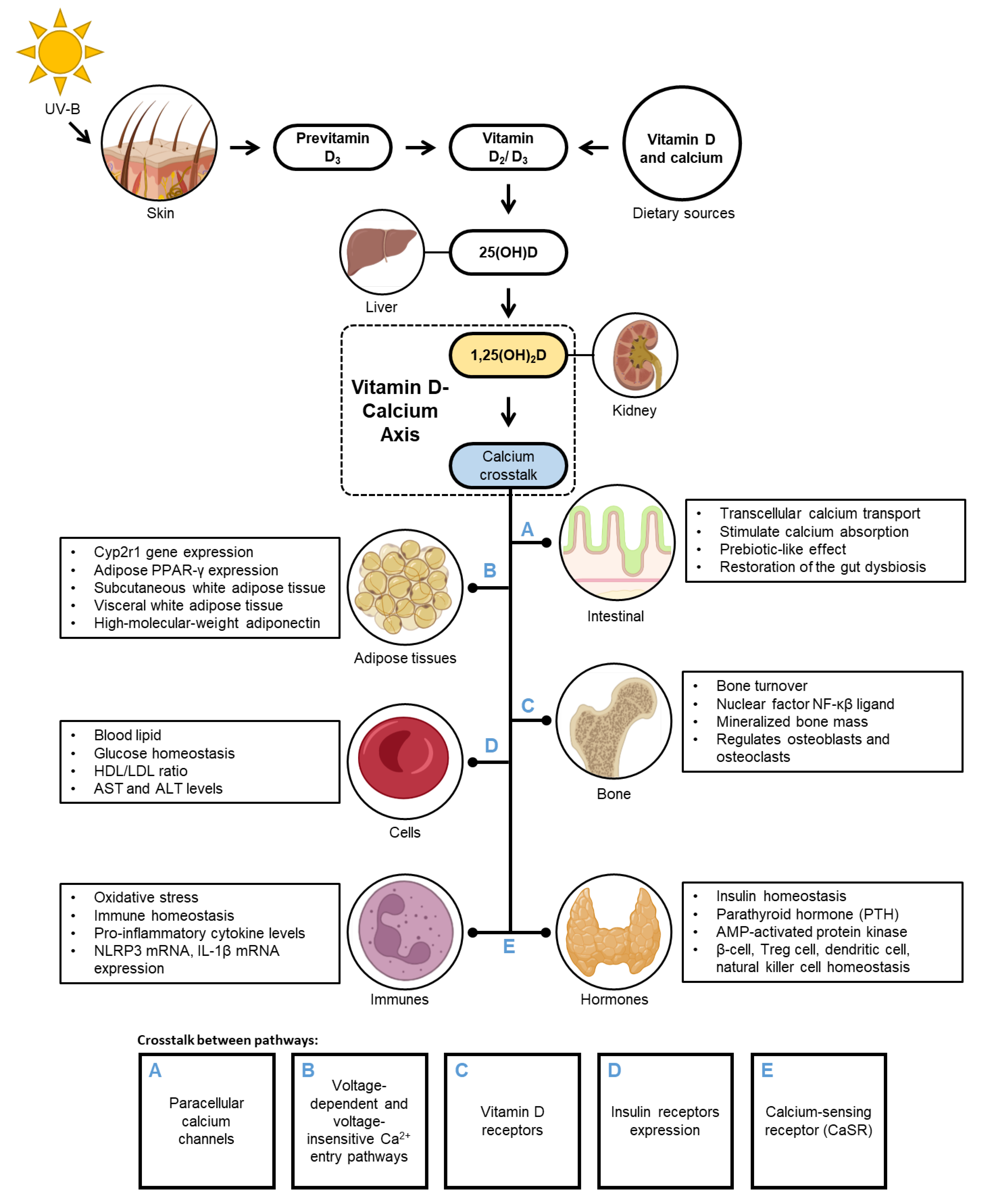
3. Physiological Association of VD–CAL
4. Interrelationship of VD–CAL in Obesity and Its Disease States
4.1. Impact of VD–CAL on Adipose Tissue
4.2. Impact of VD–CAL on Body Fatness
4.3. Impact of VD on Bone Density
4.4. Impact of VD–CAL on Inflammatory, Insulin, Hormone, and Cell Functions
4.5. Potential Applications of VD–CAL in Obesity Prevention
4.6. Inadequate Evidence for the Effect of VD–CAL on Obesity
5. Limitations
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed]
- de Souza Silva, J.; Sobrinho, S.; Elaine Pereira, S.; José Saboya Sobrinho, C.; Ramalho, A. Nutrición Hospitalaria Trabajo Original Obesidad y síndrome metabólico Obesity, related diseases and their relationship with vitamin D defi ciency in adolescents Obesidad, enfermedades relacionadas y su relación con la defi ciencia de vitamina D en adoles. Nutr. Hosp. 2016, 33, 856–864. [Google Scholar]
- Dădârlat-Pop, A.; Sitar-Tăut, A.; Zdrenghea, D.; Caloian, B.; Tomoaia, R.; Pop, D.; Buzoianu, A. Profile of obesity and comorbidities in elderly patients with heart failure. Clin. Interv. Aging 2020, 15, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.; Morris, T. Physical activity and obesity research in the Asia-Pacific: A review. Asia-Pac. J. Public Health 2012, 24, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.; Sanchez-Romero, L.M.; Brown, M.; Jaccard, A.; Jewell, J.; Galea, G.; Webber, L.; Breda, J. Forecasting Future Trends in Obesity across Europe: The Value of Improving Surveillance. Obes. Facts 2018, 11, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Yako, Y.Y.; Echouffo-Tcheugui, J.B.; Balti, E.V.; Matsha, T.E.; Sobngwi, E.; Erasmus, R.T.; Kengne, A.P. Genetic association studies of obesity in Africa: A systematic review. Obes. Rev. 2015, 16, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.J.; Morton, J.; Brethauer, S.; Mattar, S.; De Maria, E.; Benz, J.K.; Titus, J.; Sterrett, D. Obesity in America. Surg. Obes. Relat. Dis. 2017, 13, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef]
- Castro, L.N.; Jewell, J.; Whiting, S.; Rippin, H.; Farrand, C.; Wickramasinghe; Kremlin Breda, J. Nutrition, Overweight and Obesity Factsheet-Sustainable Development Goals: Health Targets; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Damms-Machado, A.; Weser, G.; Bischoff, S.C. Micronutrient deficiency in obese subjects undergoing low calorie diet. Nutr. J. 2012, 11, 34. [Google Scholar] [CrossRef]
- Thomas-Valdés, S.; Das, M.; Tostes, G.V.; Anunciação, P.C.; Da Silva, B.P.; Sant’ana, H.M.P.; Thomas-Vald Es, S.; Das Graças, M.; Tostes, V.; Anunciaçao, P.C.; et al. Association between vitamin deficiency and metabolic disorders related to obesity. Crit. Rev. Food Sci. Nutr. 2017, 57, 3332–3343. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Suliburska, J.; Cofta, S.; Gajewska, E.; Kalmus, G.; Sobieska, M.; Samborski, W.; Krejpcio, Z. The evaluation of selected serum mineral concentrations and their association with insulin resistance in obese adolescents. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2396–2400. [Google Scholar]
- Marotte, C.; Bryk, G.; Gonzales Chaves, M.M.S.; Lifshitz, F.; Pita Martín de Portela, M.L.; Zeni, S.N. Low dietary calcium and obesity: A comparative study in genetically obese and normal rats during early growth. Eur. J. Nutr. 2014, 53, 769–778. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef]
- Song, Q.; Sergeev, I.N. Calcium and vitamin D in obesity. Nutr. Res. Rev. 2012, 25, 130–141. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A.G. Vitamin D(3) is more potent than vitamin D(2) in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Arruda, A.P.; Hotamisligil, G.S. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015, 22, 381–397. [Google Scholar] [CrossRef]
- Zemel, M.B.; Thompson, W.; Milstead, A.; Morris, K.; Campbell, P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes. Res. 2004, 12, 582–590. [Google Scholar] [CrossRef]
- Vuralli, D. Clinical Approach to Hypocalcemia in Newborn Period and Infancy: Who Should Be Treated? Int. J. Pediatr. 2019, 2019, 4318075. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Vitamin D status, calcium intake and risk of developing type 2 diabetes: An unresolved issue. Nutrients 2019, 11, 642. [Google Scholar] [CrossRef]
- Arteh, J.; Narra, S.; Nair, S. Prevalence of vitamin D deficiency in chronic liver disease. Dig. Dis. Sci. 2010, 55, 2624–2628. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef]
- Mitri, J.; Dawson-Hughes, B.; Hu, F.B.; Pittas, A.G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 486–494. [Google Scholar] [CrossRef]
- Goel, R.K.; Lal, H. Role of Vitamin D Supplementation in Hypertension. Indian J. Clin. Biochem. 2011, 26, 88–90. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D deficiency: Consequence or cause of obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef]
- Duan, L.; Han, L.; Liu, Q.; Zhao, Y.; Wang, L.; Wang, Y. Effects of Vitamin D Supplementation on General and Central Obesity: Results from 20 Randomized Controlled Trials Involving Apparently Healthy Populations. Ann. Nutr. Metab. 2020, 76, 153–164. [Google Scholar] [CrossRef]
- Lu, L.; Chen, C.; Tang, W.; Jacobs, D.; Shikany, J.; He, K. Calcium Intake Is Inversely Related to the Risk of Obesity among American Young Adults over a 30-Year Follow-Up. Curr. Dev. Nutr. 2020, 4, 1445. [Google Scholar] [CrossRef]
- Pannu, P.K.; Calton, E.K.; Soares, M.J. Calcium and Vitamin D in Obesity and Related Chronic Disease, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 77. [Google Scholar]
- El-Hajj Fuleihan, G. Can the sunshine vitamin melt the fat? Metabolism 2012, 61, 603–610. [Google Scholar] [CrossRef]
- Lips, P. Interaction between Vitamin D and calcium. Scand. J. Clin. Lab. Investig. 2012, 72, 60–64. [Google Scholar] [CrossRef]
- Goltzman, D.; Mannstadt, M.; Marcocci, C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. In Vitamin D in Clinical Medicine; Karger: Basel, Switzerland, 2018; Volume 50, pp. 1–13. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Chedraui, P.; Fernández-Alonso, A.M. Vitamin D and aging: Beyond calcium and bone metabolism. Maturitas 2011, 69, 27–36. [Google Scholar] [CrossRef]
- Carmeliet, G.; Dermauw, V.; Bouillon, R. Vitamin D signaling in calcium and bone homeostasis: A delicate balance. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.; Azadbakht, L.; Faghihimani, E.; Tabesh, M.; Esmaillzadeh, A. Effects of calcium–vitamin D co-supplementation on metabolic profiles in vitamin D insufficient people with type 2 diabetes: A randomised controlled clinical trial. Diabetologia 2014, 57, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, M.; Azadbakht, L.; Faghihimani, E.; Tabesh, M.; Esmaillzadeh, A. Calcium-vitamin D cosupplementation influences circulating inflammatory biomarkers and adipocytokines in vitamin D-insufficient diabetics: A randomized controlled clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, E2485–E2493. [Google Scholar] [CrossRef] [PubMed]
- Jahn, D.; Dorbath, D.; Schilling, A.K.; Gildein, L.; Meier, C.; Vuille-dit-Bille, R.N.; Schmitt, J.; Kraus, D.; Fleet, J.C.; Hermanns, H.M.; et al. Intestinal vitamin D receptor modulates lipid metabolism, adipose tissue inflammation and liver steatosis in obese mice. Biochim. Biophys. Acta—Mol. Basis Dis. 2019, 1865, 1567–1578. [Google Scholar] [CrossRef]
- Park, C.Y.; Shin, Y.; Kim, J.H.; Zhu, S.; Jung, Y.S.; Han, S.N. Effects of high fat diet-induced obesity on vitamin D metabolism and tissue distribution in vitamin D deficient or supplemented mice. Nutr. Metab. 2020, 17, 44. [Google Scholar] [CrossRef]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.F.; Riva, C. Vitamin d supplementation improves adipose tissue inflammation and reduces hepatic steatosis in obese c57bl/6j mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef]
- Jin, W.; Cui, B.; Li, P.; Hua, F.; Lv, X.; Zhou, J.; Hu, Z.; Zhang, X. 1,25-Dihydroxyvitamin D3 protects obese rats from metabolic syndrome via promoting regulatory T cell-mediated resolution of inflammation. Acta Pharm. Sin. B 2018, 8, 178–187. [Google Scholar] [CrossRef]
- Chaplin, A.; Parra, P.; Laraichi, S.; Serra, F.; Palou, A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol. Nutr. Food Res. 2016, 60, 468–480. [Google Scholar] [CrossRef]
- Conceição, E.P.S.; Moura, E.G.; Manhães, A.C.; Carvalho, J.C.; Nobre, J.L.; Oliveira, E.; Lisboa, P.C. Calcium reduces vitamin D and glucocorticoid receptors in the visceral fat of obese male rats. J. Endocrinol. 2016, 230, 263–274. [Google Scholar] [CrossRef]
- Nobre, J.L.; Lisboa, P.C.; Peixoto-Silva, N.; Quitete, F.T.; Carvalho, J.C.; de Moura, E.G.; de Oliveira, E. Role of vitamin D in adipose tissue in obese rats programmed by early weaning and post diet calcium. Mol. Nutr. Food Res. 2016, 60, 810–822. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Song, Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol. Nutr. Food Res. 2014, 58, 1342–1348. [Google Scholar] [CrossRef]
- Das, S.; Choudhuri, D. Dietary calcium regulates the insulin sensitivity by altering the adipokine secretion in high fat diet induced obese rats. Life Sci. 2020, 250, 117560. [Google Scholar] [CrossRef]
- Belenchia, A.M.; Tosh, A.K.; Hillman, L.S.; Peterson, C.A. Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: A randomized controlled trial. World Rev. Nutr. Diet. 2014, 109, 18–19. [Google Scholar] [CrossRef]
- Rosenblum, J.L.; Castro, V.M.; Moore, C.E.; Kaplan, L.M. Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. Am. J. Clin. Nutr. 2012, 95, 101–108. [Google Scholar] [CrossRef]
- Seldeen, K.L.; Pang, M.; Rodríguez-Gonzalez, M.; Hernandez, M.; Sheridan, Z.; Yu, P.; Troen, B.R. A mouse model of Vitamin D insufficiency: Is there a relationship between 25(OH) Vitamin D levels and obesity? Nutr. Metab. 2017, 14, 26. [Google Scholar] [CrossRef]
- Drori, A.; Rotnemer-Golinkin, D.; Avni, S.; Drori, A.; Danay, O.; Levanon, D.; Tam, J.; Zolotarev, L.; Ilan, Y. Attenuating the rate of total body fat accumulation and alleviating liver damage by oral administration of vitamin D-enriched edible mushrooms in a diet-induced obesity murine model is mediated by an anti-inflammatory paradigm shift. BMC Gastroenterol. 2017, 17, 130. [Google Scholar] [CrossRef]
- Marotte, C.; Weisstaub, A.; Bryk, G.; Olguin, M.C.; Posadas, M.; Lucero, D.; Schreier, L.; Pita Martín De Portela, M.L.; Zeni, S.N. Effect of dietary calcium (Ca) on body composition and Ca metabolism during growth in genetically obese (β) male rats. Eur. J. Nutr. 2013, 52, 297–305. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Glutathione deficiency induces epigenetic alterations of vitamin D metabolism genes in the livers of high-fat diet-fed obese mice. Sci. Rep. 2019, 9, 14784. [Google Scholar] [CrossRef]
- Roizen, J.D.; Long, C.; Casella, A.; O’Lear, L.; Caplan, I.; Lai, M.; Sasson, I.; Singh, R.; Makowski, A.J.; Simmons, R.; et al. Obesity Decreases Hepatic 25-Hydroxylase Activity Causing Low Serum 25-Hydroxyvitamin D. J. Bone Miner. Res. 2019, 34, 1068–1073. [Google Scholar] [CrossRef]
- Shab-Bidar, S.; Neyestani, T.R.; Djazayery, A. Vitamin D receptor Cdx-2-dependent response of central obesity to Vitamin D intake in the subjects with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 114, 1375–1384. [Google Scholar] [CrossRef]
- Salehpour, A.; Hosseinpanah, F.; Shidfar, F.; Vafa, M.; Razaghi, M.; Dehghani, S.; Hoshiarrad, A.; Gohari, M. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr. J. 2012, 11, 78. [Google Scholar] [CrossRef]
- Wamberg, L.; Kampmann, U.; Stødkilde-Jørgensen, H.; Rejnmark, L.; Pedersen, S.B.; Richelsen, B. Effects of vitamin D supplementation on body fat accumulation, inflammation, and metabolic risk factors in obese adults with low vitamin D levels—Results from a randomized trial. Eur. J. Intern. Med. 2013, 24, 644–649. [Google Scholar] [CrossRef]
- Agbaht, K.; Mercan, Y.; Kutlu, S.; Alpdemir, M.F.; Sezgin, T. Obesity with and without metabolic syndrome: Do vitamin D and thyroid autoimmunity have a role? Diabetes Res. Clin. Pract. 2014, 106, 27–34. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The effect of vitamin D supplementation on serum 25OHD in thin and obese women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef]
- Subih, H.S.; Zueter, Z.; Obeidat, B.M.; al-Qudah, M.A.; Janakat, S.; Hammoh, F.; Sharkas, G.; Bawadi, H.A. A high weekly dose of cholecalciferol and calcium supplement enhances weight loss and improves health biomarkers in obese women. Nutr. Res. 2018, 59, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cai, D.; Wang, Y.; Lin, N.; Hu, Q.; Qi, Y.; Ma, S.; Amarasekara, S. Calcium plus vitamin D3 supplementation facilitated Fat loss in overweight and obese college students with very-low calcium consumption: A randomized controlled trial. Nutr. J. 2013, 12, 8. [Google Scholar] [CrossRef]
- Jabbour, J.; Rahme, M.; Mahfoud, Z.R.; El-Hajj Fuleihan, G. Effect of high dose vitamin D supplementation on indices of sarcopenia and obesity assessed by DXA among older adults: A randomized controlled trial. Endocrine 2022, 76, 162–171. [Google Scholar] [CrossRef]
- Villa, C.R.; Taibi, A.; Chen, J.; Ward, W.E.; Comelli, E.M. Colonic Bacteroides are positively associated with trabecular bone structure and programmed by maternal Vitamin D in male but not female offspring in an obesogenic environment. Int. J. Obes. 2018, 42, 696–703. [Google Scholar] [CrossRef]
- Villa, C.R.; Chen, J.; Wen, B.; Sacco, S.M.; Taibi, A.; Ward, W.E.; Comelli, E.M. Maternal Vitamin D beneficially programs metabolic, gut and bone health of mouse male offspring in an obesogenic environment. Int. J. Obes. 2016, 40, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Canales, B.K.; Schafer, A.L.; Shoback, D.M.; Carpenter, T.O. Gastric bypass in obese rats causes bone loss, vitamin D deficiency, metabolic acidosis, and elevated peptide YY. Surg. Obes. Relat. Dis. 2014, 10, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.S.; Park, C.Y.; Lee, S.E.; Kim, T.Y.; Han, S.N. The effects of 1,25-dihydroxyvitamin D3 on markers related to the differentiation and maturation of bone marrow-derived dendritic cells from control and obese mice. J. Nutr. Biochem. 2020, 85, 108464. [Google Scholar] [CrossRef]
- Wang, Y.; Buckendahl, P.; Sharma, K.; Miller, J.W.; Shapses, S.A. Expression of vitamin D hydroxylases and bone quality in obese mice consuming saturated or monounsaturated enriched high-fat diets. Nutr. Res. 2018, 60, 106–115. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, D.H.; Lee, G.Y.; An, J.H.; Han, S.N. The effects of dietary vitamin D supplementation and in vitro 1,25 dihydroxyvitamin D3 treatment on autophagy in bone marrow-derived dendritic cells from high-fat diet-induced obese mice. J. Nutr. Biochem. 2021, 100, 108880. [Google Scholar] [CrossRef]
- Luger, M.; Kruschitz, R.; Winzer, E.; Schindler, K.; Grabovac, I.; Kainberger, F.; Krebs, M.; Hoppichler, F.; Langer, F.; Prager, G.; et al. Changes in Bone Mineral Density Following Weight Loss Induced by One-Anastomosis Gastric Bypass in Patients with Vitamin D Supplementation. Obes. Surg. 2018, 28, 3454–3465. [Google Scholar] [CrossRef]
- Wamberg, L.; Pedersen, S.B.; Richelsen, B.; Rejnmark, L. The effect of high-dose vitamin D supplementation on calciotropic hormones and bone mineral density in obese subjects with low levels of circulating 25-hydroxyvitamin D: Results from a randomized controlled study. Calcif. Tissue Int. 2013, 93, 69–77. [Google Scholar] [CrossRef]
- Gil-Cosano, J.J.; Gracia-Marco, L.; Ubago-Guisado, E.; Migueles, J.H.; Mora-Gonzalez, J.; Escolano-Margarit, M.V.; Gómez-Vida, J.; Maldonado, J.; Ortega, F.B. Muscular fitness mediates the association between 25-hydroxyvitamin D and areal bone mineral density in children with overweight/obesity. Nutrients 2019, 11, 2760. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chen, Y.P.; Yang, X.; Li, C.Q. Vitamin D3 levels and NLRP3 expression in murine models of obese asthma: Association with asthma outcomes. Braz. J. Med. Biol. Res. 2018, 51, e6841. [Google Scholar] [CrossRef]
- Ittichaicharoen, J.; Apaijai, N.; Tanajak, P.; Sa-nguanmoo, P.; Chattipakorn, N.; Chattipakorn, S.C. Impaired Mitochondria and Intracellular Calcium Transients in the Salivary Glands of Obese Rats. Appl. Physiol. Nutr. Metab. 2016, 42, 420–429. [Google Scholar] [CrossRef]
- Kang, M.S.; Park, C.Y.; Lee, G.Y.; Cho, D.H.; Kim, S.J.; Han, S.N. Effects of in vitro vitamin D treatment on function of T cells and autophagy mechanisms in high-fat diet-induced obese mice. Nutr. Res. Pract. 2021, 15, 673. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Nameni, G.; Hajiluian, G.; Shahabi, P. The effects of vitamin D administration on brain inflammatory markers in high fat diet induced obese rats. BMC Neurosci. 2017, 18, 81. [Google Scholar] [CrossRef]
- Nameni, G.; Hajiluian, G.; Shahabi, P.; Farhangi, M.A.; Mesgari-Abbasi, M.; Hemmati, M.R.; Vatandoust, S.M. The Impact of Vitamin D Supplementation on Neurodegeneration, TNF-α Concentration in Hypothalamus, and CSF-to-Plasma Ratio of Insulin in High-Fat-Diet-Induced Obese Rats. J. Mol. Neurosci. 2017, 61, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hajiluian, G.; Nameni, G.; Shahabi, P.; Mesgari-Abbasi, M.; Sadigh-Eteghad, S.; Farhangi, M.A. Vitamin D administration, cognitive function, BBB permeability and neuroinflammatory factors in high-fat diet-induced obese rats. Int. J. Obes. 2017, 41, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Park, C.Y.; Cha, K.S.; Lee, S.E.; Pae, M.; Han, S.N. Differential effect of dietary vitamin D supplementation on natural killer cell activity in lean and obese mice. J. Nutr. Biochem. 2018, 55, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jahn, D.; Dorbath, D.; Kircher, S.; Nier, A.; Bergheim, I.; Lenaerts, K.; Hermanns, H.M.; Geier, A. Beneficial effects of vitamin D treatment in an obese mouse model of non-alcoholic steatohepatitis. Nutrients 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Benetti, E.; Mastrocola, R.; Chiazza, F.; Nigro, D.; D’Antona, G.; Bordano, V.; Fantozzi, R.; Aragno, M.; Collino, M.; Minetto, M.A. Effects of vitamin D on insulin resistance and myosteatosis in diet-induced obese mice. PLoS ONE 2018, 13, e0189707. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Cho, D.H.; Lee, G.Y.; Kang, M.S.; Kim, S.J.; Han, S.N. Effects of vitamin d supplementation on CD4+ t cell subsets and mtor signaling pathway in high-fat-diet-induced obese mice. Nutrients 2021, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhu, L.; Ge, Y.; Zhang, H. Improving effect of vitamin Dsupplementation on obesity-related diabetes in rats. Minerva Endocrinol. 2020, 45, 29–35. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Nameni, G.; Hajiluian, G.; Mesgari-Abbasi, M. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat- diet induced obese rats. BMC Cardiovasc. Disord. 2017, 17, 161. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Mesgari-Abbasi, M.; Shahabi, P. Hepatic Complications of High-Fat Diet-Induced Obesity: Reduction of Increased Hepatic Oxidative Stress and Tumor Necrosis Factor-Alpha Concentration by Vitamin D Administration. Curr. Top. Nutraceutical Res. 2020, 18, 360–365. [Google Scholar]
- Conceição, E.P.S.; Moura, E.G.; Soares, P.N.; Ai, X.X.; Figueiredo, M.S.; Oliveira, E.; Lisboa, P.C. High calcium diet improves the liver oxidative stress and microsteatosis in adult obese rats that were overfed during lactation. Food Chem. Toxicol. 2016, 92, 245–255. [Google Scholar] [CrossRef]
- Kim, H.; Chandler, P.; Ng, K.; Manson, J.E.; Giovannucci, E. Obesity and efficacy of vitamin D3 supplementation in healthy black adults. Cancer Causes Control. 2020, 31, 303–307. [Google Scholar] [CrossRef]
- Torres, M.R.S.G.; Sanjuliani, A.F. Effects of weight loss from a high-calcium energy-reduced diet on biomarkers of inflammatory stress, fibrinolysis, and endothelial function in obese subjects. Nutrition 2013, 29, 143–151. [Google Scholar] [CrossRef][Green Version]
- Lotfi-Dizaji, L.; Mahboob, S.; Aliashrafi, S.; Vaghef-Mehrabany, E.; Ebrahimi-Mameghani, M.; Morovati, A. Effect of vitamin D supplementation along with weight loss diet on meta-inflammation and fat mass in obese subjects with vitamin D deficiency: A double-blind placebo-controlled randomized clinical trial. Clin. Endocrinol. 2019, 90, 94–101. [Google Scholar] [CrossRef]
- Khosravi, Z.; Kafeshani, M.; Tavasoli, P.; Zadeh, A.; Entezari, M. Effect of Vitamin D supplementation on weight loss, glycemic indices, and lipid profile in obese and overweight women: A clinical trial study. Int. J. Prev. Med. 2018, 9, 63. [Google Scholar] [CrossRef]
- Mesinovic, J.; Mousa, A.; Wilson, K.; Scragg, R.; Plebanski, M.; de Courten, M.; Scott, D.; Naderpoor, N.; de Courten, B. Effect of 16-weeks vitamin D replacement on calcium-phosphate homeostasis in overweight and obese adults. J. Steroid Biochem. Mol. Biol. 2019, 186, 169–175. [Google Scholar] [CrossRef]
- Varshney, S.; Khadgawat, R.; Gahlot, M.; Khandelwal, D.; Oberoi, A.; Yadav, R.; Sreenivas, V.; Gupta, N.; Tandon, N. Effect of high-dose Vitamin D supplementation on beta cell function in obese Asian-Indian children and adolescents: A randomized, double blind, active controlled study. Indian J. Endocrinol. Metab. 2019, 23, 545–551. [Google Scholar] [CrossRef]
- Palaniswamy, S.; Gill, D.; De Silva, N.M.; Lowry, E.; Jokelainen, J.; Karhu, T.; Mutt, S.J.; Dehghan, A.; Sliz, E.; Chasman, D.I.; et al. Could Vitamin D reduce obesity-Associated inflammation? Observational and Mendelian randomization study. Am. J. Clin. Nutr. 2020, 111, 1036–1047. [Google Scholar] [CrossRef]
- Shah, S.; Wilson, D.M.; Bachrach, L.K. Large doses of vitamin D fail to increase 25-hydroxyvitamin D levels or to alter cardiovascular risk factors in obese adolescents: A pilot study. J. Adolesc. Health 2015, 57, 19–23. [Google Scholar] [CrossRef]
- Mangge, H.; Zelzer, S.; Meinitzer, A.; Stelzer, I.; Schnedl, W.; Weghuber, D.; Fuchs, D.; Postolache, T.; Aigner, E.; Datz, C.; et al. 25OH-Vitamin D3 Levels in Obesity and Metabolic Syndrome—Unaltered in Young and not Correlated to Carotid IMT in All Ages. Curr. Pharm. Des. 2015, 21, 2243–2249. [Google Scholar] [CrossRef]
- Esteghamati, A.; Aryan, Z.; Esteghamati, A.; Nakhjavani, M. Differences in vitamin D concentration between metabolically healthy and unhealthy obese adults: Associations with inflammatory and cardiometabolic markers in 4391 subjects. Diabetes Metab. 2014, 40, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Moschonis, G.; Androutsos, O.; Hulshof, T.; Dracopoulou, M.; Chrousos, G.P.; Manios, Y. Vitamin D insufficiency is associated with insulin resistance independently of obesity in primary schoolchildren. The healthy growth study. Pediatr. Diabetes 2018, 19, 866–873. [Google Scholar] [CrossRef]
- Pirgon, O.; Cekmez, F.; Bilgin, H.; Eren, E.; Dundar, B. Low 25-hydroxyvitamin D level is associated with insulin sensitivity in obese adolescents with non-alcoholic fatty liver disease. Obes. Res. Clin. Pract. 2013, 7, e275–e283. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, J.D.; Vasaitis, T.S.; Streeten, E.; Ryan, A.S.; Goldberg, A.P. Evidence for threshold effects of 25-hydroxyvitamin D on glucose tolerance and insulin resistance in black and white obese postmenopausal women. J. Nutr. 2014, 144, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Amini, Z.; Bryant, S.; Smith, C.; Singh, R.; Kumar, S. Is the serum vitamin d-parathyroid hormone relationship influenced by obesity in children? Horm. Res. Paediatr. 2013, 80, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Marziou, A.; Aubert, B.; Couturier, C.; Astier, J.; Philouze, C.; Obert, P.; Landrier, J.-F.; Riva, C. Combined Beneficial Effect of Voluntary Physical Exercise and Vitamin D Supplementation in Diet-induced Obese C57BL/6J Mice. Med. Sci. Sport. Exerc. 2021, 53, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Nandan, P.; Nayanatara, A.K.; Poojary, R.; Bhagyalakshmi, K.; Nirupama, M.; Kini, R.D. Protective Role of Co-administration of Vitamin D in Monosodium Glutamate Induced Obesity in Female Rats. J. Natl. Med. Assoc. 2018, 110, 98–102. [Google Scholar] [CrossRef]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.J.; Bendahan, D.; Bernard, M.; Martin, J.C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef]
- Yoo, J.S.; Park, C.Y.; Seo, Y.K.; Woo, S.H.; Kim, D.Y.; Han, S.N. Vitamin D supplementation partially affects colonic changes in dextran sulfate sodium–induced colitis obese mice but not lean mice. Nutr. Res. 2019, 67, 90–99. [Google Scholar] [CrossRef]
- Hou, C.; Wang, Y.; Zhu, E.; Yan, C.; Zhao, L.; Wang, X.; Qiu, Y.; Shen, H.; Sun, X.; Feng, Z.; et al. Coral calcium hydride prevents hepatic steatosis in high fat diet-induced obese rats: A potent mitochondrial nutrient and phase II enzyme inducer. Biochem. Pharmacol. 2016, 103, 85–97. [Google Scholar] [CrossRef]
- Valle, M.; Mitchell, P.L.; Pilon, G.; Varin, T.; Hénault, L.; Rolin, J.; McLeod, R.; Gill, T.; Richard, D.; Vohl, M.C.; et al. Salmon peptides limit obesity-associated metabolic disorders by modulating a gut-liver axis in vitamin D-deficient mice. Obesity 2021, 29, 1635–1649. [Google Scholar] [CrossRef]
- Rock, C.L.; Emond, J.A.; Flatt, S.W.; Heath, D.D.; Karanja, N.; Pakiz, B.; Sherwood, N.E.; Thomson, C.A. Weight loss is associated with increased serum 25-hydroxyvitamin D in overweight or obese women. Obesity 2012, 20, 2296–2301. [Google Scholar] [CrossRef]
- Carrillo, A.E.; Flynn, M.G.; Pinkston, C.; Markofski, M.M.; Jiang, Y.; Donkin, S.S.; Teegarden, D. Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin. Nutr. 2013, 32, 375–381. [Google Scholar] [CrossRef]
- Faghih, S.H.; Abadi, A.R.; Hedayati, M.; Kimiagar, S.M. Comparison of the effects of cows’ milk, fortified soy milk, and calcium supplement on weight and fat loss in premenopausal overweight and obese women. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 499–503. [Google Scholar] [CrossRef]
- Carrillo, A.E.; Flynn, M.G.; Pinkston, C.; Markofski, M.M.; Jiang, Y.; Donkin, S.S.; Teegarden, D. Vitamin D supplementation during exercise training does not alter inflammatory biomarkers in overweight and obese subjects. Eur. J. Appl. Physiol. 2012, 112, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- Sharifan, P.; Ziaee, A.; Darroudi, S.; Rezaie, M.; Safarian, M.; Eslami, S.; Khadem-Rezaiyan, M.; Tayefi, M.; Mohammadi Bajgiran, M.; Ghazizadeh, H.; et al. Effect of low-fat dairy products fortified with 1500IU nano encapsulated vitamin D3 on cardiometabolic indicators in adults with abdominal obesity: A total blinded randomized controlled trial. Curr. Med. Res. Opin. 2021, 37, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Vinet, A.; Morrissey, C.; Perez-Martin, A.; Goncalves, A.; Raverdy, C.; Masson, D.; Gayrard, S.; Carrere, M.; Landrier, J.F.; Amiot, M.J. Effect of vitamin D supplementation on microvascular reactivity in obese adolescents: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Naderpoor, N.; Mousa, A.; de Courten, M.; Scragg, R.; de Courten, B. The relationship between 25-hydroxyvitamin D concentration and liver enzymes in overweight or obese adults: Cross-sectional and interventional outcomes. J. Steroid Biochem. Mol. Biol. 2018, 177, 193–199. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Hernández-Aguilera, A.; de Vries, M.A.; Burggraaf, B.; van der Zwan, E.; Pouw, N.; Joven, J.; Cabezas, M.C. Effect of vitamin D3 on the postprandial lipid profile in obese patients: A non-targeted lipidomics study. Nutrients 2019, 11, 1194. [Google Scholar] [CrossRef]
- Harris, S.S.; Pittas, A.G.; Palermo, N.J. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes. Metab. 2012, 14, 789–794. [Google Scholar] [CrossRef]
- Chacko, S.J.; Pauwaa, S.; Barengolts, E.; Ciubotaru, I.; Kansal, M.M. Vitamin D Attenuates Left Atrial Volume Changes in African American Males with Obesity and Prediabetes. Echocardiography 2016, 33, 681–685. [Google Scholar] [CrossRef]
- El Hajj, C.; Walrand, S.; Helou, M.; Yammine, K. Effect of Vitamin D Supplementation on Inflammatory Markers in Non-Obese Lebanese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2020, 12, 2033. [Google Scholar] [CrossRef]
- Chen, L.; Dong, Y.; Bhagatwala, J.; Raed, A.; Huang, Y.; Zhu, H. Vitamin D3 Supplementation Increases Long-Chain Ceramide Levels in Overweight/Obese African Americans: A Post-Hoc Analysis of a Randomized Controlled Trial. Nutrients 2020, 12, 981. [Google Scholar] [CrossRef]
- Cefalo, C.M.A.; Conte, C.; Sorice, G.P.; Moffa, S.; Sun, V.A.; Cinti, F.; Salomone, E.; Muscogiuri, G.; Brocchi, A.A.G.; Pontecorvi, A.; et al. Effect of Vitamin D Supplementation on Obesity-Induced Insulin Resistance: A Double-Blind, Randomized, Placebo-Controlled Trial. Obesity 2018, 26, 651–657. [Google Scholar] [CrossRef]
- Motlaghzadeh, Y.; Sayarifard, F.; Allahverdi, B.; Rabbani, A.; Setoodeh, A.; Sayarifard, A.; Abbasi, F.; Haghi-Ashtiani, M.T.; Rahimi-Froushani, A. Assessment of vitamin D status and response to vitamin D3 in obese and non-obese iranian children. J. Trop. Pediatr. 2016, 62, 269–275. [Google Scholar] [CrossRef]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016, 4, 16041. [Google Scholar] [CrossRef]
- Thomas, A.P.; Dunn, T.N.; Drayton, J.B.; Oort, P.J.; Adams, S.H. A high calcium diet containing nonfat dry milk reduces weight gain and associated adipose tissue inflammation in diet-induced obese mice when compared to high calcium alone. Nutr. Metab. 2012, 9, 3. [Google Scholar] [CrossRef]
- Wood, A.D.; Secombes, K.R.; Thies, F.; Aucott, L.S.; Black, A.J.; Reid, D.M.; Mavroeidi, A.; Simpson, W.G.; Fraser, W.D.; Macdonald, H.M. A parallel group double-blind RCT of vitamin D3 assessing physical function: Is the biochemical response to treatment affected by overweight and obesity? Osteoporos. Int. 2014, 25, 305–315. [Google Scholar] [CrossRef]
- Salehpour, A.; Shidfar, F.; Hosseinpanah, F.; Vafa, M.; Razaghi, M.; Amiri, F. Does vitamin D3 supplementation improve glucose homeostasis in overweight or obese women? A double-blind, randomized, placebo-controlled clinical trial. Diabet. Med. 2013, 30, 1477–1481. [Google Scholar] [CrossRef]
- Brzeziński, M.; Jankowska, A.; Słomińska-Frączek, M.; Metelska, P.; Wiśniewski, P.; Socha, P.; Szlagatys-Sidorkiewicz, A. Long-term effects of vitamin d supplementation in obese children during integrated weight–loss programme—A double blind randomized placebo–controlled trial. Nutrients 2020, 12, 1093. [Google Scholar] [CrossRef]
- Jones, K.W.; Eller, L.K.; Parnell, J.A.; Doyle-Baker, P.K.; Edwards, A.L.; Reimer, R.A. Effect of a dairy-and calcium-rich diet on weight loss and appetite during energy restriction in overweight and obese adults: A randomized trial. Eur. J. Clin. Nutr. 2013, 67, 371–376. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Roberts, M.D.; Campbell, B.I.; Galbreath, M.M.; Taylor, L.W.; Wilborn, C.D.; Lee, A.; Dove, J.; Bunn, J.W.; Rasmussen, C.J.; et al. Differential impact of calcium and vitamin d on body composition changes in post-menopausal women following a restricted energy diet and exercise program. Nutrients 2020, 12, 713. [Google Scholar] [CrossRef]
- van Schoor, N.; Lips, P. Worldwide Vitamin D Status. Vitam. D Fourth Ed. 2017, 2, 15–40. [Google Scholar] [CrossRef]
- Balk, E.M.; Adam, G.P.; Langberg, V.N.; Earley, A.; Clark, P.; Ebeling, P.R.; Mithal, A.; Rizzoli, R.; Zerbini, C.A.F.; Pierroz, D.D.; et al. Global dietary calcium intake among adults: A systematic review. Osteoporos. Int. 2017, 28, 3315–3324. [Google Scholar] [CrossRef]
- Van Der Meer, I.M.; Middelkoop, B.J.C.; Boeke, A.J.P.; Lips, P. Prevalence of vitamin D deficiency among Turkish, Moroccan, Indian and sub-Sahara African populations in Europe and their countries of origin: An overview. Osteoporos. Int. 2011, 22, 1009–1021. [Google Scholar] [CrossRef]
- Radhakishun, N.; van Vliet, M.; von Rosenstiel, I.; Weijer, O.; Diamant, M.; Beijnen, J.; Brandjes, D. High prevalence of vitamin D insufficiency/deficiency in Dutch multi-ethnic obese children. Eur. J. Pediatr. 2015, 174, 183–190. [Google Scholar] [CrossRef]
- Guessous, I.; Dudler, V.; Glatz, N.; Theler, J.M.; Zoller, O.; Paccaud, F.; Burnier, M.; Bochud, M. Vitamin D levels and associated factors: A population-based study in Switzerland. Swiss Med. Wkly. 2012, 142, w13719. [Google Scholar] [CrossRef]
- Yeum, K.J.; Song, B.C.; Joo, N.S. Impact of geographic location on vitamin D status and bone mineral density. Int. J. Environ. Res. Public Health 2016, 13, 184. [Google Scholar] [CrossRef]
- De Lucia, F.; Minisola, S.; Romagnoli, E.; Pepe, J.; Cipriani, C.; Scillitani, A.; Parikh, N.; Rao, D.S. Effect of gender and geographic location on the expression of primary hyperparathyroidism. J. Endocrinol. Investig. 2013, 36, 123–126. [Google Scholar] [CrossRef]
- Heravi, A.S.; Michos, E.D. Vitamin D and Calcium Supplements: Helpful, Harmful, or Neutral for Cardiovascular Risk? Methodist Debakey Cardiovasc. J. 2019, 15, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Silk, L.N.; Greene, D.A.; Baker, M.K. The effect of calcium or calcium and Vitamin D supplementation on bone mineral density in healthy males: A systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, M.O.; Dickin, K.L.; O’Brien, K.O.; Neufeld, L.M.; De Regil, L.M.; Stoltzfus, R.J. Calcium supplementation to prevent preeclampsia: Translating guidelines into practice in low-income countries. Adv. Nutr. 2016, 7, 275–278. [Google Scholar] [CrossRef] [PubMed]
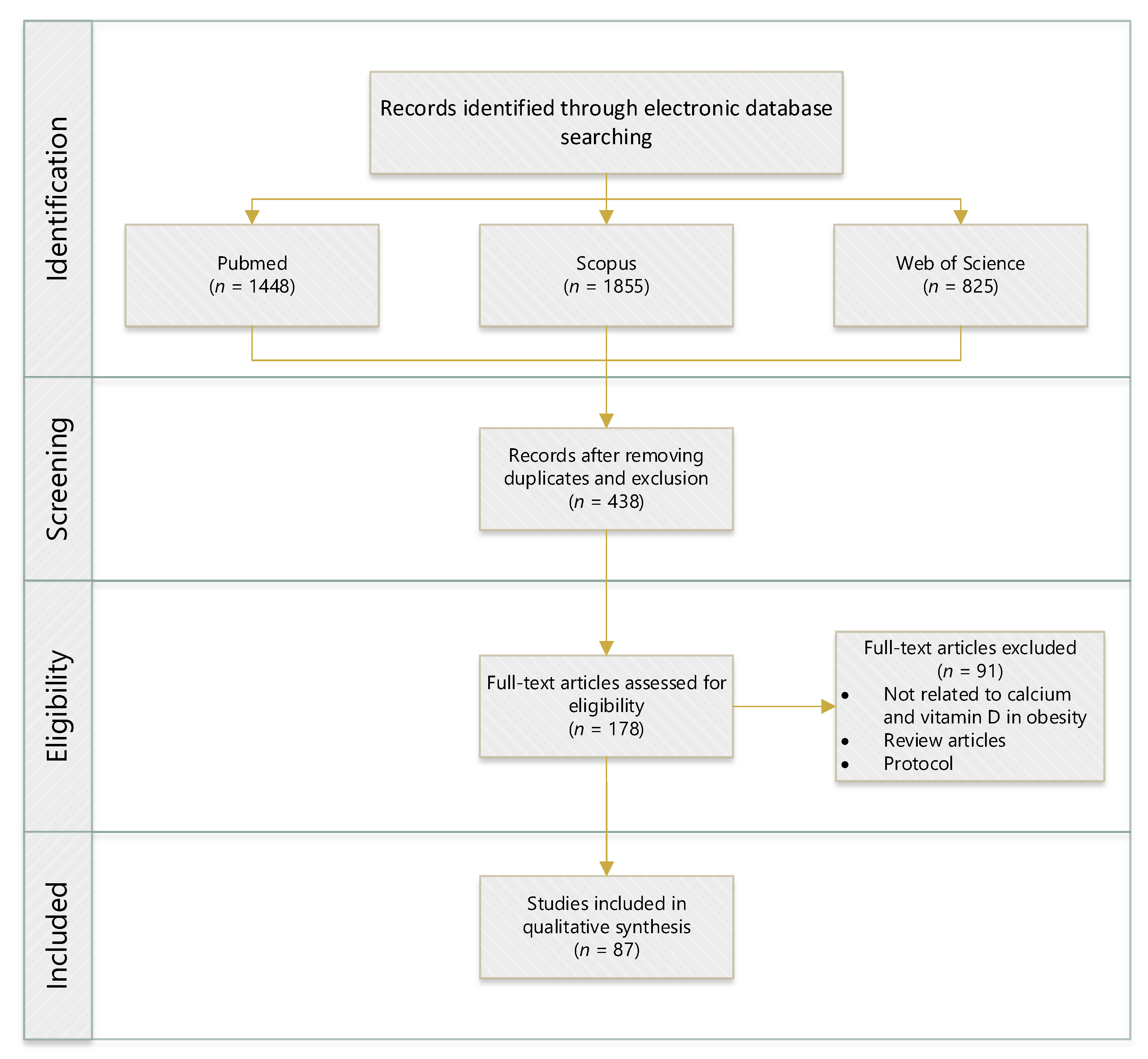
| Constraint | Inclusion | Exclusion |
|---|---|---|
| Type of articles | Scientific articles on animal experiments and human investigations | In vitro assessment |
| Relationship | Studies related to the association of either VD, CAL, or both in obese | Studies not related to the association of either VD, CAL, or both in obese |
| Comparator | Placebo or no comparator | — |
| Outcomes | There was scientific evidence of the association of either VD, CAL, or both with obesity | There was no scientific evidence of the association of either VD, CAL, or both with obesity |
| Study design | Evidence-based interventions, including multi-level factors, types of action, outcomes, and unintended negative consequences | Repetition articles, review journals, protocol records, case studies, second analysis reports, inadequate explanation of VD–CAL, neglect of English language, and issued earlier than 2011 |
| Reference | Experimental Design | Diet Intervention | Comorbidities | Highlight Result |
|---|---|---|---|---|
| Animal study | ||||
| [37] | Male C57BL/6J wild-type mice aged eight weeks old | A high-fat diet with 500 IU/kg diet followed by a 10,000 IU/kg diet of VD3 | Obesity | Gut and adipose tissue cross-talked in VDRs signalling associated with lipid homeostasis. |
| [38] | C57BL/6 mice aged six weeks old | High-fat-diet with 25,000 IU of VD3 | Obesity | ↑ VD3 concentrations in liver and adipose tissue |
| [39] | Male C57BL/6J mice aged six weeks old | High-fat and high-sucrose with 15,000 IU/kg of VD | Obesity | ↓ chemokine mRNA concentrations in adipose tissue; ↓ lipid droplets and triglyceride accumulation in the liver; ↓ hepatic DNL and fatty acid oxidation expression. |
| [40] | New-born Wistar rats | 1 μg/kg of 1,25(OH)2D3 | Obesity | ↓ macrophages in pancreas and adipose tissue inflitration; ↑ Tgf-β1 in liver tissue expression; ↑ Treg cells and insulin-targeted tissues inflitration. |
| [41] | Obese C57BL/6J mice aged five weeks old | A high-fat diet with 4 g/kg of CAL | Obesity | ↓ endotoxin concentrations; ↑ angiopoietin-like 4 expression; ↓ hepatic lipid; ↑ adipose tissue expression and inflammation. |
| [42] | Obese Wistar rats | 10 g CaCO3/kg | Obesity | ↓ VAT fat acid synthase, ↓ leptinemia; and ↓ Vdr. |
| [43] | Early weaning Wistar rats | 10 g CaCO3/kg | Obesity | ↑ calbidin, VDR and prevented adipose tissue dysfunction |
| [44] | Four-week-old C57BL/6J male mice | High CAL, high VD | Obesity | ↓ weight gain (body and fat), ↑ adiposity and ↑ plasma glucose, insulin, adiponectin, calcifediol, calcitriol, PTH. |
| [45] | Obese male Wistar rats with CAL deficiency | 1000 mg of CAL carbonate per 100 g high-fat diet | Obesity | ↓ body weight, ↓ adiposity, ↓ glucose, ↓ insulin, ↓ HOMA-IR, ↓ TNF-α, IL-6, MCP-1, Leptin, ↓ hepatic lipid, ↓ hepatic macrophage, ↓ adipocyte hypertrophy, and ↑ adiponectin level |
| Human study | ||||
| [46] | Obese adolescent patients aged 14.1 + 2.8 years old | 4000 IU of VD3 | Obesity | ↑ serum 25(OH)D; ↑ fasting insulin; ↑ HOMA-IR; ↑ leptin-to-adiponectin ratio; No changes in inflammatory signs. |
| [47] | Men and women with overweight and obese aged 18–65 years old | 350 mg of CAL and 100 IU of VD3 | Obesity | Significant correlation of VD–CAL on visceral adipose tissue. |
| Reference | Experimental Design | Diet Intervention | Comorbidities | Highlight Result |
|---|---|---|---|---|
| Animal Study | ||||
| [48] | Obese male C57BL/6J mice aged twenty four weeks old | 162 IU, 1282 IU and 5169 IU of VD3/kg | Obesity | ↓ serum calcitriol; ↑ serum parathyroid hormone. |
| [49] | Male C57BL/6 mice aged ten weeks old | A high-fat diet with VD-enriched mushrooms extract | Obesity | ↓ total body fat; ↓ hepatic fat content; ↑ immunomodulatory effect (CD4/CD8 lymphocyte ratio). |
| [50] | Wistar and genetically predisposed obese IIMb/b rats | A low dose of 0.2% CaCO3. | Obesity | ↑ body fat, ↑ liver weight, ↑ perigonadal and retroperitoneal fat in low CAL intake; ↓ body ashes and ↓ total skeleton bone mineral in low CAL intake. |
| [51] | Male C57BL/6J mice aged five weeks old | A high-fat diet | Obesity | ↓ CYP2R1 and CYP27A1, CYP27B1, VDR; ↑ CYP24A1 (24-hydroxylase); ↑ DNA methylation, Dnmt activity, and 5-methylcytosine; ↓ Tet activity and 5-hydroxymethylcytosine. |
| [52] | C57BL/6 mice | A high-fat diet | Obesity | ↓ Cyp2r1 mRNA; Positive relationship between Cyp2r1 mRNA levels with 25(OH)D levels and cholecalciferol ratio. |
| Human Study | ||||
| [53] | Type 2 diabetes patients aged 30–60 years old | Fortified yogurt drink by 170 mg CAL with 12.5 μg VD3/250 mL twice a day | Diabetic type 2 | ↓ waist circumference, ↓ fat mass, ↓ truncal fat, and ↓ visceral adipose tissue |
| [54] | Healthy overweight or obese women with an average age of 38 ± 8.1 years old | Cholecalciferol 25 μg | Obesity | ↓ serum iPTH levels; ↓ fat mass; No alterations in body weight and waist circumference. |
| [55] | Healthy adults aged 18–50 years old with BMI > 30 kg/m2 | 7000 IU of VD each day | Obesity | No alterations in body fat, intramyocellular lipids, VAT, intrahepatic, subcutaneous, HOMA, blood pressure, plasma lipids, and hsCRP. |
| [56] | Males and females aged 35–51 years old with BMI ≥30 kg/m2 | No intervention of VD | Obesity | Negative correlation among 25(OH)D levels, BMI, and percentage body fat. |
| [57] | Thin and obese women aged 57–90 years old with VD insufficiency (50 nmol/L) | Daily doses of VD3 of 400, 800, 1600, 2400, 3200, 4000, and 4800 IU | Obesity | No significantly different in total body fat mass. |
| [58] | Obese women aged 18–48 years old | 50,000 IU of cholecalciferol/week, 1200 mg/dL CAL/day, and cholecalciferol plus CAL | Obesity | ↓ body fat percentage, ↓ fasting blood glucose, ↓ PTH, ↓ cholesterol, and ↓ triglycerides in cholecalciferol plus CAL intake. |
| [59] | Overweight or obese male aged 18–25 years old | CAL carbonate (600 mg) and VD3 (125 IU) | Obesity | ↓ fat mass loss; ↓ visceral fat mass and ↓ visceral fat area. |
| [60] | Overweight and obese males and females aged greater than 65 years old | 600 IU and 3750 IU of VD | Obesity | No significant differences in muscle and visceral adiposity |
| Reference | Experimental Design | Diet Intervention | Comorbidities | Highlight Result |
|---|---|---|---|---|
| Animal study | ||||
| [61] | C57BL/6J male and female offspring | High and low VD | Obesity | Positive correlation between Bacteroides and LPS, trabecular femur peak load, vertebral trabecular separation, trabecular number, and bone volume fraction in both |
| [62] | Three-week-old C57BL/6J mice | High or low VD; high fat and sucrose | Obesity | ↓ lower intestinal permeability; ↑ trabecular number and ↓ trabecular separation; No effects on IL-6 and TNF-α serum concentrations. |
| [63] | Twenty-one-week-old male obese Sprague Dawley rats underwent Roux-en-Y gastric bypass | High-fat diet | Obesity | ↓ serum bicarbonate, calcium, 25-hydroxyvitamin D, insulin, and leptin levels; ↑ serum osteocalcin; ↓ P1NP levels; ↓ the volume, number, and thickness of trabecular bone. |
| [64] | Male C57BL/6N obese mice aged five weeks old | High-fat diet | Obesity | ↓ Il12b mRNA levels in stimulated bone marrow-derived dendritic cells |
| [65] | Female C57BL/6J mice aged eight months old | Saturated fatty acids and VD 1000 IU/kg diet | Obesity | ↓ hepatic Cyp2r1 and renal Cyp24a1 mRNA expression |
| [66] | C57BL/6 males aged ten weeks old | High-fat diet, VD 1000 and 10,000 IU/kg diet | Obesity | ↓ phenotypes related to dendritic cells function expression; ↓ production of IL-12p70 by bone marrow-derived dendritic cells; ↑ LC3 Ⅱ/Ⅰ and VPS34 protein concentrations; ↓ p62 expression; ↓ Vdr mRNA levels. |
| Human study | ||||
| [67] | Healthy overweight and obese women | VD3 doses (100,000 IU; 3420 IU; and 3420 IU) | Obesity | ↓ BMD; ↑ bone turnover markers; normal parathyroid hormone concentrations; No association between weight loss and changes in BMD; Positive association between low levels of bone loss and high levels of 25(OH)D. |
| [68] | Healthy males and females aged 18–50 years old | 7000 IU cholecalciferol | Obesity | ↑ plasma 25OHD, ↓ PTH, ↓ CTX; Inverse correlation between alterations in plasma 25(OH)D, bone-specific alkaline phosphatase, and CTX; Inverse correlation between alterations in CTX and in spine BMD; Association between ↑ levels of 25OHD with ↓ PTH, ↓ bone turnover, and ↑ BMD at the forearm. |
| [69] | Overweight and obese children aged 8–11 years old | No intervention of VD | Obesity | A link between 25(OH)D and areal BMD. |
| Reference | Experimental Design | Diet Intervention | Comorbidities | Highlight Result |
|---|---|---|---|---|
| Animal study | ||||
| [70] | Male BALB/C mice | A high-fat diet | Obesity | ↑ cytokine levels and IL-1b mRNA expression; Negative correlation between VD levels, inflammatory cells, IL-1b and IL-17 levels. |
| [71] | Male Wistar rats | A high-fat diet | Obesity | ↓ intracellular CAL transient rates, ↓ rates and amplitude of salivary acinar cells |
| [72] | Males C57BL/6 mice aged five weeks old | Diet-induced obesity | Obesity | ↓ autophagy of T cells, ↑ dysregulation of T cell homeostasis |
| [73] | Male Wistar rats | VD 500 IU/kg diet | Obesity | ↓ food intake and weight gain; ↓ hippocampus acetylcholine concentrations; ↑ hippocampus IL-6 concentrations. |
| [74] | Male Wistar rats | VD 500 IU/kg diet | Obesity | ↓ weight and food intake; ↓ insulin resistance; No influence of VD on degenerated neurons and TNF-α concentration. |
| [75] | Hippocampus male Wistar rats | VD 500 IU/kg diet | Obesity | ↓ body weight, NF-κB concentrations, blood–brain barrier permeability; ↑ increased brain-derived neurotrophic factor concentrations. |
| [76] | Male C57BL/6 mice aged five weeks old | VD 10,000 IU/kg diet | Obesity | ↓ natural killer cells |
| [77] | C57BL/6J mice | A high-fat/high-sugar diet with low amounts of VD | Obesity | ↓ steatosis and fibrosis; ↓ inflammatory and pro-fibrotic genes; ↓ intestinal inflammation. |
| [78] | Male C57BL/6J mice aged four weeks old | A high fat-high sugar with VD | Obesity | ↓ NF-κB activation, TNF-alpha level, SCAP/SREBP lipogenic pathway activation, CML protein adducts level, and RAGE expression. |
| [79] | Male C57BL/6N mice aged ten weeks old | A high-fat diet with 1000 or 10,000 IU of VD/kg diet | Obesity | ↑ CD4 + IL-17 + T cells; ↑ CD4 + CD25 + Foxp3 + T cells; ↓ phospho-p70S6K/total-p70S6K ratio; ↑ phospho-AKT/total-AKT ratio; ↓ Hif1α mRNA levels. |
| [80] | Obesity-related diabetes rats | VD | Obesity | ↓ parathormone and adipocytokines |
| [81] | Obese male Wistar rats | 500 IU/kg VD | Obesity | ↑ superoxide dismutase activity; ↓ catalase activity; ↓ TNF-α concentration in heart tissue. |
| [82] | Obese male Wistar rats | 500 IU/kg VD | Obesity | ↓ glutathione peroxidase activity; ↓ hepatic tumour necrosis factor concentrations; ↑ superoxide dismutase activity; No effects on glutathione peroxidase or catalase activity. |
| [83] | Female Wistar rats | 10 g CaCO3/kg diet | Obesity | ↑ fatty acid synthase, ↑ steatosis, and ↓ protein kinase B (Akt) |
| Human study | ||||
| [84] | Men and women aged 30–80 years old | 1000, 2000, and 4000 IU VD3 per day | Obesity | ↓ PTH levels with 1000 IU per day; ↑ PTH levels with 2000 to 4000 IU per day. |
| [85] | Patients aged 20–60 years old | A high-CAL diet (1200–1300 mg/d) and low-CAL diet (<500 mg/d) | Obesity | ↓ inflammation markers, ↓ fibrinolysis, and ↓ endothelial dysfunction. |
| [86] | Males and females aged 18–59 years old | 50,000 IU of VD per week | Obesity | ↓ PTH, MCP-1, IL-1β and TLR-4 |
| [87] | Obese and overweight women aged 20–40 years old | 50,000 IU VD per week | Obesity | No alterations in C-reactive protein, insulin, insulin resistance, and waist to hip ratio. |
| [88] | Overweight and obese adults (aged 32 ± 8.5 years old) | Cholecalciferol doses 100,000 IU followed by 4000 IU per day | Obesity | No influence in iPTH and cFGF-23; Inverse correlations between serum iPTH and cFGF-23. |
| [89] | Obese children and adolescents aged 12.89 ± 1.63 years old | VD 120,000 IU/month and 12,000 IU/month | Obesity | No significant difference in insulin resistance, sensitivity, inflammatory cytokines, and pulse wave velocity |
| [90] | Pregnant women | 400 IU/d to 150,000 IU per three months | Obesity | Positive association: BMI and 9 inflammatory biomarkers; Positive association: BMI and sICAM-1, hs-CRP, and AGP; Inverse association: 25(OH)D and sICAM-1, hs-CRP, and AGP; No impact of VD intake on inflammatory biomarkers. |
| [91] | Overweight and obese youth aged 11–17.99 years old | 150,000 IU ergocalciferol per 3 months | Obesity | No alteration in inflammatory markers |
| [92] | Obese patients aged between 10 and 65 years. | No intervention of VD | Obesity | ↓ blood levels of VD; ↑ US-CRP, IL-6, HOMA |
| [93] | Obese patients aged > 18 years old | No intervention of VD | Obesity | ↓ metabolic status, ↑ liver enzymes, ↑ inflammatory markers |
| [94] | Healthy children aged 9–13 years old | No intervention of VD | Obesity | ↑ VD insufficiency occurrence in children with IR |
| [95] | Obese adolescents aged 12.7 ± 1.3 years old | No intervention of VD | Obesity | Negative association: 25(OH)D and HOMA-IR with alanine aminotransferase |
| [96] | Obese and overweight postmenopausal women without diabetes | No intervention of VD | Obesity | Inverse association: 25(OH)D and fasting and 2-h insulin, HOMA-IR, and PTH |
| [97] | Overweight and obese children aged 2-18-year-old | No intervention of vitamin D | Obesity | ↓ serum 25(OH) D level; ↓ PTH |
| Reference | Experimental Design | Diet Intervention | Comorbidities | Highlight Result |
|---|---|---|---|---|
| Animal study | ||||
| [98] | Male C57BL/6J mice aged ten weeks old | High-fat/sucrose (HFS), physical exercise, and VD | Obesity | ↑ insulin sensitivity and hepatic steatosis in combining physical exercise and VD; ↓ hepatic de novo lipogenesis in combining physical exercise and VD. |
| [99] | Adult Wistar strain albino female rats | Monosodium glutamate and calcitriol | Obesity | ↓ body weight, food, and water intake |
| [100] | Male C57BL/6J mice aged six weeks old | VD3 15,000 IU/kg diet | Obesity | ↑ lipid oxidation; ↑ upregulation of fatty acid oxidation and mitochondrial metabolism genes; ↑ expenditure on energy. |
| [101] | Male C57BL/6N mice aged five weeks old | VD 1000 and 10,000 IU/kg diet | Obesity | ↑ colonic Cldn1 and Cyp27b1 mRNA levels; Negative relationship: 25(OH)D levels and histology score. |
| [102] | Male Sprague Dawley rats aged six weeks old with high-fat-diet-induced NAFLD | 50 mg/kg coral CAL and 50 mg/kg coral CAL hydride | Obesity | ↓ body weight gain, ↑ hepatic mitochondrial dysfunction, ↓ oxidative stress, and activated phase II enzymes in coral CAL hydride treatment. |
| [103] | Low-density lipoprotein receptor (LDLr) mice | Salmon peptide fraction and VD3 (15,000 IU/kg of diet) | Obesity | ↑ metabolic syndrome via a gut-liver axis ↑ Mogibacterium and Muribaculaceae) |
| Human study | ||||
| [104] | Overweight and obese women | 400 IU of VD per day | Obesity | An association weight loss and ↑ 25(OH)D levels. |
| [105] | Overweight and obese adults aged 26.1 ± 4.7 years old with a BMI 31.3 ± 3.2 kg/m2 | 4000 IU of VD per day | Obesity | ↑ 25(OH) D; ↓ PTH; Inverse relationship between 25(OH)D and waist-to-hip ratio. |
| [106] | Overweight or obese premenopausal women aged 20–50 years old | 500–600 mg/day CAL, 800 mg/day CAL, low-fat milk (1.5%), and soy milk fortified CAL 1200 and 1300 mg/day. | Obesity | ↓ weight and BMI in all interventions |
| [107] | Overweight and obese patients | 4000 IU of VD per day with exercise training | Obesity | A significant correlation between percent body fat and CRP, and between serum 25OHD and CRP; A significant reduction in unstimulated TNFα production both groups. |
| [108] | Adults aged 30–50 years old with abdominal obesity | Fortified low-fat yogurt (1500 IU VD3 per 150 g/d); fortified low-fat milk (1500 IU VD3 per 200 g/d) | Obesity | ↑ 25(OH)D serum levels; ↓ weight to hip ratio; ↑ triglyceride and HDL-C ↓ fasting serum insulin ↑ HOMA-IR and quantitative insulin sensitivity |
| [109] | Obese adolescents aged 12–17 years old and with BMI 31.2–36.2 kg/m2 | Fruit juice with VD 4000 IU per day | Obesity | ↑ total and free 25(OH)D; ↓ HOMA-IR and CRP; ↑ endothelium-dependent microvascular. |
| [110] | Overweight, obese patients aged 18–60 years old | 4000 IU cholecalciferol/day | NAFLD and NASH with VD-deficient | No impact between VD and hepatic enzymes |
| [111] | Obese, pre-menopausal adult women | 75,000 IU cholecalciferol | Cardiovascular with VD-deficient | ↑ activity of neutral sphingomyelinases, ↓ chylomicrons, ↓ LDL and VLDL, ↓ postprandial inflammation and endothelial macrophage adhesion |
| [112] | Overweight and obese African Americans aged 57.0 ± 10.4 years old | VD3 (4000 IU/day) | Prediabetes with VD-deficient | ↑ 25OHD level; No impact of VD on post-load glucose or other glycemic measures. |
| [113] | Obese male veterans aged 35–85 years old | 50,000 IU ergocalciferol/week | Prediabetes with 25(OH)D level 5.0–29 ng/mL | ↑ left atrial volume ergocalciferol, No significant difference in blood pressure and other diastolic parameters |
| [114] | Diabetic patients aged 66.3 ± 4.4 years old | 30,000 IU cholecalciferol/week | Type 2 diabetes with VD deficient or insufficient | ↓ hs-CRP and TNF-α concentrations |
| [115] | Male and female 30–60-year-olds with obesity and type 2 diabetes | Stage 1: 6000 IU of VD/day; Stage 2: 3000 IU of VD/day; Stage 3: 2200 IU of VD/day | Obesity and type 2 diabetes | No associations between VD supplementation with weight, fat mass, or waist circumference |
| [116] | Obese males and females aged 18–70 years old | Cholecalciferol 25,000 IU/week | Obesity with VD deficient | ↑ insulin sensitivity |
| [117] | Obese children aged 2–14 years old | 50,000 IU cholecalciferol/week | Obesity with VD deficiency | ↑ VD deficiency status after consuming the dose of cholecalciferol |
| Reference | Experimental Design | Diet Intervention | Comorbidities | Highlight Result |
|---|---|---|---|---|
| Animal study | ||||
| [119] | Obese male C57BL/6J mice aged four weeks old | A high-fat diet, high-CAL, and high-CAL + non-fat dry milk | Obesity | ↓ body weight and adiposity; ↑ adiposity in high-CAL diet; ↑ feed efficiency and hyperphagia develop obesity in high-Ca mice; A strong correlation between mRNA markers of macrophages. |
| Human study | ||||
| [120] | Healthy post-menopausal women aged 60–70 years old | 400 and 1000 IU of VD3 | Obesity | No improvement in physical function (grip strength or falls) |
| [121] | Overweight and obese women aged 38 + 8 years old with a BMI 29.9 + 4.2 kg/m2 | 25 μg of VD3 | Obesity | ↓ fasting blood glucose concentrations; ↑ 25(OH)D concentrations; ↓ percentage of HbA1c; Significant association: HbA1c and 25(OH)D concentrations. |
| [122] | Overweight and obese children aged 6–15 years old | 1200 IU of VD3 | Obesity | ↓ BMI not significant; ↓ fat mass not significant; No impact on body weight reduction |
| [123] | Men and women aged 20–60 years old with a BMI 27–37 kg/m2 | Dairy with 700 mg/day CAL, and 1400 mg/day CAL | Obesity | ↑ weight loss and ↑ plasma levels of peptide tyrosine tyrosine (PYY) in high CAL fortification. |
| [124] | Overweight females with postmenopausal aged around 51.3 years old | Daily 800 mg of CAL citrate and malate + 400 IU of VD | Overweight with postmenopausal | Negligible impact on body composition |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harahap, I.A.; Landrier, J.-F.; Suliburska, J. Interrelationship between Vitamin D and Calcium in Obesity and Its Comorbid Conditions. Nutrients 2022, 14, 3187. https://doi.org/10.3390/nu14153187
Harahap IA, Landrier J-F, Suliburska J. Interrelationship between Vitamin D and Calcium in Obesity and Its Comorbid Conditions. Nutrients. 2022; 14(15):3187. https://doi.org/10.3390/nu14153187
Chicago/Turabian StyleHarahap, Iskandar Azmy, Jean-François Landrier, and Joanna Suliburska. 2022. "Interrelationship between Vitamin D and Calcium in Obesity and Its Comorbid Conditions" Nutrients 14, no. 15: 3187. https://doi.org/10.3390/nu14153187
APA StyleHarahap, I. A., Landrier, J.-F., & Suliburska, J. (2022). Interrelationship between Vitamin D and Calcium in Obesity and Its Comorbid Conditions. Nutrients, 14(15), 3187. https://doi.org/10.3390/nu14153187