Different Effects of Low Selenite and Selenium-Nanoparticle Supplementation on Adipose Tissue Function and Insulin Secretion in Adolescent Male Rats
Abstract
:1. Introduction
2. Materials and Methods
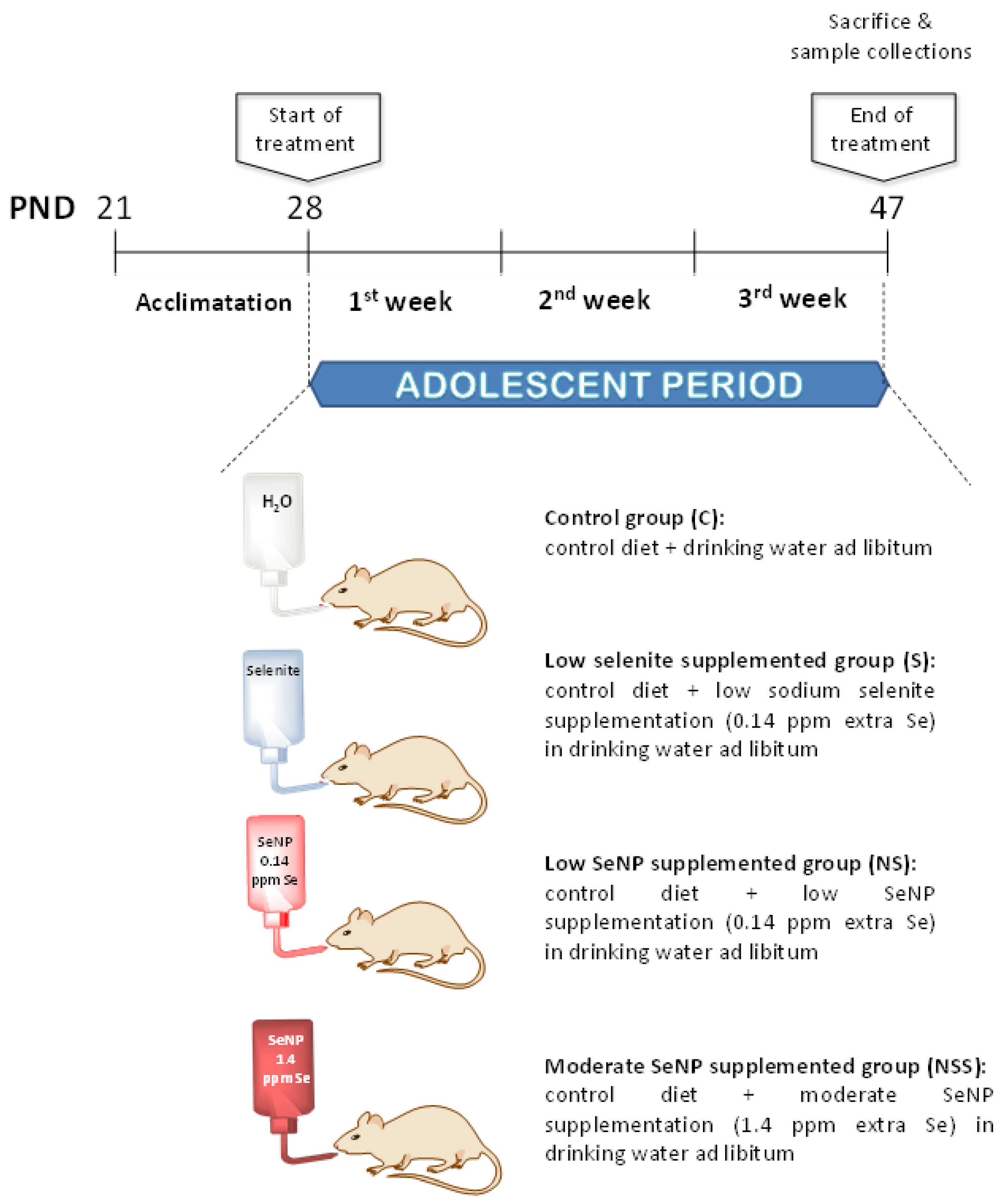
2.1. Animals
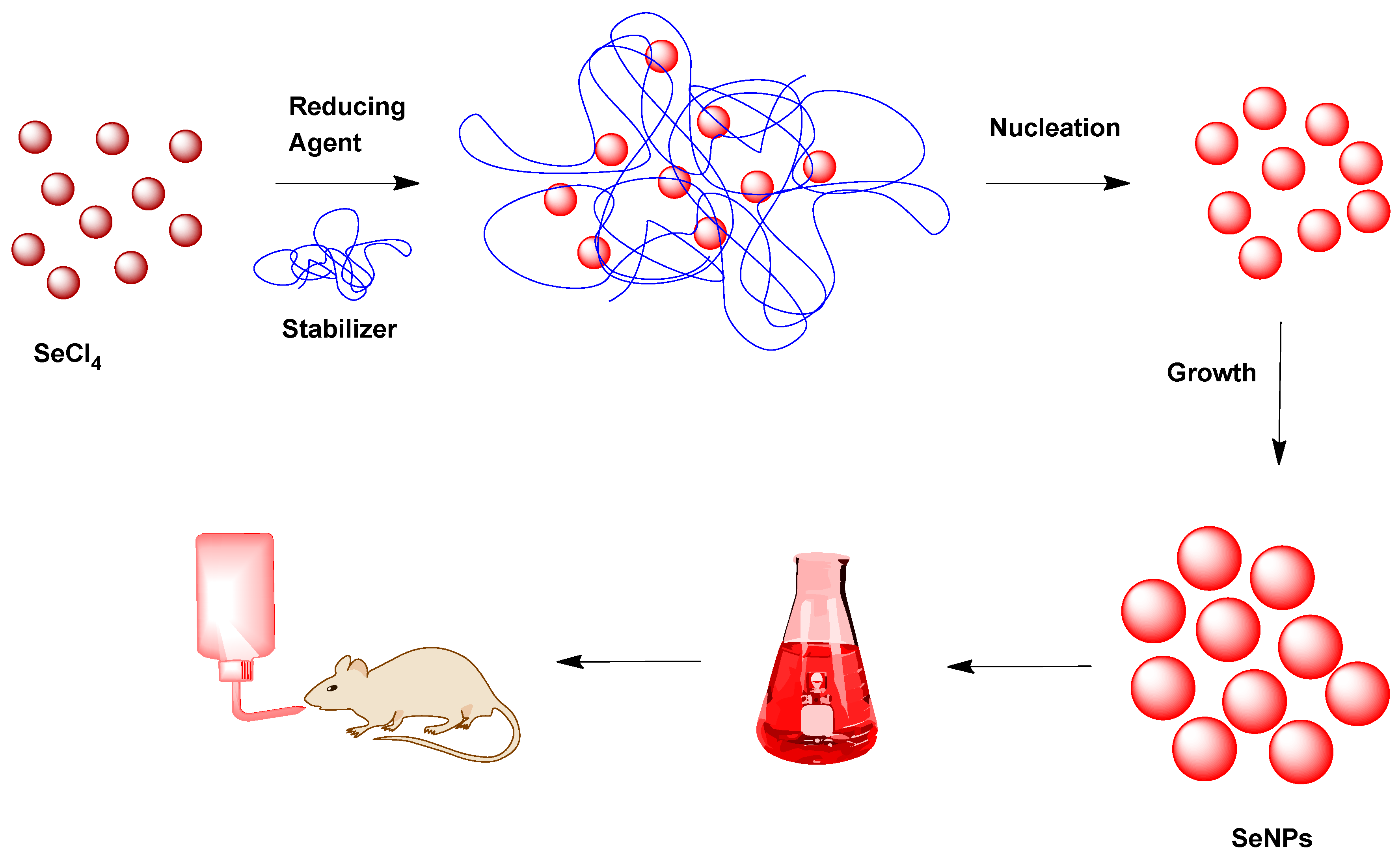
2.2. SeNP Development
2.3. Nutritional Control
2.4. Samples and Anthropometric Measurements
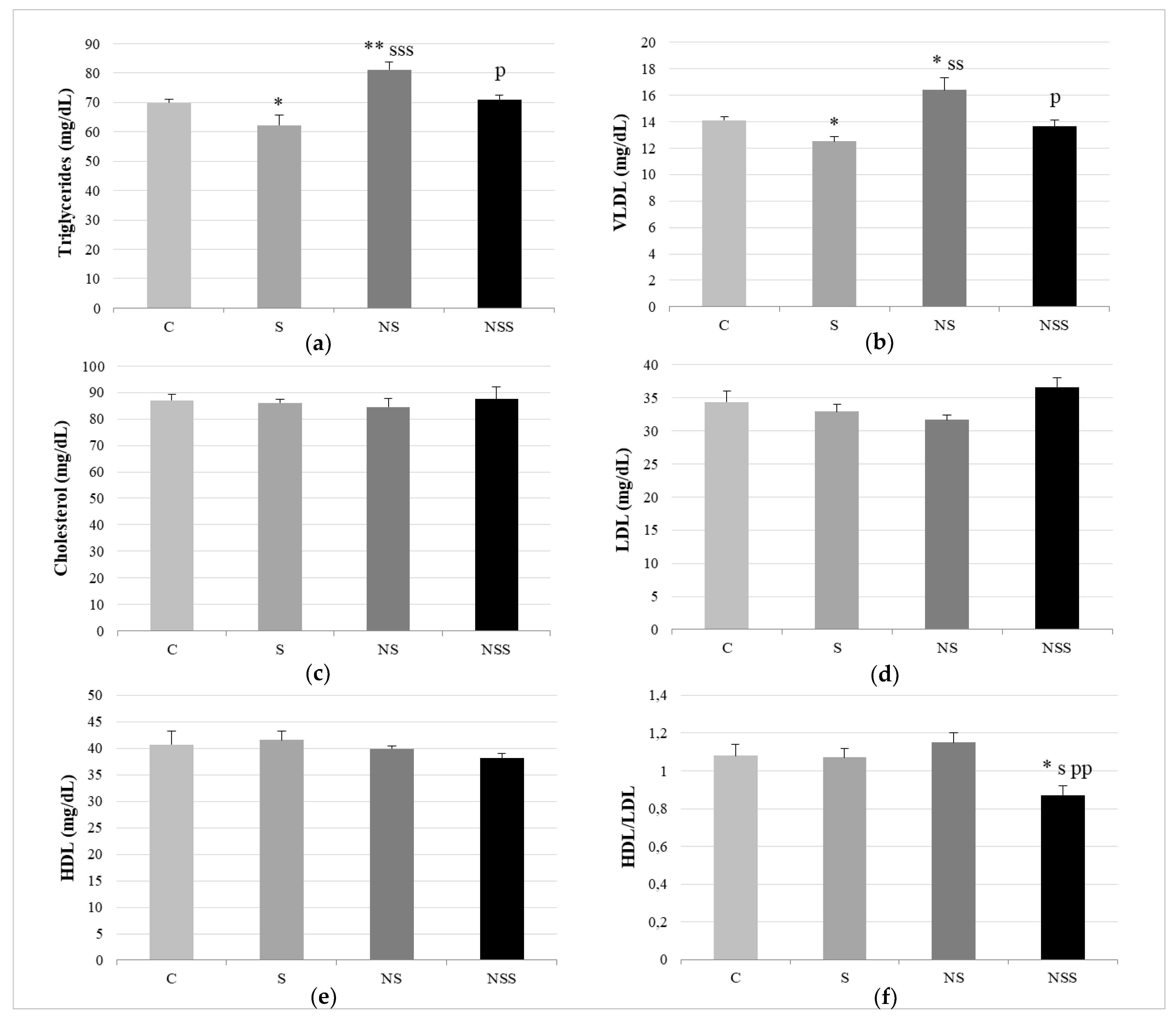
2.5. Biochemical Measurements in Serum
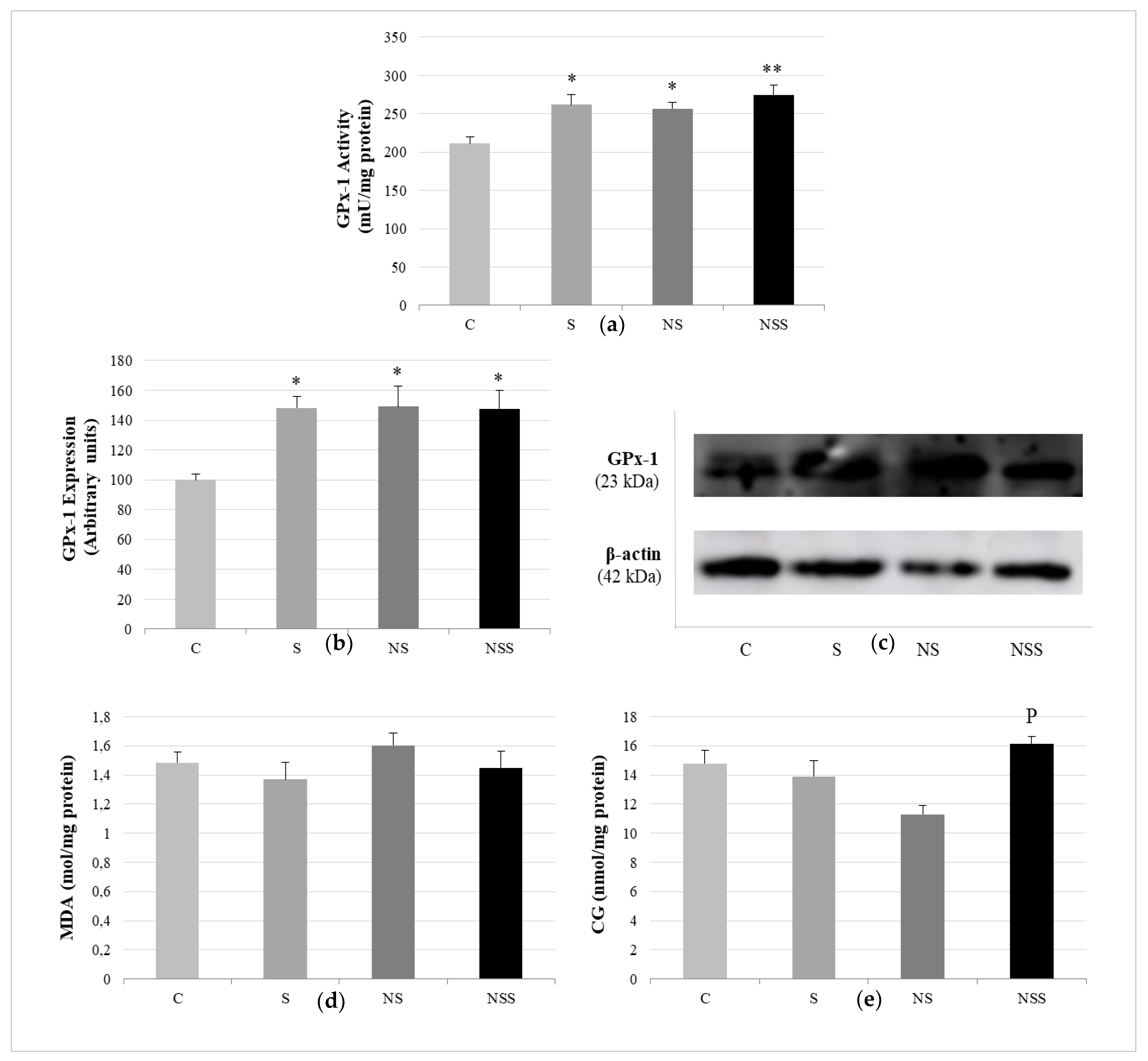
2.6. Antioxidant GPx Activity and Oxidative Stress Markers in WAT
2.7. Immunoblotting Assays
2.8. Adipocyte Size
2.9. Adipokines
2.10. Statistical Analysis
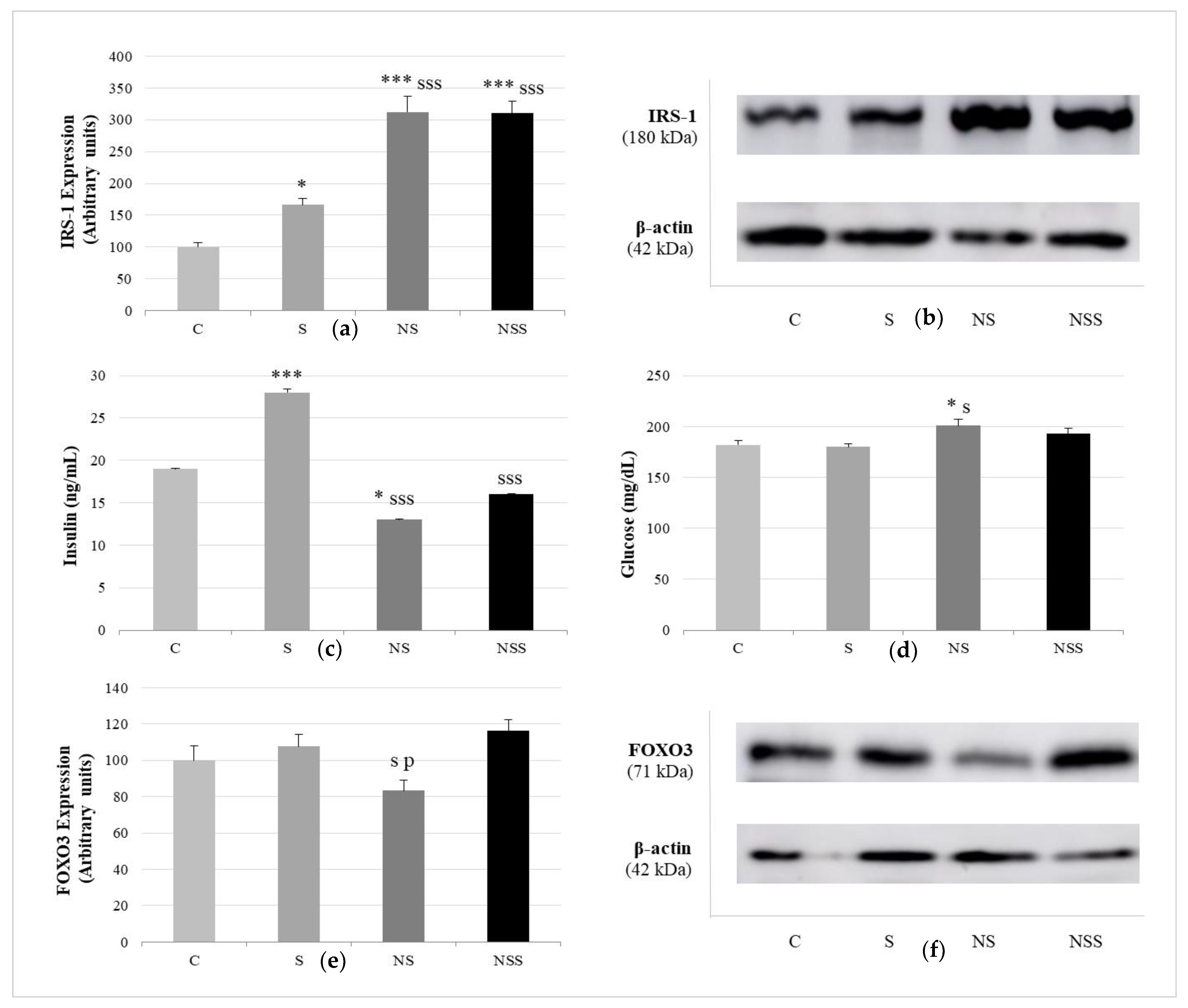
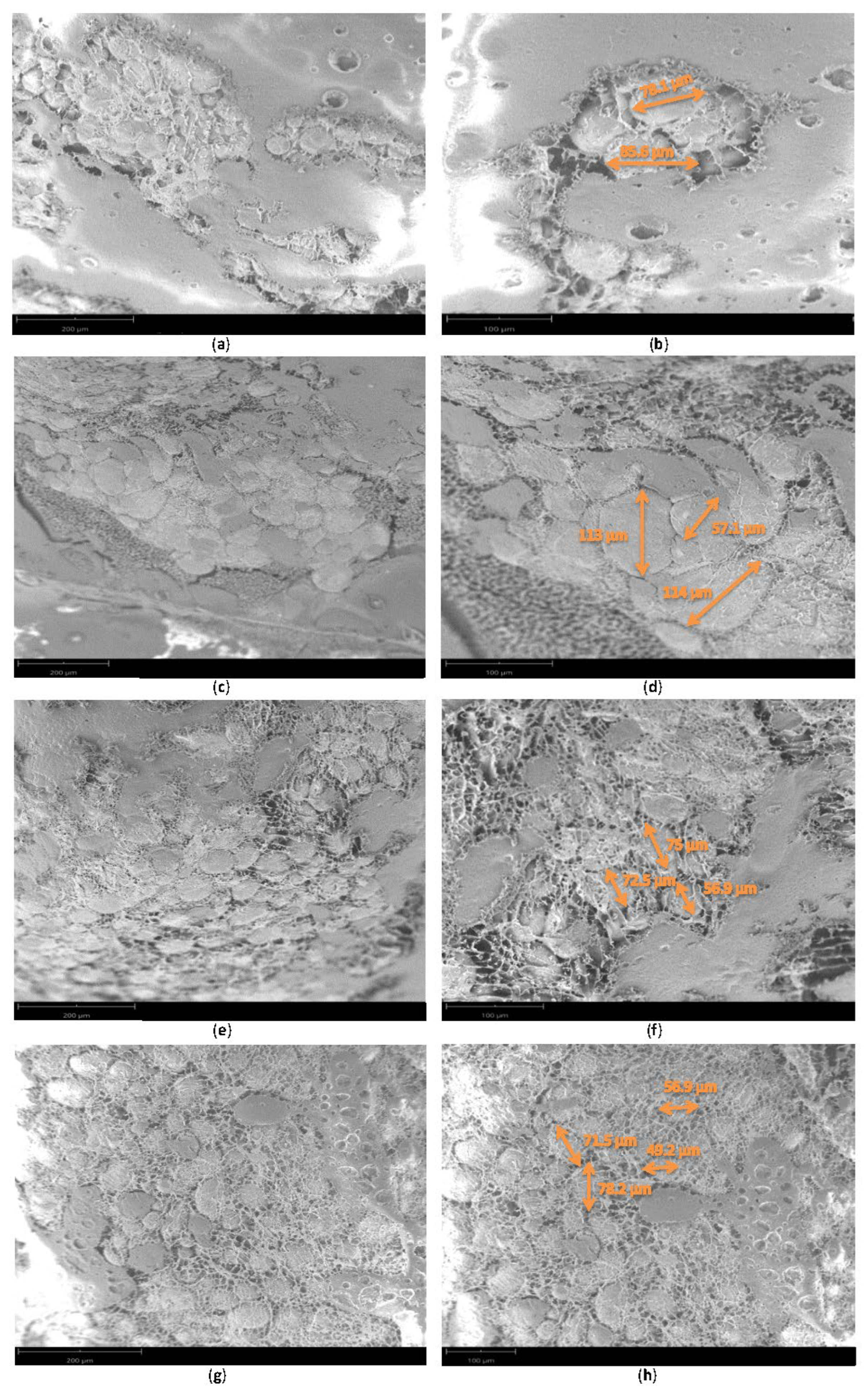
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Steinbeck, K.S.; Lister, N.B.; Gow, M.L.; Baur, L.A. Treatment of Adolescent Obesity. Nat. Rev. Endocrinol. 2018, 14, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Tzanninis, S.; Ntanasis-Stathopoulos, I.; Panotopoulos, G.; Kostopoulou, M.; Tzanninis, I.G.; Tsagianni, A.; Sergentanis, T.N. Prevention and Treatment of Childhood and Adolescent Obesity: A Systematic Review of Meta-Analyses. World J. Pediatr. 2019, 15, 350–381. [Google Scholar] [CrossRef] [PubMed]
- Herpertz-Dahlmann, B. Adolescent Eating Disorders: Update on Definitions, Symptomatology, Epidemiology, and Comorbidity. Child Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Faje, A.T.; Bredella, M.A.; Polineni, S.; Russell, S.; Resulaj, M.; Rosen, C.J.; Klibanski, A. Changes in Marrow Adipose Tissue with Short-Term Changes in Weight in Premenopausal Women with Anorexia Nervosa. Eur. J. Endocrinol. 2019, 180, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White Adipose Tissue Dysfunction in Obesity and Aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef]
- Philipsen, A.; Jørgensen, M.E.; Vistisen, D.; Sandbaek, A.; Almdal, T.P.; Christiansen, J.S.; Lauritzen, T.; Witte, D.R. Associations between Ultrasound Measures of Abdominal Fat Distribution and Indices of Glucose Metabolism in a Population at High Risk of Type 2 Diabetes: The ADDITION-PRO Study. PLoS ONE 2015, 10, e0123062. [Google Scholar] [CrossRef]
- Misra, A.; Vikram, N.K. Clinical and Pathophysiological Consequences of Abdominal Adiposity and Abdominal Adipose Tissue Depots. Nutrition 2003, 19, 457–466. [Google Scholar] [CrossRef]
- Mau, T.; Yung, R. Adipose Tissue Inflammation in Aging. Exp. Gerontol. 2018, 105, 27–31. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.-C.; Hsieh, P.-S. The Role of Adipocyte Hypertrophy and Hypoxia in the Development of Obesity-Associated Adipose Tissue Inflammation and Insulin Resistance. Adiposity Omics Mol. Underst. 2017. [Google Scholar] [CrossRef]
- Dhawan, D.; Sharma, S. Abdominal Obesity, Adipokines and Non-Communicable Diseases. J. Steroid Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Cox, A.R.; Chernis, N.; Kim, K.H.; Masschelin, P.M.; Saha, P.K.; Briley, S.M.; Sharp, R.; Li, X.; Felix, J.B.; Sun, Z.; et al. Ube2i Deletion in Adipocytes Causes Lipoatrophy in Mice. Mol. Metab. 2021, 48, 101221. [Google Scholar] [CrossRef]
- García, P.M.; Flores, R.R. Revista de Gastroenterología de México; Elsevier: Mexico DC, Mexico, 2010; pp. 205–207. [Google Scholar]
- Karageorgiou, V.; Furukawa, T.A.; Tsigkaropoulou, E.; Karavia, A.; Gournellis, R.; Soureti, A.; Bellos, I.; Douzenis, A.; Michopoulos, I. Adipokines in Anorexia Nervosa: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2020, 112, 104485. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The Role of Selenium in Type-2 Diabetes Mellitus and Its Metabolic Comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Hatfield, D.L.; Gladyshev, V.N. Understanding Selenoprotein Function and Regulation through the Use of Rodent Models. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1633–1642. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Filippini, T.; Zhou, J.-C.; Lei, X.G.; Gatiatulina, E.R.; Michalke, B.; Skalnaya, M.G.; Vinceti, M.; Aschner, M.; et al. Selenium and Selenoproteins in Adipose Tissue Physiology and Obesity. Biomolecules 2020, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Magd, N.F.; Barbosa, P.O.; Nick, J.; Covalero, V.; Grignetti, G.; Bermano, G. Selenium, as Selenite, Prevents Adipogenesis by Modulating Selenoproteins Gene Expression and Oxidative Stress–Related Genes. Nutrition 2022, 93, 111424. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant Selenoenzymes and Beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Ducluzeau, P.H.; Priou, M.; Weitheimer, M.; Flamment, M.; Duluc, L.; Iacobazi, F.; Soleti, R.; Simard, G.; Durand, A.; Rieusset, J.; et al. Dynamic Regulation of Mitochondrial Network and Oxidative Functions during 3T3-L1 Fat Cell Differentiation. J. Physiol. Biochem. 2011, 67, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Schieke, S.M.; Von Montfort, C.; Buchczyk, D.P.; Timmer, A.; Grether-Beck, S.; Krutmann, J.; Holbrook, N.J.; Klotz, L.O. Singlet Oxygen-Induced Attenuation of Growth Factor Signaling: Possible Role of Ceramides. Free Radic. Res. 2004, 38, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, H.M.; Pearson, K.J.; Gizard, F.; Zhao, Y.; Qing, H.; Jones, K.L.; Cohn, D.; Heywood, E.B.; de Cabo, R.; Bruemmer, D. Oxidative Stress Accumulates in Adipose Tissue during Aging and Inhibits Adipogenesis. PLoS ONE 2011, 6, e18532. [Google Scholar] [CrossRef]
- Duarte, G.B.S.; Reis, B.Z.; Rogero, M.M.; Vargas-Mendez, E.; Júnior, F.B.; Cercato, C.; Cozzolino, S.M.F. Consumption of Brazil Nuts with High Selenium Levels Increased Inflammation Biomarkers in Obese Women: A Randomized Controlled Trial. Nutrition 2019, 63–64, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Puchau, B.; Bressan, J.; Martínez, J.A. Association of Retinol-Binding Protein-4 with Dietary Selenium Intake and Other Lifestyle Features in Young Healthy Women. Nutrition 2009, 25, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Amirkhizi, F.; Siassi, F.; Djalali, M.; Shahraki, S.H. Impaired Enzymatic Antioxidant Defense in Erythrocytes of Women with General and Abdominal Obesity. Obes. Res. Clin. Pract. 2014, 8, e26–e34. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.R.; Kari, Z.A.; Abdul Razab, M.K.A.; Ahmed, H.A.; Alagawany, M.; Gewaily, M.S. Selenium Nanoparticles as a Natural Antioxidant and Metabolic Regulator in Aquaculture: A Review. Antioxidants 2021, 10, 1364. [Google Scholar] [CrossRef]
- Hassan, R.M.; Elsayed, M.; Kholief, T.E.; Hassanen, N.H.M.; Gafer, J.A.; Attia, Y.A. Mitigating Effect of Single or Combined Administration of Nanoparticles of Zinc Oxide, Chromium Oxide, and Selenium on Genotoxicity and Metabolic Insult in Fructose/Streptozotocin Diabetic Rat Model. Environ. Sci. Pollut. Res. Int. 2021, 28, 48517–48534. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the Cytotoxic Effect of Selenium Nanoparticles in Different Human Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef]
- Gangadoo, S.; Stanley, D.; Hughes, R.J.; Moore, R.J.; Chapman, J. The Synthesis and Characterisation of Highly Stable and Reproducible Selenium Nanoparticles. Inorg. Nano Metal Chem. 2017, 47, 1568–1576. [Google Scholar] [CrossRef]
- Collery, P. Strategies for the Development of Selenium-Based Anticancer Drugs. J. Trace Elem. Med. Biol. 2018, 50, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Yoo, M.H.; Carlson, B.A.; Gladyshev, V.N. Selenoproteins That Function in Cancer Prevention and Promotion. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Al-Quraishy, S.; Adkhil, M.; Moneim, A.E.A. Anti-Hyperglycemic Activity of Selenium Nanoparticles in Streptozotocin-Induced Diabetic Rats. Int. J. Nanomed. 2015, 10, 6741. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Nogales, F.; del Carmen Gallego-López, M.; Carreras, O. Binge Drinking during the Adolescence Period Causes Oxidative Damage-Induced Cardiometabolic Disorders: A Possible Ameliorative Approach with Selenium Supplementation. Life Sci. 2022, 301, 120618. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.G.; Hill, K.E.; Burk, R.F. Dietary Selenium Intake Controls Rat Plasma Selenoprotein P Concentration. J. Nutr. 1989, 119, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione Peroxidase Activity in Selenium-Deficient Rat Liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde Determination as Index of Lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [CrossRef]
- Reznick, A.Z.; Packer, L. Oxidative Damage to Proteins: Spectrophotometric Method for Carbonyl Assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randal, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Xia, I.F.; Kong, H.K.; Wu, M.M.H.; Lu, Y.; Wong, K.H.; Kwok, K.W.H. Selenium Nanoparticles (SeNPs) Immunomodulation is More Than Redox Improvement: Serum Proteomics and Transcriptomic Analyses. Antioxidants 2022, 11, 964. [Google Scholar] [CrossRef]
- Liu, H.; Bian, W.; Liu, S.; Huang, K. Selenium Protects Bone Marrow Stromal Cells against Hydrogen Peroxide-Induced Inhibition of Osteoblastic Differentiation by Suppressing Oxidative Stress and ERK Signaling Pathway. Biol. Trace Elem. Res. 2012, 150, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Gregoire, B.R.; Zeng, H. Selenium Deficiency Decreases Antioxidative Capacity and is Detrimental to Bone Microarchitecture in Mice. J. Nutr. 2012, 142, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Kim, J.M.; Kim, M.Y.; Kim, J.R.; Lee, H.W.; Chung, Y.W.; Shin, H.I.; Kim, T.; Park, E.S.; et al. Selenoprotein W Ensures Physiological Bone Remodeling by Preventing Hyperactivity of Osteoclasts. Nat. Commun. 2021, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, M.L.; Nogales, F.; Romero-Herrera, I.; Carreras, O. Fetal Programming Is Deeply Related to Maternal Selenium Status and Oxidative Balance; Experimental Offspring Health Repercussions. Nutrients 2021, 13, 2085. [Google Scholar] [CrossRef]
- Hofstee, P.; Bartho, L.A.; McKeating, D.R.; Radenkovic, F.; McEnroe, G.; Fisher, J.J.; Holland, O.J.; Vanderlelie, J.J.; Perkins, A.V.; Cuffe, J.S.M. Maternal Selenium Deficiency during Pregnancy in Mice Increases Thyroid Hormone Concentrations, Alters Placental Function and Reduces Fetal Growth. J. Physiol. 2019, 597, 5597–5617. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Carreras, O.; Díaz-Castro, J.; Murillo, M.L.; Nogales, F. High- and Low-Selenium Diets Affect Endocrine Energy Balance during Early Programming. Toxicol. Appl. Pharmacol. 2019, 382, 114744. [Google Scholar] [CrossRef]
- Ma, P.; Hu, Z.; Li, L.; Li, D.; Tang, R. Dietary Selenium Promotes the Growth Performance through Growth Hormone-Insulin-like Growth Factor and Hypothalamic-Pituitary-Thyroid Axes in Grass Carp (Ctenopharyngodon Idella). Fish Physiol. Biochem. 2021, 47, 1313–1327. [Google Scholar] [CrossRef]
- Javdani, M.; Habibi, A.; Shirian, S.; Kojouri, G.A.; Hosseini, F. Effect of Selenium Nanoparticle Supplementation on Tissue Inflammation, Blood Cell Count, and IGF-1 Levels in Spinal Cord Injury-Induced Rats. Biol. Trace Elem. Res. 2019, 187, 202–211. [Google Scholar] [CrossRef]
- Abd El-Kader, M.F.; Fath El-Bab, A.F.; Abd-Elghany, M.F.; Abdel-Warith, A.W.A.; Younis, E.M.; Dawood, M.A.O. Selenium Nanoparticles Act Potentially on the Growth Performance, Hemato-Biochemical Indices, Antioxidative, and Immune-Related Genes of European Seabass (Dicentrarchus Labrax). Biol. Trace Elem. Res. 2021, 199, 3126–3134. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Pourmozaffar, S.; Adeshina, I.; Mahmoudi, R.; Erfanifar, E.; Ajdari, A. Selenium Nanoparticle and Selenomethionine as Feed Additives: Effects on Growth Performance, Hepatic Enzymes’ Activity, Mucosal Immune Parameters, Liver Histology, and Appetite-Related Gene Transcript in Goldfish (Carassius Auratus). Fish Physiol. Biochem. 2021, 47, 639–652. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist Circumference as a Vital Sign in Clinical Practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Shen, F.; Ye, T.; Zhou, Q.; Deng, H.; Gu, X. Waist-to-Height Ratio Is an Appropriate Index for Identifying Cardiometabolic Risk in Chinese Individuals with Normal Body Mass Index and Waist Circumference. J. Diabetes 2014, 6, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, S.R. Selenium: An Insulin-Mimetic. Cell. Mol. Life Sci. 2000, 57, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 292. [Google Scholar] [CrossRef]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87, S168–S177. [Google Scholar] [CrossRef]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological Effects of a Nano Red Elemental Selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of Short-Term Toxicity between Nano-Se and Selenite in Mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef]
- Klein, J. Probing the Interactions of Proteins and Nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2029–2030. [Google Scholar] [CrossRef]
- Khan, K.U.; Zuberi, A.; Nazir, S.; Fernandes, J.B.K.; Jamil, Z.; Sarwar, H. Effects of Dietary Selenium Nanoparticles on Physiological Andbiochemical Aspects of Juvenile Tor Putitora. Turk. J. Zool. 2016, 40, 704–712. [Google Scholar] [CrossRef]
- Holben, D.H.; Smith, A.M. The Diverse Role of Selenium within Selenoproteins: A Review. J. Am. Diet. Assoc. 1999, 99, 836–843. [Google Scholar] [CrossRef]
- Shetty, S.; Marsicano, J.R.; Copeland, P.R. Uptake and Utilization of Selenium from Selenoprotein P. Biol. Trace Elem. Res. 2018, 181, 54. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y. Selenium Transport Mechanism via Selenoprotein P—Its Physiological Role and Related Diseases. Front. Nutr. 2021, 8, 685517. [Google Scholar] [CrossRef] [PubMed]
- Yiannikouris, F.; Wang, Y.; Shoemaker, R.; Larian, N.; Thompson, J.; English, V.L.; Charnigo, R.; Su, W.; Gong, M.; Cassis, L.A. Deficiency of Angiotensinogen in Hepatocytes Markedly Decreases Blood Pressure in Lean and Obese Male Mice. Hypertension 2015, 66, 836–842. [Google Scholar] [CrossRef]
- Urs, S.; Smith, C.; Campbell, B.; Saxton, A.M.; Taylor, J.; Zhang, B.; Snoddy, J.; Voy, B.J.; Moustaid-Moussa, N. Gene Expression Profiling in Human Preadipocytes and Adipocytes by Microarray Analysis. J. Nutr. 2004, 134, 762–770. [Google Scholar] [CrossRef]
- Cabezas, F.; Lagos, J.; Céspedes, C.; Vio, C.P.; Bronfman, M.; Marzolo, M.P. Megalin/LRP2 Expression Is Induced by Peroxisome Proliferator-Activated Receptor -Alpha and -Gamma: Implications for PPARs’ Roles in Renal Function. PLoS ONE 2011, 6, e16794. [Google Scholar] [CrossRef]
- Donma, M.M.; Donma, O. Promising Link between Selenium and Peroxisome Proliferator Activated Receptor Gamma in the Treatment Protocols of Obesity as Well as Depression. Med. Hypotheses 2016, 89, 79–83. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.; Long, Z.; Sun, Q.; Liu, J.; Liu, Y.; Hai, C. Differential Effect of Se on Insulin Resistance: Regulation of Adipogenesis and Lipolysis. Mol. Cell. Biochem. 2016, 415, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, F.; Singh, M. Selenium Nanoparticles: Potential in Cancer Gene and Drug Delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Shafeev, G.A.; Glinushkin, A.P.; Shkirin, A.V.; Barmina, E.V.; Rakov, I.I.; Simakin, A.V.; Kislov, A.V.; Astashev, M.E.; Vodeneev, V.A.; et al. Production and Use of Selenium Nanoparticles as Fertilizers. ACS Omega 2020, 5, 17767–17774. [Google Scholar] [CrossRef]
- Ullrey, D.E. Biochemical and Physiological Indicators of Selenium Status in Animals. J. Anim. Sci. 1987, 65, 1712–1726. [Google Scholar] [CrossRef]
- Hauffe, R.; Stein, V.; Chudoba, C.; Flore, T.; Rath, M.; Ritter, K.; Schell, M.; Wardelmann, K.; Deubel, S.; Kopp, J.F.; et al. GPx3 Dysregulation Impacts Adipose Tissue Insulin Receptor Expression and Sensitivity. JCI Insight 2020, 5, e136283. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Softic, S.; El Ouaamari, A.; Krumpoch, M.T.; Kleinridders, A.; Kulkarni, R.N.; O’Neill, B.T.; Kahn, C.R. Differential Roles of Insulin and IGF-1 Receptors in Adipose Tissue Development and Function. Diabetes 2016, 65, 2201–2213. [Google Scholar] [CrossRef]
- Committee on Medical Aspects of Food Policy. Dietary Reference Values for Food Energy and Nutrients for the United Kingdom; Committee on Medical Aspects of Food Policy: London, UK, 1991; ISBN 9780113213979. [Google Scholar]
- Harmon, J.S.; Bogdani, M.; Parazzoli, S.D.; Mak, S.S.M.; Oseid, E.A.; Berghmans, M.; LeBoeuf, R.C.; Robertson, R.P. β-Cell-Specific Overexpression of Glutathione Peroxidase Preserves Intranuclear MafA and Reverses Diabetes in Db/Db Mice. Endocrinology 2009, 150, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dai, C.; Guo, M.; Taylor, B.; Harmon, J.S.; Sander, M.; Robertson, R.P.; Powers, A.C.; Stein, R. Inactivation of Specific β Cell Transcription Factors in Type 2 Diabetes. J. Clin. Investig. 2013, 123, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Vatamaniuk, M.Z.; Wang, S.K.; Roneker, C.A.; Simmons, R.A.; Lei, X.G. Molecular Mechanisms for Hyperinsulinaemia Induced by Overproduction of Selenium-Dependent Glutathione Peroxidase-1 in Mice. Diabetologia 2008, 51, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Hauffe, R.; Rath, M.; Agyapong, W.; Jonas, W.; Vogel, H.; Schulz, T.J.; Schwarz, M.; Kipp, A.P.; Blüher, M.; Kleinridders, A. Obesity Hinders the Protective Effect of Selenite Supplementation on Insulin Signaling. Antioxidants 2022, 11, 862. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Zhang, X.; Guo, K.; Zhang, X.; Zhou, Z. FOXO3a Regulates Lipid Accumulation and Adipocyte Inflammation in Adipocytes through Autophagy: Role of FOXO3a in Obesity. Inflamm. Res. 2021, 70, 591–603. [Google Scholar] [CrossRef]
- Luo, W.; Cao, J.; Li, J.; He, W. Adipose Tissue-Specific PPARγ Deficiency Increases Resistance to Oxidative Stress. Exp. Gerontol. 2008, 43, 154–163. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium Nanoparticles: A Review on Synthesis and Biomedical Applications. Mater. Adv. 2022, 3, 1415–1431. [Google Scholar] [CrossRef]
- Ryu, K.Y.; Jeon, E.J.; Leem, J.; Park, J.H.; Cho, H. Regulation of Adipsin Expression by Endoplasmic Reticulum Stress in Adipocytes. Biomolecules 2020, 10, 314. [Google Scholar] [CrossRef] [Green Version]
- Tafere, G.G.; Wondafrash, D.Z.; Zewdie, K.A.; Assefa, B.T.; Ayza, M.A. Plasma Adipsin as a Biomarker and Its Implication in Type 2 Diabetes Mellitus. Diabetes. Metab. Syndr. Obes. 2020, 13, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. Adipose Tissue-Derived Plasminogen Activator Inhibitor-1 Function and Regulation. Compr. Physiol. 2016, 6, 1873–1896. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Risé, P.; Mussoni, L.; Galli, C.; Tremoli, E. Linoleic Acid Enhances the Secretion of Plasminogen Activator Inhibitor Type 1 by HepG2 Cells. J. Lipid Res. 1997, 38, 860–869. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhou, J.C.; Wu, Y.Y.; Ren, F.Z.; Lei, X.G. Role of Glutathione Peroxidase 1 in Glucose and Lipid Metabolism-Related Diseases. Free Radic. Biol. Med. 2018, 127, 108–115. [Google Scholar] [CrossRef]
- Al Jaberi, S.; Cohen, A.; D’Souza, C.; Abdulrazzaq, Y.M.; Ojha, S.; Bastaki, S.; Adeghate, E.A. Lipocalin-2: Structure, Function, Distribution and Role in Metabolic Disorders. Biomed. Pharmacother. 2021, 142, 112002. [Google Scholar] [CrossRef]
- Yan, Q.W.; Yang, Q.; Mody, N.; Graham, T.E.; Hsu, C.H.; Xu, Z.; Houstis, N.E.; Kahn, B.B.; Rosen, E.D. The Adipokine Lipocalin 2 Is Regulated by Obesity and Promotes Insulin Resistance. Diabetes 2007, 56, 2533–2540. [Google Scholar] [CrossRef]
- Li, J.; Kim, S.Y.; Lainez, N.M.; Coss, D.; Nair, M.G. Macrophage-Regulatory T Cell Interactions Promote Type 2 Immune Homeostasis Through Resistin-Like Molecule α. Front. Immunol. 2021, 12, 710406. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, L.; Zhang, Z.; Xu, J.; Wan, Z.; Qin, L. Resistin Induces Lipolysis and Suppresses Adiponectin Secretion in Cultured Human Visceral Adipose Tissue. Regul. Pept. 2014, 194–195, 49–54. [Google Scholar] [CrossRef]
- Yanai, H.; Yoshida, H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef]
- Wang, C.; Mao, X.; Wang, L.; Liu, M.; Wetzel, M.D.; Guan, K.L.; Dong, L.Q.; Liu, F. Adiponectin Sensitizes Insulin Signaling by Reducing P70 S6 Kinase-Mediated Serine Phosphorylation of IRS-1. J. Biol. Chem. 2007, 282, 7991–7996. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Magkos, F.; Mantzoros, C.S.; Kang, E.S. Effects of Leptin and Adiponectin on Pancreatic β-Cell Function. Metabolism 2011, 60, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Tural, U.; Iosifescu, D.V. Adiponectin in Anorexia Nervosa and Its Modifiers: A Meta-Regression Study. Int. J. Eat. Disord. 2022. [Google Scholar] [CrossRef] [PubMed]

| Parameter | C | S | NS | NSS |
|---|---|---|---|---|
| Body weight increase (g/day) | 6.01–0.1 | 6.07–0.2 | 5.98–0.2 | 5.13–0.2 * s |
| Solid intake (g/day) | 18.26–0.35 | 18.58–0.18 | 18.28–0.09 | 16.54–0.19 *** sss ppp |
| Liquid intake (mL/day) | 21.4–0.66 | 21.54–0.7 | 19.16–0.77 | 16.67–0.45 *** sss pp |
| Total Se intake (µg/day) | 3.48–0.08 | 6.81–0.14 ** | 6.59–0.09 ** | 31.35–1.43 *** sss ppp |
| Serum GPx activity (mU/mg protein) | 59.7–2.2 | 73.3–3.5 * | 74.8–3.4 * | 80.4–3.7 ** |
| BMI (Kg/m2) | 51.65–0.8 | 48.72–00.5 | 47.46–0.9 ** | 47.99–0.6 * |
| CCL (cm) | 18.22–0.11 | 19.68–0.19 *** | 19.6–0.2 *** | 18.87–0.18 s p |
| AC (cm) | 10.56–0.46 | 11.52–0.26 * | 10.16–0.35 ss | 10.75–0.25 |
| AC/CCL | 0.57–0.01 | 0.59–0.01 | 0.51–0.01 ** sss | 0.57–0.01 p |
| LSI (%) | 3.73–0.09 | 3.65–0.1 | 3.93–0.09 | 3.97–0.06 |
| KSI (%) | 1.04–0.04 | 0.99–0.03 | 0.98–0.02 | 1.19–0.03 *** ss ppp |
| HSI (%) | 0.51–0.02 | 0.49–0.01 | 0.53–0.01 | 0.56–0.02 s |
| PSI (%) | 0.72–0.02 | 0.89–0.03 ** | 0.70–0.02 ss | 0.81–0.02 |
| BSI (%) | 1.08–0.02 | 1.17–0.02 | 1.07–0.02 | 1.14–0.03 |
| WATSI (%) | 0.62–0.02 | 0.59–0.03 | 0.50–0.02 * | 0.59–0.02 |
| BATSI (%) | 0.24–0.01 | 0.28–0.01 * | 0.25–0.02 s | 0.22–0.01 |
| BAT/WAT | 0.4–0.02 | 0.48–0.02 * | 0.49–0.02 * | 0.38–0.02 s p |
| BMI (Kg/m2) | 51.65–0.8 | 48.72–00.5 | 47.46–0.9 ** | 47.99–0.6 * |
| Adipokine | C | S | NS | NSS |
|---|---|---|---|---|
| Adiponectin (pg/mL) | 71.1–4.9 | 29.2–1.1 ** | 99.2–8.1 * sss | 362–9.7 *** sss ppp |
| Resistin (pg/mL) | 48.1–2.9 | 41.5–1 | 53.5–1.9 ss | 54.1–1.9 ss |
| Adipsin (pg/mL) | 319–12 | 112.7–1 *** | 108–1.6 *** | 112–3.5 *** |
| Lipocalin (LCN2) (pg/mL) | 26.3–1.5 | 13.8–0.7 *** | 29.4–1.1 sss | 28.9–1.1 sss |
| PAI-1 (pg/mL) | 70.2–1.1 | 90.8–1.8 *** | 85.2–1.5 *** | 83.4–0.7 *** s |
| TNF-alfa (pg/mL) | 13.7–0.8 | 9.9–0.3 ** | 2.7–0.2 *** s | 3.1–0.2 *** s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda, M.L.; Nogales, F.; Carreras, O.; Pajuelo, E.; Gallego-López, M.d.C.; Romero-Herrera, I.; Begines, B.; Moreno-Fernández, J.; Díaz-Castro, J.; Alcudia, A. Different Effects of Low Selenite and Selenium-Nanoparticle Supplementation on Adipose Tissue Function and Insulin Secretion in Adolescent Male Rats. Nutrients 2022, 14, 3571. https://doi.org/10.3390/nu14173571
Ojeda ML, Nogales F, Carreras O, Pajuelo E, Gallego-López MdC, Romero-Herrera I, Begines B, Moreno-Fernández J, Díaz-Castro J, Alcudia A. Different Effects of Low Selenite and Selenium-Nanoparticle Supplementation on Adipose Tissue Function and Insulin Secretion in Adolescent Male Rats. Nutrients. 2022; 14(17):3571. https://doi.org/10.3390/nu14173571
Chicago/Turabian StyleOjeda, María Luisa, Fátima Nogales, Olimpia Carreras, Eloísa Pajuelo, María del Carmen Gallego-López, Inés Romero-Herrera, Belén Begines, Jorge Moreno-Fernández, Javier Díaz-Castro, and Ana Alcudia. 2022. "Different Effects of Low Selenite and Selenium-Nanoparticle Supplementation on Adipose Tissue Function and Insulin Secretion in Adolescent Male Rats" Nutrients 14, no. 17: 3571. https://doi.org/10.3390/nu14173571
APA StyleOjeda, M. L., Nogales, F., Carreras, O., Pajuelo, E., Gallego-López, M. d. C., Romero-Herrera, I., Begines, B., Moreno-Fernández, J., Díaz-Castro, J., & Alcudia, A. (2022). Different Effects of Low Selenite and Selenium-Nanoparticle Supplementation on Adipose Tissue Function and Insulin Secretion in Adolescent Male Rats. Nutrients, 14(17), 3571. https://doi.org/10.3390/nu14173571










