Putative Biomarkers of Environmental Enteric Disease Fail to Correlate in a Cross-Sectional Study in Two Study Sites in Sub-Saharan Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setup and Sample Collection
2.2. Measurement of Biomarkers in Feces, Duodenum and Blood
2.2.1. Blood Biomarkers
2.2.2. Duodenal and Fecal Biomarkers
2.2.3. Urine Biomarkers
2.2.4. Cutoffs Used for the Categorization of the Biomarkers
2.3. Statistical Analysis
2.3.1. Correlation of Biomarkers across Body Sites and Between Each Other
2.3.2. Factors Associated with Anemia and Height-for-Age Z-Score
2.3.3. Factors Associated with Putative Biomarkers of EED
3. Results
3.1. Description of Study Population and Biomarkers Measured
3.1.1. Enterocyte Mass and (Intestinal) Inflammation
3.1.2. Gut Leakiness and Bacterial Translocation
3.1.3. General Markers of Undernutrition
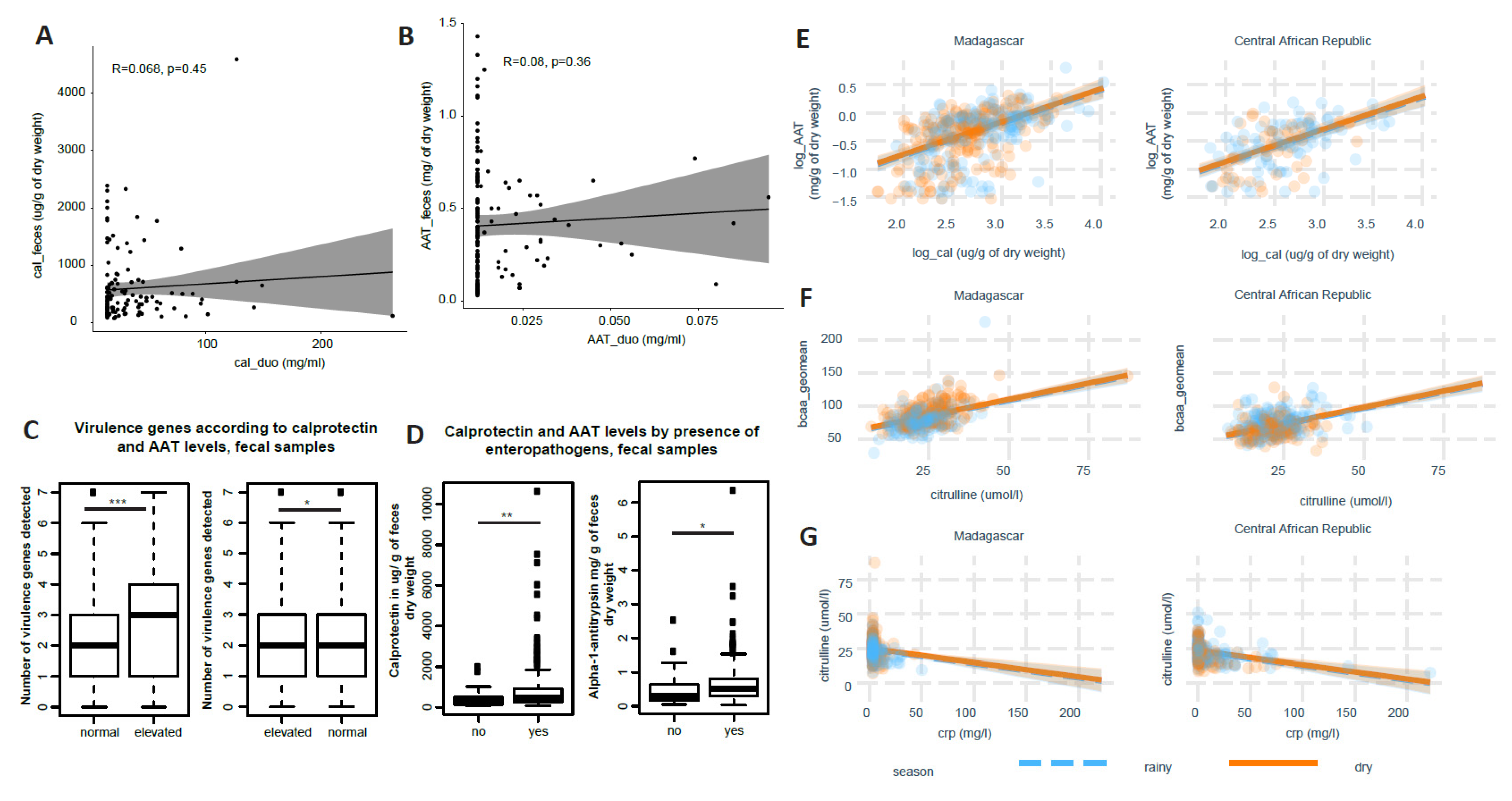
3.2. Little Correlation of Biomarkers across Body Sites and between Each Other
3.2.1. Correlation across Body Sites
3.2.2. Correlation between Different Biomarkers
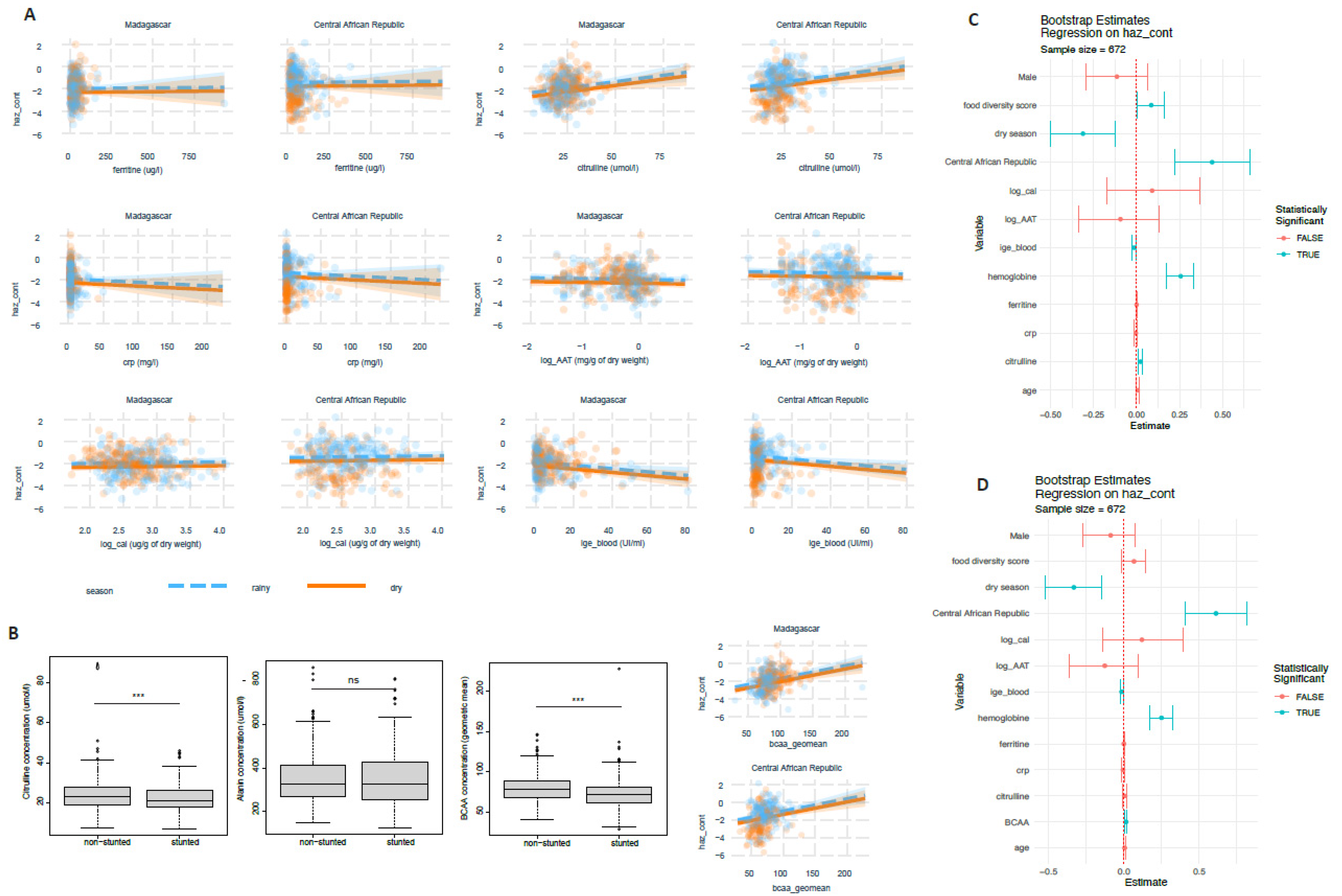
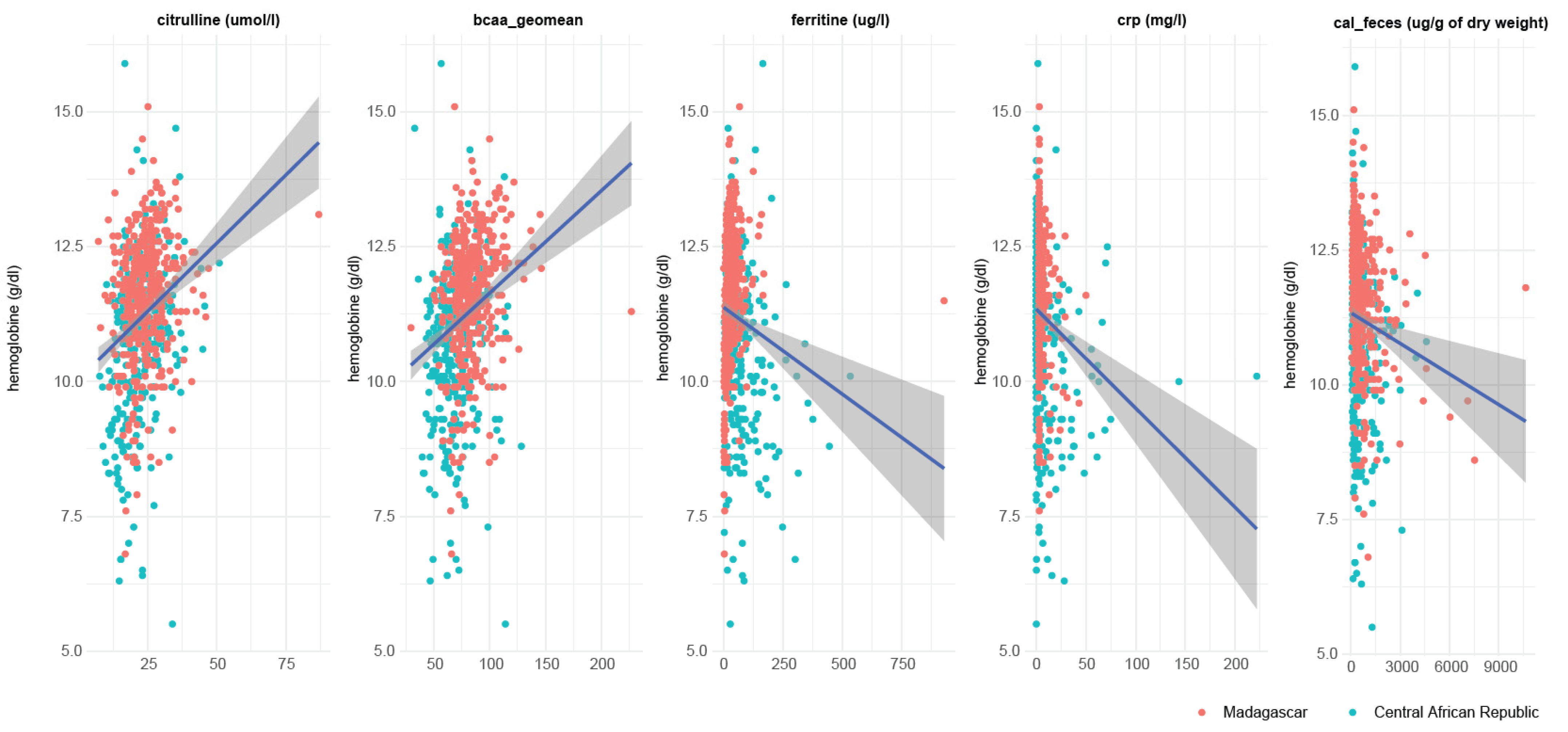
3.3. Blood Amino Acid Levels, Blood IgE and Ferritine Are Associated with Stunted Child Growth
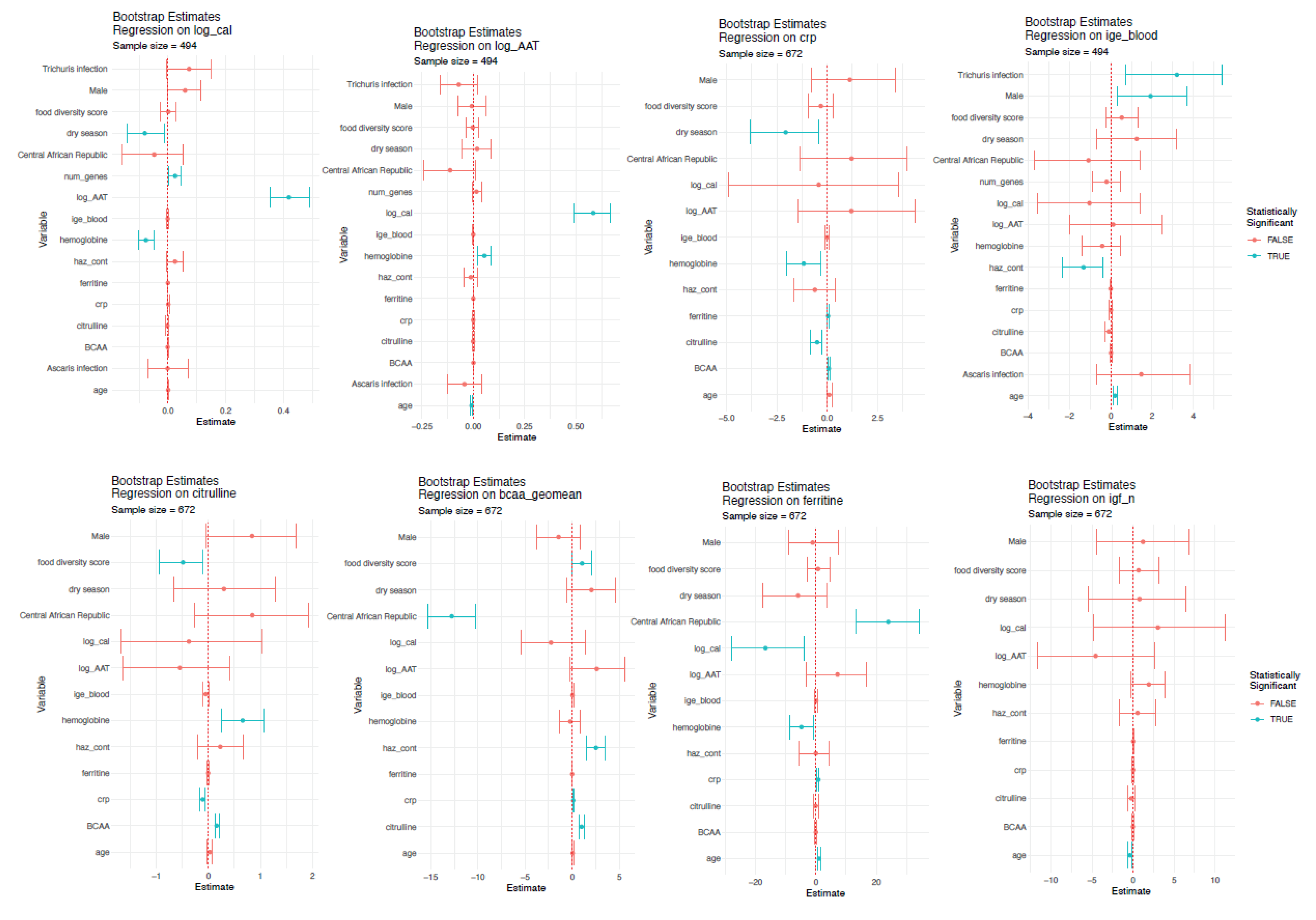
3.4. Factors Associated with Putative Biomarkers of EED
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Watanabe, K.; Petri, W.A. Environmental Enteropathy: Elusive but Significant Subclinical Abnormalities in Developing Countries. EBioMedicine 2016, 10, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Keusch, G.T.; Plaut, A.G.; Troncale, F.J. Subclinical malabsorption in Thailand. II. Intestinal absorption in American military and Peace Corps personnel1. Am. J. Clin. Nutr. 1972, 25, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Lindenbaum, J.; Gerson, C.D.; Kent, T.H. Recovery of small-intestinal structure and function after residence in the tropics. I. Studies in Peace Corps volunteers. Ann. Intern. Med. 1971, 74, 218–222. [Google Scholar] [CrossRef]
- Kosek, M.N.; Ahmed, T.; Bhutta, Z.; Caulfield, L.; Guerrant, R.; Houpt, E.; Kang, G.; Kosek, M.; Lee, G.; Lima, A.; et al. Causal Pathways from Enteropathogens to Environmental Enteropathy: Findings from the MAL-ED Birth Cohort Study. EBioMedicine 2017, 18, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Vonaesch, P.; Morien, E.; Andrianonimiadana, L.; Sanke, H.; Mbecko, J.R.; Huus, K.E.; Naharimanananirina, T.; Gondje, B.P.; Nigatoloum, S.N.; Vondo, S.S.; et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl. Acad. Sci. USA 2018, 115, E8489–E8498. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Y.; Kung, V.L.; Das, S.; Hossain, M.S.; Hibberd, M.C.; Guruge, J.; Mahfuz, M.; Begum, S.K.N.; Rahman, M.M.; Fahim, S.M.; et al. Duodenal Microbiota in Stunted Undernourished Children with Enteropathy. N. Engl. J. Med. 2020, 383, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Arndt, M.B.; Richardson, B.A.; Ahmed, T.; Mahfuz, M.; Haque, R.; John-Stewart, G.C.; Denno, D.M.; Petri, W.A., Jr.; Kosek, M.; Walson, J.L. Fecal Markers of Environmental Enteropathy and Subsequent Growth in Bangladeshi Children. Am. J. Trop. Med. Hyg. 2016, 95, 694–701.e1. [Google Scholar] [CrossRef]
- Yu, J.; Ordiz, M.I.; Stauber, J.; Shaikh, N.; Trehan, I.; Barnell, E.; Head, R.D.; Maleta, K.; Tarr, P.I.; Manary, M.J. Environmental Enteric Dysfunction Includes a Broad Spectrum of Inflammatory Responses and Epithelial Repair Processes. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 158–174.e1. [Google Scholar] [CrossRef]
- Iqbal, N.T.; Sadiq, K.; Syed, S.; Akhund, T.; Umrani, F.; Ahmed, S.; Yakoob, M.Y.; Rahman, N.; Qureshi, S.; Xin, W.; et al. Promising Biomarkers of Environmental Enteric Dysfunction: A Prospective Cohort study in Pakistani Children. Sci. Rep. 2018, 8, 2966. [Google Scholar] [CrossRef]
- Guerrant, R.L.; Leite, A.M.; Pinkerton, R.; Medeiros, P.H.; Cavalcante, P.A.; DeBoer, M.; Kosek, M.; Duggan, C.; Gewirtz, A.; Kagan, J.C.; et al. Biomarkers of Environmental Enteropathy, Inflammation, Stunting, and Impaired Growth in Children in Northeast Brazil. PLoS ONE 2016, 11, e0158772. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, J.; Naylor, C.; Kirkpatrick, B.D.; Haque, R.; Petri, W.A.; Ma, J.Z. Application of penalized linear regression methods to the selection of environmental enteropathy biomarkers. Biomark. Res. 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.A.; McCormick, B.J.; Murray-Kolb, L.E.; Lee, G.O.; Seidman, J.C.; Mahfuz, M.; Ahmed, T.; Guerrant, R.L.; Petri, W.A., Jr.; Rogawski, E.T.; et al. Enteric dysfunction and other factors associated with attained size at 5 years: MAL-ED birth cohort study findings. Am. J. Clin. Nutr. 2019, 110, 131–138. [Google Scholar] [CrossRef]
- Singh, A.; Ghosh, S.; Ward, H.; Manary, M.J.; Rogers, B.L.; Rosenberg, I.H. Biomarkers of environmental enteric dysfunction are differently associated with recovery and growth among children with moderate acute malnutrition in Sierra Leone. Am. J. Clin. Nutr. 2021, 113, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Haberman, Y.; Iqbal, N.T.; Ghandikota, S.; Mallawaarachchi, I.; Braun, T.; Dexheimer, P.J.; Rahman, N.; Hadar, R.; Sadiq, K.; Ahmad, Z.; et al. Mucosal Genomics Implicate Lymphocyte Activation and Lipid Metabolism in Refractory Environmental Enteric Dysfunction. Gastroenterology 2021, 160, 2055–2071.e0. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.M.; Mutasa, M.; Prendergast, A.J.; Humphrey, J.; Manges, A.R. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Negl. Trop. Dis. 2018, 12, e0006205. [Google Scholar] [CrossRef]
- Lauer, J.M.; McDonald, C.M.; Kisenge, R.; Aboud, S.; Fawzi, W.W.; Liu, E.; Tran, H.Q.; Gewirtz, A.T.; Manji, K.P.; Duggan, C.P. Markers of Systemic Inflammation and Environmental Enteric Dysfunction Are Not Reduced by Zinc or Multivitamins in Tanzanian Infants: A Randomized, Placebo-Controlled Trial. J. Pediatrics 2019, 210, 34–40.e1. [Google Scholar] [CrossRef]
- Fahim, S.M.; Das, S.; Sanin, K.I.; Gazi, M.A.; Mahfuz, M.; Islam, M.M.; Ahmed, T. Association of Fecal Markers of Environmental Enteric Dysfunction with Zinc and Iron Status among Children at First Two Years of Life in Bangladesh. Am. J. Trop. Med. Hyg. 2018, 99, 489–494. [Google Scholar] [CrossRef]
- Smith, H.E.; Ryan, K.N.; Stephenson, K.B.; Westcott, C.; Thakwalakwa, C.; Maleta, K.; Cheng, J.Y.; Brenna, J.T.; Shulman, R.J.; Trehan, I.; et al. Multiple micronutrient supplementation transiently ameliorates environmental enteropathy in Malawian children aged 12–35 months in a randomized controlled clinical trial. J. Nutr. 2014, 144, 2059–2065. [Google Scholar] [CrossRef]
- Vonaesch, P.; Randremanana, R.; Gody, J.C.; Collard, J.M.; Giles-Vernick, T.; Doria, M.; Vigan-Womas, I.; Rubbo, P.A.; Etienne, A.; Andriatahirintsoa, E.J.; et al. Identifying the etiology and pathophysiology underlying stunting and environmental enteropathy: Study protocol of the AFRIBIOTA project. BMC Pediatr. 2018, 18, 236. [Google Scholar] [CrossRef] [PubMed]
- Vonaesch, P.; Djorie, S.G.; Kandou, K.J.E.; Rakotondrainipiana, M.; Schaeffer, L.; Andriatsalama, P.V.; Randriamparany, R.; Gondje, B.P.; Nigatoloum, S.; Vondo, S.S.; et al. Factors Associated with Stunted Growth in Children Under Five Years in Antananarivo, Madagascar and Bangui, Central African Republic. Matern. Child Health J. 2021, 25, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Collard, J.M.; Andrianonimiadana, L.; Habib, A.; Rakotondrainipiana, M.; Andriantsalama, P.; Randriamparany, R.; Rabenandrasana, M.A.N.; Weill, F.X.; Sauvonnet, N.; Randremanana, R.V.; et al. High prevalence of small intestine bacteria overgrowth and asymptomatic carriage of enteric pathogens in stunted children in Antananarivo, Madagascar. PLoS Negl. Trop. Dis. 2022, 16, e0009849. [Google Scholar] [CrossRef]
- World Health Organization. WHO Child Growth Standards: Head Circumference-for-Age, Arm Circumference-for-Age, Triceps Skinfold-for-Age and Subscapular Skinfold-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2007.
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef]
- Centers for Disease, C. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb. Mortal. Wkly. Rep. 1989, 38, 400–404. [Google Scholar]
- Sullivan, K.M.; Mei, Z.; Grummer-Strawn, L.; Parvanta, I. Haemoglobin adjustments to define anaemia. Trop. Med. Int. Health 2008, 13, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Dutta, U.; Noor, M.T.; Taneja, N.; Kochhar, R.; Sharma, M.; Singh, K. Endoscopic jejunal biopsy culture: A simple and effective method to study jejunal microflora. Indian J. Gastroenterol. Off. J. Indian Soc. Gastroenterol. 2010, 29, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Huus, K.E.; Rodriguez-Pozo, A.; Kapel, N.; Nestoret, A.; Habib, A.; Dede, M.; Manges, A.; Collard, J.M.; Sansonetti, P.J.; Vonaesch, P.; et al. Immunoglobulin recognition of fecal bacteria in stunted and non-stunted children: Findings from the Afribiota study. Microbiome 2020, 8, 113. [Google Scholar] [CrossRef]
- Habib, A.; Andrianonimiadana, L.; Rakotondrainipiana, M.; Andriantsalama, P.; Randriamparany, R.; Randremanana, R.V.; Rakotoarison, R.; Vigan-Womas, I.; Rafalimanantsoa, A.; Vonaesch, P.; et al. High prevalence of intestinal parasite infestations among stunted and control children aged 2 to 5 years old in two neighborhoods of Antananarivo, Madagascar. PLoS Negl. Trop. Dis. 2021, 15, e0009333. [Google Scholar] [CrossRef]
- Kalach, N.; Kapel, N.; Waligora-Dupriet, A.J.; Castelain, M.C.; Cousin, M.O.; Sauvage, C.; Ba, F.; Nicolis, I.; Campeotto, F.; Butel, M.J.; et al. Intestinal permeability and fecal eosinophil-derived neurotoxin are the best diagnosis tools for digestive non-IgE-mediated cow’s milk allergy in toddlers. Clin. Chem. Lab. Med. 2013, 51, 351–361. [Google Scholar] [CrossRef]
- OMS. Concentrations en Hémoglobine Permettant de Diagnostiquer L’anémie et D’en Évaluer la Sévérité. 2011. Available online: https://apps.who.int/iris/handle/10665/85841 (accessed on 9 May 2019).
- Onis, M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar]
- WHO. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. In Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Lauer, J.M.; Ghosh, S.; Ausman, L.M.; Webb, P.; Bashaasha, B.; Agaba, E.; Turyashemererwa, F.M.; Tran, H.Q.; Gewirtz, A.T.; Erhardt, J.; et al. Markers of Environmental Enteric Dysfunction Are Associated with Poor Growth and Iron Status in Rural Ugandan Infants. J. Nutr. 2020, 150, 2175–2182. [Google Scholar] [CrossRef]
- Ott, C.; Scholmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, Z.; Yalew, A.W.; Bitew, B.D.; Lee, J.; Bisesi, M. Fecal biomarkers of environmental enteric dysfunction and associated factors among children aged 24–59 months in east Dembiya district, northwest Ethiopia. BMC Gastroenterol. 2022, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- McCormick, B.J.; Lee, G.O.; Seidman, J.C.; Haque, R.; Mondal, D.; Quetz, J.; Lima, A.A.; Babji, S.; Kang, G.; Shrestha, S.K.; et al. Dynamics and Trends in Fecal Biomarkers of Gut Function in Children from 1-24 Months in the MAL-ED Study. Am. J. Trop. Med. Hyg. 2017, 96, 465–472. [Google Scholar] [CrossRef]
- Hasan, M.M.; Gazi, M.A.; Das, S.; Fahim, S.M.; Hossaini, F.; Alam, M.A.; Mahfuz, M.; Ahmed, T. Association of lipocalin-2 and low-density lipoprotein receptor-related protein-1 (LRP1) with biomarkers of environmental enteric dysfunction (EED) among under 2 children in Bangladesh: Results from a community-based intervention study. BMJ Paediatr. Open 2021, 5, e001138. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.I.; Hossain, M.; Islam, S.; Akter, A.; Nishat, N.S.; Nila, T.A.; Rafique, T.A.; Leung, D.T.; Calderwood, S.B.; Ryan, E.T.; et al. An assessment of potential biomarkers of environment enteropathy and its association with age and microbial infections among children in Bangladesh. PLoS ONE 2021, 16, e0250446. [Google Scholar] [CrossRef]
- Victora, C.G.; De Onis, M.; Hallal, P.C.; Blössner, M.; Shrimpton, R. Worldwide Timing of Growth Faltering: Revisiting Implications for Interventions. Pediatrics 2010, 125, e473–e480. [Google Scholar] [CrossRef] [PubMed]
- Denno, D.M.; VanBuskirk, K.; Nelson, Z.C.; Musser, C.A.; Hay Burgess, D.C.; Tarr, P.I. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: A systematic review. Clin. Infect. Dis. 2014, 59, S213–S219. [Google Scholar] [CrossRef]
- Agapova, S.; Stephenson, K.; Manary, M.; Weisz, A.; Tarr, P.I.; Mkakosya, R.; Maleta, K.; Shulman, R.J.; Manary, M.; Shaikh, N. Detection of low-concentration host mRNA transcripts in Malawian children at risk for environmental enteropathy. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 66–71. [Google Scholar] [CrossRef]
- Peterson, K.M.; Buss, J.; Easley, R.; Yang, Z.; Korpe, P.S.; Niu, F.; Ma, J.Z.; Olortegui, M.P.; Haque, R.; Kosek, M.N.; et al. REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am. J. Clin. Nutr. 2013, 97, 1129–1133. [Google Scholar] [CrossRef]
- Colston, J.M.; Peñataro Yori, P.; Colantuoni, E.; Moulton, L.H.; Ambikapathi, R.; Lee, G.; Rengifo Trigoso, D.; Siguas Salas, M.; Kosek, M.N. A methodologic framework for modeling and assessing biomarkers of environmental enteropathy as predictors of growth in infants: An example from a Peruvian birth cohort. Am. J. Clin. Nutr. 2017, 106, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; VanBuskirk, K.; Ali, S.A.; Kelly, M.P.; Holtz, L.R.; Yilmaz, O.H.; Sadiq, K.; Iqbal, N.; Amadi, B.; Syed, S.; et al. A novel histological index for evaluation of environmental enteric dysfunction identifies geographic-specific features of enteropathy among children with suboptimal growth. PLoS Negl. Trop. Dis. 2020, 14, e0007975. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.T.; Syed, S.; Sadiq, K.; Khan, M.N.; Iqbal, J.; Ma, J.Z.; Umrani, F.; Ahmed, S.; Maier, E.A.; Denson, L.A.; et al. Study of Environmental Enteropathy and Malnutrition (SEEM) in Pakistan: Protocols for biopsy based biomarker discovery and validation. BMC Pediatrics 2019, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Chama, M.; Amadi, B.C.; Chandwe, K.; Zyambo, K.; Besa, E.; Shaikh, N.; Ndao, I.M.; Tarr, P.I.; Storer, C.; Head, R.; et al. Transcriptomic analysis of enteropathy in Zambian children with severe acute malnutrition. EBioMedicine 2019, 45, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.; Tembo, M.; Kelly, P. Intestinal Biopsies for the Evaluation of Environmental Enteropathy and Environmental Enteric Dysfunction. J. Infect. Dis. 2021, 224, S856–S863. [Google Scholar] [CrossRef] [PubMed]
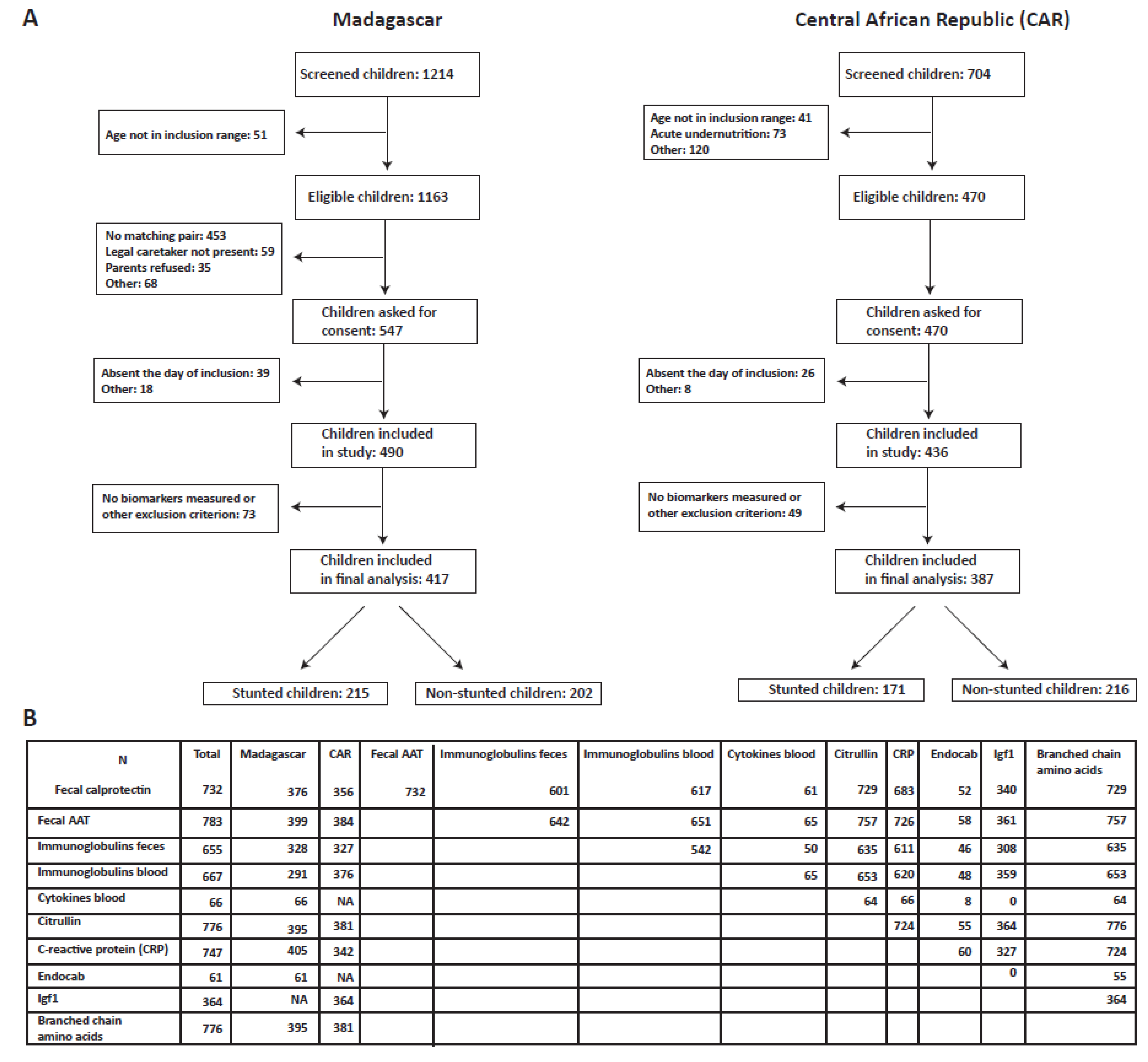
| Antananarivo, Madagascar | Bangui, Central African Republic | |
|---|---|---|
| (n = 417) | (n = 387) | |
| Height-for-age z-score | ||
| Moderately stunted | 109 (26.1%) | 85 (22.0%) |
| Mon-stunted | 215 (51.6%) | 216 (55.8%) |
| Severely stunted | 93 (22.3%) | 86 (22.2%) |
| Mean (SD) | −2.03 (1.11) | −1.83 (1.40) |
| Median [Min, Max] | −1.97 [−5.23, 2.03] | −1.75 [−5.67, 2.17] |
| Age (months) | ||
| Mean (SD) | 42.8 (10.7) | 40.0 (10.2) |
| Median [Min, Max] | 43.2 [24.2, 60.0] | 38.8 [24.2, 60.8] |
| Hemoglobin (g/L) | ||
| Mean (SD) | 11.6 (1.15) | 10.8 (1.42) |
| Median [Min, Max] | 11.7 [6.80, 15.1] | 11.0 [5.50, 15.9] |
| Missing values | 9 (2.2%) | 47 (12.1%) |
| Anemia (Hb < 11 g/L) | ||
| No | 308 (73.9%) | 181 (46.8%) |
| Yes | 100 (24.0%) | 159 (41.1%) |
| Missing values | 9 (2.2%) | 47 (12.1%) |
| Ascaris infestation | ||
| No | 205 (49.2%) | 337 (87.1%) |
| Yes | 209 (50.1%) | 1 (0.3%) |
| Missing values | 3 (0.7%) | 49 (12.7%) |
| Trichuris infestation | ||
| No | 138 (33.1%) | 338 (87.3%) |
| Yes | 276 (66.2%) | 0 (0%) |
| Missing values | 3 (0.7%) | 49 (12.7%) |
| Giardia infestation | ||
| No | 316 (75.8%) | 274 (70.8%) |
| Yes | 98 (23.5%) | 64 (16.5%) |
| Missing values | 3 (0.7%) | 49 (12.7%) |
| Number of virulence genes detected | ||
| Mean (SD) | 2.51 (1.52) | 0.824 (0.947) |
| Median [Min, Max] | 2.00 [0, 7.00] | 1.00 [0, 4.00] |
| Missing values | 3 (0.7%) | 205 (53.0%) |
| Food diversity score | ||
| Mean (SD) | 3.90 (1.11) | 3.63 (1.24) |
| Median [Min, Max] | 4.00 [1.00, 7.00] | 4.00 [1.00, 7.00] |
| Season of inclusion | ||
| Rainy season | 199 (47.7%) | 228 (58.9%) |
| Dry season | 218 (52.3%) | (41.1%) |
| Antananarivo, Madagascar | Bangui, Central African Republic | |||
|---|---|---|---|---|
| Non-Stunted | Stunted | Non-Stunted | Stunted | |
| (n = 215) | (n = 202) | (n = 216) | (n = 171) | |
| Ferritin (ng/mL) | ||||
| Mean (SD) | 35.7 (29.6) | 35.0 (68.5) | 65.5 (74.9) | 67.7 (62.7) |
| Median [Min, Max] | 28.0 [1.88, 170] | 25.4 [1.27, 929] | 40.0 [3.00, 534] | 52.0 [4.00, 343] |
| Missing values | 4 (1.9%) | 8 (4.0%) | 17 (7.9%) | 22 (12.9%) |
| Ferritin level | ||||
| Missing values | 4 (1.9%) | 8 (4.0%) | 17 (7.9%) | 22 (12.9%) |
| No | 175 (81.4%) | 158 (78.2%) | 189 (87.5%) | 138 (80.7%) |
| Yes | 36 (16.7%) | 36 (17.8%) | 10 (4.6%) | 11 (6.4%) |
| Citrulline (umol/l) | ||||
| Mean (SD) | 25.1 (7.52) | 23.2 (6.79) | 22.5 (6.52) | 21.0 (6.61) |
| Median [Min, Max] | 24.4 [10.5, 87.0] | 22.1 [7.00, 46.0] | 21.9 [7.50, 50.9] | 20.8 [7.55, 44.9] |
| Missing | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Citrulline level | ||||
| Missing | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Normal | 200 (93.0%) | 182 (90.1%) | 200 (92.6%) | 152 (88.9%) |
| Too low | 8 (3.7%) | 5 (2.5%) | 14 (6.5%) | 15 (8.8%) |
| Valin (umol/L) | ||||
| Mean (SD) | 173 (30.6) | 160 (32.8) | 134 (27.8) | 118 (29.1) |
| Median [Min, Max] | 168 [102, 267] | 157 [62.9, 360] | 132 [59.9, 223] | 116 [52.8, 215] |
| Missing values | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Valin level | ||||
| Low | 32 (14.9%) | 57 (28.2%) | 124 (57.4%) | 129 (75.4%) |
| Normal | 176 (81.9%) | 129 (63.9%) | 90 (41.7%) | 38 (22.2%) |
| Elevated | 0 (0%) | 1 (0.5%) | 0 (0%) | 0 (0%) |
| Missing values | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Alanine (umol/L) | ||||
| Mean (SD) | 361 (116) | 354 (131) | 337 (102) | 340 (132) |
| Median [Min, Max] | 348 [174, 862] | 328 [130, 807] | 316 [146, 659] | 327 [124, 811] |
| Missing | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Alanine levels | ||||
| Low | 0 (0%) | 3 (1.5%) | 0 (0%) | 5 (2.9%) |
| Normal | 166 (77.2%) | 144 (71.3%) | 170 (78.7%) | 131 (76.6%) |
| Elevated | 42 (19.5%) | 40 (19.8%) | 44 (20.4%) | 31 (18.1%) |
| Missing values | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Isoleucine (umol/L) | ||||
| Mean (SD) | 47.4 (10.2) | 43.9 (12.5) | 40.2 (10.1) | 36.6 (10.0) |
| Median [Min, Max] | 46.0 [28.9, 87.0] | 42.1 [14.9, 161] | 39.0 [18.9, 81.0] | 35.6 [14.0, 74.0] |
| Missing values | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Isoleucine levels | ||||
| Low | 24 (11.2%) | 42 (20.8%) | 80 (37.0%) | 94 (55.0%) |
| Normal | 182 (84.7%) | 144 (71.3%) | 134 (62.0%) | 73 (42.7%) |
| Elevated | 2 (0.9%) | 1 (0.5%) | 0 (0%) | 0 (0%) |
| Missing values | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Leucine (umol/L) | ||||
| Mean (SD) | 82.1 (16.5) | 74.3 (17.1) | 71.2 (15.8) | 63.1 (17.1) |
| Median [Min, Max] | 79.4 [49.7, 139] | 72.9 [26.5, 203] | 69.9 [36.6, 124] | 60.5 [31.6, 137] |
| Missing values | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| Leucine levels | ||||
| Low | 34 (15.8%) | 60 (29.7%) | 93 (43.1%) | 113 (66.1%) |
| Normal | 174 (80.9%) | 126 (62.4%) | 121 (56.0%) | 54 (31.6%) |
| Elevated | 0 (0%) | 1 (0.5%) | 0 (0%) | 0 (0%) |
| Missing values | 7 (3.3%) | 15 (7.4%) | 2 (0.9%) | 4 (2.3%) |
| AAT in feces (mg/g) | ||||
| Mean (SD) | 0.571 (0.603) | 0.611 (0.491) | 0.402 (0.335) | 0.389 (0.298) |
| Median [Min, Max] | 0.480 [0.0300, 6.36] | 0.550 [0.0300, 3.52] | 0.340 [0.0100, 1.80] | 0.315 [0.0300, 1.48] |
| Missing values | 11 (5.1%) | 7 (3.5%) | 2 (0.9%) | 1 (0.6%) |
| AAT levels in feces | ||||
| Elevated | 3 (1.4%) | 2 (1.0%) | 0 (0%) | 0 (0%) |
| Grey zone | 9 (4.2%) | 11 (5.4%) | 5 (2.3%) | 3 (1.8%) |
| Missing | 9 (4.2%) | 4 (2.0%) | 2 (0.9%) | 1 (0.6%) |
| Normal | 194 (90.2%) | 185 (91.6%) | 209 (96.8%) | 167 (97.7%) |
| Calprotectin in feces (ug/g) | ||||
| Mean (SD) | 651 (700) | 888 (1280) | 588 (695) | 500 (580) |
| Median [Min, Max] | 404 [55.0, 4530] | 515 [54.0, 10600] | 303 [56.0, 4050] | 309 [72.0, 4600] |
| Missing values | 19 (8.8%) | 22 (10.9%) | 17 (7.9%) | 14 (8.2%) |
| Calprotectin levels in feces | ||||
| Elevated | 66 (30.7%) | 72 (35.6%) | 56 (25.9%) | 28 (16.4%) |
| Normal | 130 (60.5%) | 107 (53.0%) | 142 (65.7%) | 128 (74.9%) |
| Missing values | 19 (8.8%) | 23 (11.4%) | 18 (8.3%) | 15 (8.8%) |
| CRP (mg/L) | ||||
| Mean (SD) | 4.54 (4.38) | 5.16 (5.97) | 6.76 (20.0) | 9.36 (18.5) |
| Median [Min, Max] | 3.00 [3.00, 31.0] | 3.00 [3.00, 50.0] | 0.440 [0.0100, 222] | 1.11 [0.0100, 144] |
| Missing values | 4 (1.9%) | 8 (4.0%) | 22 (10.2%) | 23 (13.5%) |
| CRP level | ||||
| Elevated | 14 (6.5%) | 20 (9.9%) | 30 (13.9%) | 38 (22.2%) |
| Normal | 197 (91.6%) | 174 (86.1%) | 164 (75.9%) | 110 (64.3%) |
| Missing values | 4 (1.9%) | 8 (4.0%) | 22 (10.2%) | 23 (13.5%) |
| Insulin-like growth factor 1 (Igf1) (ng/mL) * | ||||
| Mean (SD) | 56.5 (24.7) | 54.2 (19.8) | ||
| Median [Min, Max] | 56.0 [1.00, 101] | 50.5 [8.00, 98.0] | ||
| Missing values | 10 (4.6%) | 13 (7.6%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vonaesch, P.; Winkel, M.; Kapel, N.; Nestoret, A.; Barbot-Trystram, L.; Pontoizeau, C.; Barouki, R.; Rakotondrainipiana, M.; Kandou, K.; Andriamanantena, Z.; et al. Putative Biomarkers of Environmental Enteric Disease Fail to Correlate in a Cross-Sectional Study in Two Study Sites in Sub-Saharan Africa. Nutrients 2022, 14, 3312. https://doi.org/10.3390/nu14163312
Vonaesch P, Winkel M, Kapel N, Nestoret A, Barbot-Trystram L, Pontoizeau C, Barouki R, Rakotondrainipiana M, Kandou K, Andriamanantena Z, et al. Putative Biomarkers of Environmental Enteric Disease Fail to Correlate in a Cross-Sectional Study in Two Study Sites in Sub-Saharan Africa. Nutrients. 2022; 14(16):3312. https://doi.org/10.3390/nu14163312
Chicago/Turabian StyleVonaesch, Pascale, Munir Winkel, Nathalie Kapel, Alison Nestoret, Laurence Barbot-Trystram, Clément Pontoizeau, Robert Barouki, Maheninasy Rakotondrainipiana, Kaleb Kandou, Zo Andriamanantena, and et al. 2022. "Putative Biomarkers of Environmental Enteric Disease Fail to Correlate in a Cross-Sectional Study in Two Study Sites in Sub-Saharan Africa" Nutrients 14, no. 16: 3312. https://doi.org/10.3390/nu14163312
APA StyleVonaesch, P., Winkel, M., Kapel, N., Nestoret, A., Barbot-Trystram, L., Pontoizeau, C., Barouki, R., Rakotondrainipiana, M., Kandou, K., Andriamanantena, Z., Andrianonimiadana, L., Habib, A., Rodriguez-Pozo, A., Hasan, M., Vigan-Womas, I., Collard, J.-M., Gody, J.-C., Djorie, S., Sansonetti, P. J., ... on behalf of the Afribiota Investigators. (2022). Putative Biomarkers of Environmental Enteric Disease Fail to Correlate in a Cross-Sectional Study in Two Study Sites in Sub-Saharan Africa. Nutrients, 14(16), 3312. https://doi.org/10.3390/nu14163312






