Impaired Height Growth Associated with Vitamin D Deficiency in Young Children from the Japan Environment and Children’s Study
Abstract
:1. Introduction
2. Materials and Methods
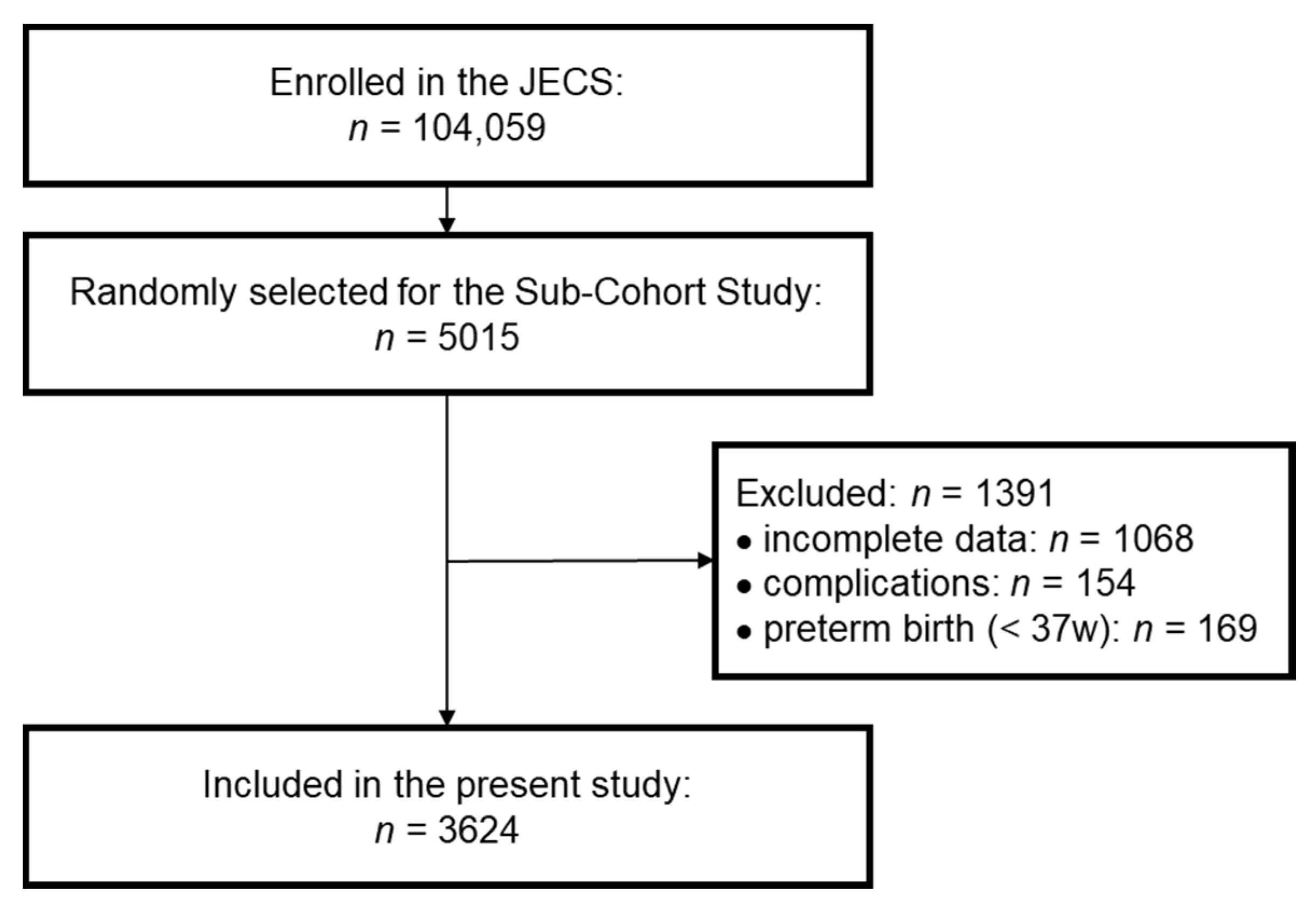
2.1. Study Participants
2.2. Data Collection
2.3. Calculation of Standard Scores and Height Growth
2.4. Statistical Analysis
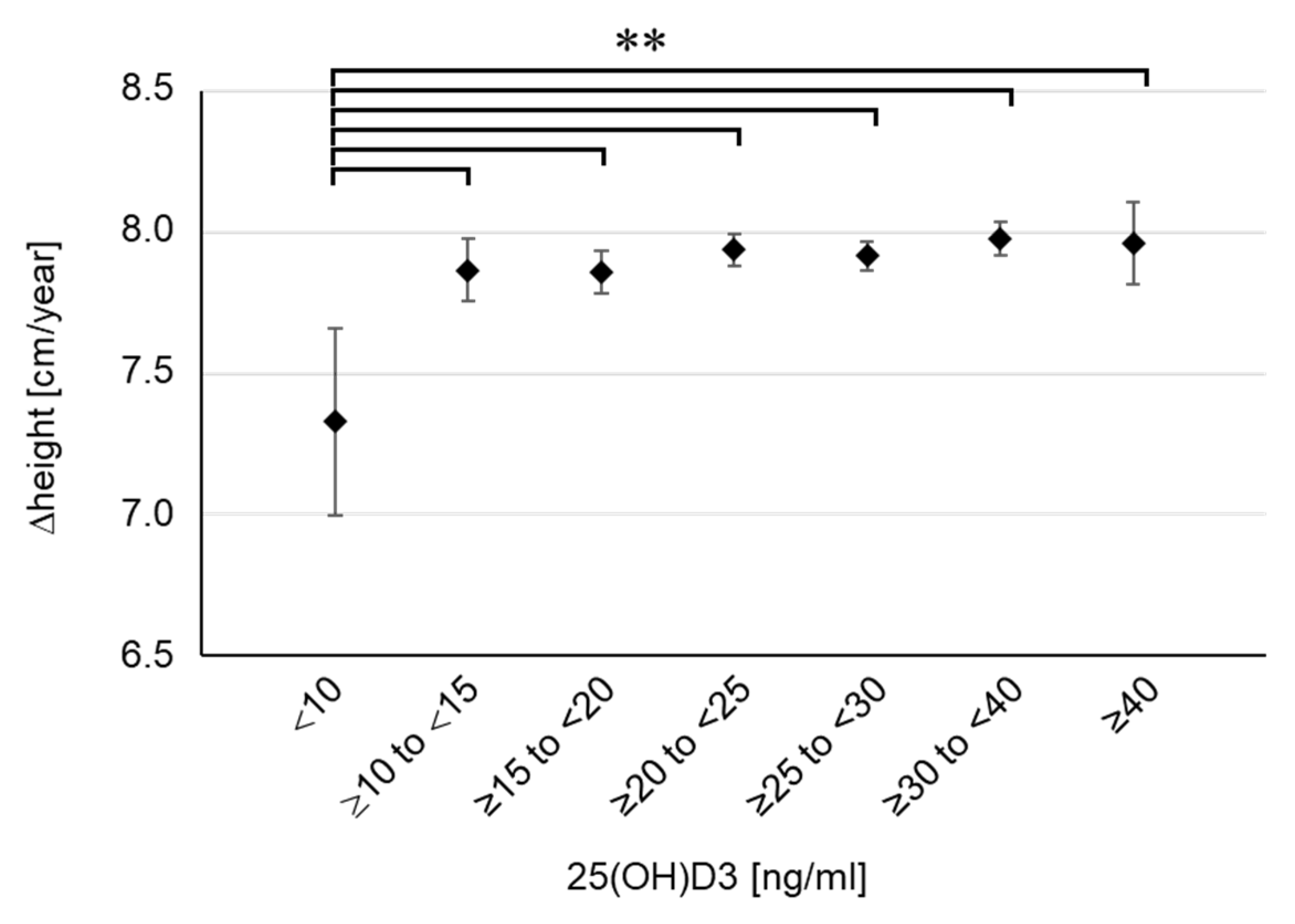
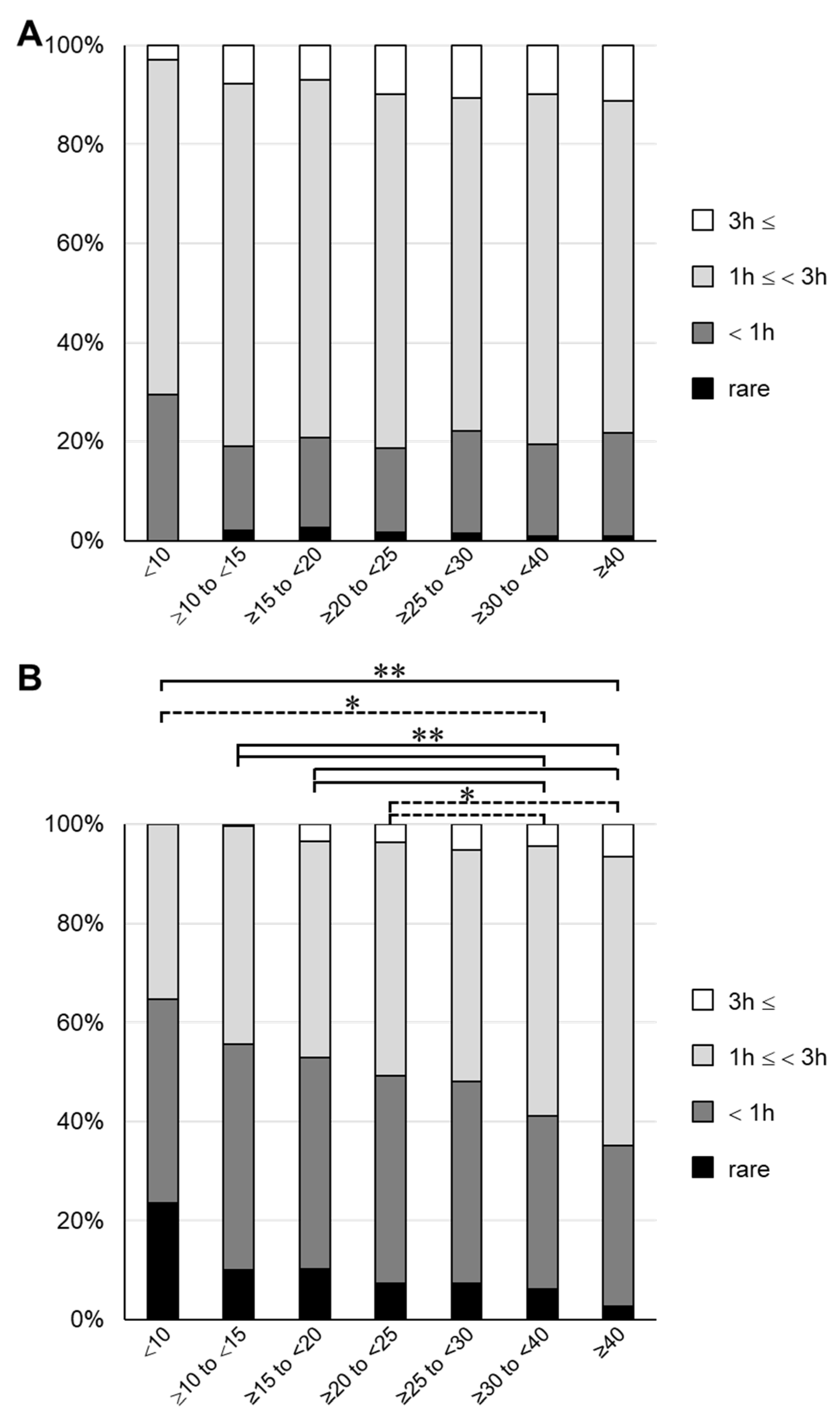
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Antonucci, R.; Locci, C.; Clemente, M.G.; Chicconi, E.; Antonucci, L. Vitamin D Deficiency in Childhood: Old Lessons and Current Challenges. J. Pediatr. Endocrinol. Metab. 2018, 31, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Jones, G. Pharmacokinetics of Vitamin D Toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D Effects on Musculoskeletal Health, Immunity, Autoimmunity, Cardiovascular Disease, Cancer, Fertility, Pregnancy, Dementia and Mortality—A Review of Recent Evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Koo, W.; Walyat, N. Vitamin D and Skeletal Growth and Development. Curr. Osteoporos. Rep. 2013, 11, 188–193. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, H.-C. Vitamin D and Health—The Missing Vitamin in Humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Rabufetti, A.; Milani, G.P.; Lava, S.A.G.; Edefonti, V.; Bianchetti, M.G.; Stettbacher, A.; Muggli, F.; Simonetti, G. Vitamin D Status Among Male Late Adolescents Living in Southern Switzerland: Role of Body Composition and Lifestyle. Nutrients 2019, 11, 2727. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Dietary Reference Intakes for Calcium and Vitamin D. Pediatrics 2012, 130, e1424. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D Supplementation Guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Juonala, M.; Voipio, A.; Pahkala, K.; Viikari, J.S.A.; Mikkilä, V.; Kähönen, M.; Hutri-Kähönen, N.; Jula, A.; Burgner, D.; Sabin, M.A.; et al. Childhood 25-OH Vitamin D Levels and Carotid Intima-Media Thickness in Adulthood: The Cardiovascular Risk in Young Finns Study. J. Clin. Endocrinol. Metab. 2015, 100, 1469–1476. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Brett, N.; Lavery, P.; Agellon, S.; Vanstone, C.; Goruk, S.; Field, C.; Weiler, H. Vitamin D Status and Immune Health Outcomes in a Cross-Sectional Study and a Randomized Trial of Healthy Young Children. Nutrients 2018, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Sofianopoulou, E.; Kaptoge, S.K.; Afzal, S.; Jiang, T.; Gill, D.; Gundersen, T.E.; Bolton, T.R.; Allara, E.; Arnold, M.G.; Mason, A.M.; et al. Estimating Dose-Response Relationships for Vitamin D with Coronary Heart Disease, Stroke, and All-Cause Mortality: Observational and Mendelian Randomisation Analyses. Lancet Diabetes Endocrinol. 2021, 9, 837–846. [Google Scholar] [CrossRef]
- Pham, H.; Waterhouse, M.; Baxter, C.; Duarte Romero, B.; McLeod, D.S.A.; Armstrong, B.K.; Ebeling, P.R.; English, D.R.; Hartel, G.; Kimlin, M.G.; et al. The Effect of Vitamin D Supplementation on Acute Respiratory Tract Infection in Older Australian Adults: An Analysis of Data from the D-Health Trial. Lancet Diabetes Endocrinol. 2021, 9, 69–81. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Jorde, R.; Dieffenbach, A.K.; Lu, C.; Alves, A.C.; Heerspink, H.J.L.; Tikkanen, E.; Eriksson, J.; et al. Association of Vitamin D Status with Arterial Blood Pressure and Hypertension Risk: A Mendelian Randomisation Study. Lancet Diabetes Endocrinol. 2014, 2, 719–729. [Google Scholar] [CrossRef]
- Afzal, S.; Brøndum-Jacobsen, P.; Bojesen, S.E.; Nordestgaard, B.G. Vitamin D Concentration, Obesity, and Risk of Diabetes: A Mendelian Randomisation Study. Lancet Diabetes Endocrinol. 2014, 2, 298–306. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D Status and Ill Health: A Systematic Review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Xu, B.; Feng, Y.; Gan, L.; Zhang, Y.; Jiang, W.; Feng, J.; Yu, L. Vitamin D Status in Children With Short Stature: Accurate Determination of Serum Vitamin D Components Using High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Front. Endocrinol. 2021, 12, 707283. [Google Scholar] [CrossRef]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and Study Design of the Japan Environment and Children’s Study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef]
- Sekiyama, M.; Yamazaki, S.; Michikawa, T.; Nakayama, S.F.; Nitta, H.; Taniguchi, Y.; Suda, E.; Isobe, T.; Kobayashi, Y.; Iwai-Shimada, M.; et al. Study Design and Participants’ Profile in the Sub-Cohort Study in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2022, 32, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Hanada, K.; Futamura, M.; Kitazawa, H.; Ohya, Y.; Kobayashi, F.; Kusuda, T.; Sanefuji, M.; Oda, M.; Mitsubuchi, H.; Shibata, E.; et al. Relieving Pain and Distress during Venipuncture: Pilot Study of the Japan Environment and Children’s Study (JECS). Pediatr. Int. 2015, 57, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Ayabe, T.; Yamamoto-Hanada, K.; Mezawa, H.; Konishi, M.; Ishitsuka, K.; Saito, M.; Fukami, M.; Michikawa, T.; Yamazaki, S.; Senju, A.; et al. Regional Differences in Infant 25-Hydroxyvitamin D: Pilot Study of the Japan Environment and Children’s Study. Pediatr. Int. 2018, 60, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.P.; Simonetti, G.D.; Edefonti, V.; Lava, S.A.G.; Agostoni, C.; Curti, M.; Stettbacher, A.; Bianchetti, M.G.; Muggli, F. Seasonal Variability of the Vitamin D Effect on Physical Fitness in Adolescents. Sci. Rep. 2021, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sato, M.; Saito-Abe, M.; Irahara, M.; Nishizato, M.; Sasaki, H.; Konishi, M.; Ishitsuka, K.; Mezawa, H.; Yamamoto-Hanada, K.; et al. 25-Hydroxyvitamin D Levels among 2-Year-Old Children: Findings from the Japan Environment and Children’s Study (JECS). BMC Pediatr. 2021, 21, 539. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. Vitamin D Deficiency in Children and Its Management: Review of Current Knowledge and Recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef]
- Narumi, S.; Ohnuma, T.; Takehara, K.; Morisaki, N.; Urayama, K.Y.; Hattori, T. Evaluating the Seasonality of Growth in Infants Using a Mobile Phone Application. npj Digit. Med. 2020, 3, 138. [Google Scholar] [CrossRef]

| Subgroup by Serum 25(OH)D3 Concentrations | |||||||
|---|---|---|---|---|---|---|---|
| 25(OH)D3 [ng/mL] | <10 | ≥10 to <15 | ≥15 to <20 | ≥20 to <25 | ≥25 to <30 | ≥30 to <40 | ≥40 |
| ··· | (n = 40) | (n = 254) | (n = 524) | (n = 913) | (n = 925) | (n = 846) | (n = 122) |
| Sex | ··· | ||||||
| Male (%) | 20 (50.0) | 122 (48.0) | 243 (46.4) | 425 (46.5) | 488 (52.8) | 458 (54.1) | 72 (59.0) |
| Female (%) | 20 (50.0) | 132 (52.0) | 281 (53.6) | 488 (53.5) | 437 (47.2) | 388 (45.9) | 50 (41.0) |
| Birth information | |||||||
| pregnancy period [weeks] | 39.7 | 39.4 | 39.5 | 39.5 | 39.5 | 39.6 | 39.5 |
| birth weight [g] | 3093 | 3066 | 3095 | 3073 | 3073 | 3089 | 3068 |
| (95% CI) | (2991, 3195) | (3019, 3113) | (3062, 3129) | (3049, 3098) | (3048, 3097) | (3064, 3113) | (2998, 3139) |
| birth height [cm] | 49.26 | 49.17 | 49.26 | 49.11 | 49.10 | 49.26 | 49.10 |
| (95% CI) | (48.73, 49.79) | (48.92, 49.42) | (49.10, 49.43) | (48.99, 49.24) | (48.98, 49.22) | (49.12, 49.39) | (48.74, 49.46) |
| At 2 years of age | |||||||
| height [cm] | 83.49 | 83.78 | 83.89 | 83.96 | 83.86 | 84.07 | 84.34 |
| (95% CI) | (82.28, 84.70) | (83.40, 84.16) | (83.65, 84.13) | (83.78, 84.14) | (83.67, 84.06) | (83.88, 84.27) | (83.83, 84.85) |
| height SDS | −0.55 | −0.33 | −0.31 | −0.31 | −0.37 | −0.30 | −0.25 |
| (95% CI) | (−0.92, −0.18) | (−0.46, −0.20) | (−0.39, −0.23) | (−0.37, −0.24) | (−0.43, −0.31) | (−0.37, −0.24) | (−0.43, −0.07) |
| weight [kg] | 11.47 | 11.48 | 11.5 | 11.46 | 11.49 | 11.65 | 11.57 |
| (95% CI) | (11.00, 11.93) | (11.33, 11.62) | (11.40, 11.60) | (11.39, 11.54) | (11.41, 11.57) | (11.40, 11.90) | (11.36, 11.78) |
| BMI | 16.4 | 16.32 | 16.32 | 16.24 | 16.3 | 16.46 | 16.24 |
| (95% CI) | (16.00, 16.81) | (16.19, 16.46) | (16.22, 16.42) | (16.17, 16.32) | (16.23, 16.37) | (16.11, 16.81) | (16.03, 16.45) |
| At 4 years of age | |||||||
| height [cm] | 98.5 | 99.62 | 99.75 | 99.87 | 99.77 | 100.09 | 100.26 |
| (95% CI) | (96.99, 100.00) | (99.14, 100.11) | (99.42, 100.08) | (99.63, 100.10) | (99.52, 100.01) | (99.85, 100.34) | (99.59, 100.93) |
| height SDS | −0.5 | −0.07 | −0.05 | −0.01 | −0.06 | 0.02 | 0.06 |
| (95% CI) | (−0.87, −0.12) | (−0.19, −0.06) | (−0.14, 0.03) | (−0.07, 0.05) | (−0.12, 0.00) | (−0.04, 0.09) | (−0.11, 0.24) |
| weight [kg] | 15.22 | 15.44 | 15.53 | 15.57 | 15.45 | 15.5 | 15.51 |
| (95% CI) | (14.55, 15.89) | (15.23, 15.65) | (15.38, 15.69) | (15.45, 15.69) | (15.34, 15.56) | (15.39, 15.61) | (15.19, 15.83) |
| BMI | 15.62 | 15.53 | 15.58 | 15.58 | 15.49 | 15.45 | 15.4 |
| (95% CI) | (15.28, 15.96) | (15.40, 15.66) | (15.48, 15.68) | (15.50, 15.66) | (15.42, 15.56) | (15.38, 15.52) | (15.18, 15.62) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuraoka, S.; Oda, M.; Mitsubuchi, H.; Nakamura, K.; Katoh, T.; Japan Environment and Children’s Study (JECS) Group. Impaired Height Growth Associated with Vitamin D Deficiency in Young Children from the Japan Environment and Children’s Study. Nutrients 2022, 14, 3325. https://doi.org/10.3390/nu14163325
Kuraoka S, Oda M, Mitsubuchi H, Nakamura K, Katoh T, Japan Environment and Children’s Study (JECS) Group. Impaired Height Growth Associated with Vitamin D Deficiency in Young Children from the Japan Environment and Children’s Study. Nutrients. 2022; 14(16):3325. https://doi.org/10.3390/nu14163325
Chicago/Turabian StyleKuraoka, Shohei, Masako Oda, Hiroshi Mitsubuchi, Kimitoshi Nakamura, Takahiko Katoh, and Japan Environment and Children’s Study (JECS) Group. 2022. "Impaired Height Growth Associated with Vitamin D Deficiency in Young Children from the Japan Environment and Children’s Study" Nutrients 14, no. 16: 3325. https://doi.org/10.3390/nu14163325
APA StyleKuraoka, S., Oda, M., Mitsubuchi, H., Nakamura, K., Katoh, T., & Japan Environment and Children’s Study (JECS) Group. (2022). Impaired Height Growth Associated with Vitamin D Deficiency in Young Children from the Japan Environment and Children’s Study. Nutrients, 14(16), 3325. https://doi.org/10.3390/nu14163325






