Discovery of Pinostrobin as a Melanogenic Agent in cAMP/PKA and p38 MAPK Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Lines and Pinostrobin Treatment
2.3. Cell Viability Assay
2.4. Measurement of Melanin Content
2.5. Evaluation of Tyrosinase Activity
2.6. Western Blot Analysis
2.7. In Silico Pharmacokinetic Prediction and Docking Simulation
2.8. Statistical Analysis
3. Results
3.1. Effects of Pinostrobin on Melanin Production and Tyrosinase Activity in B16F10 Cells
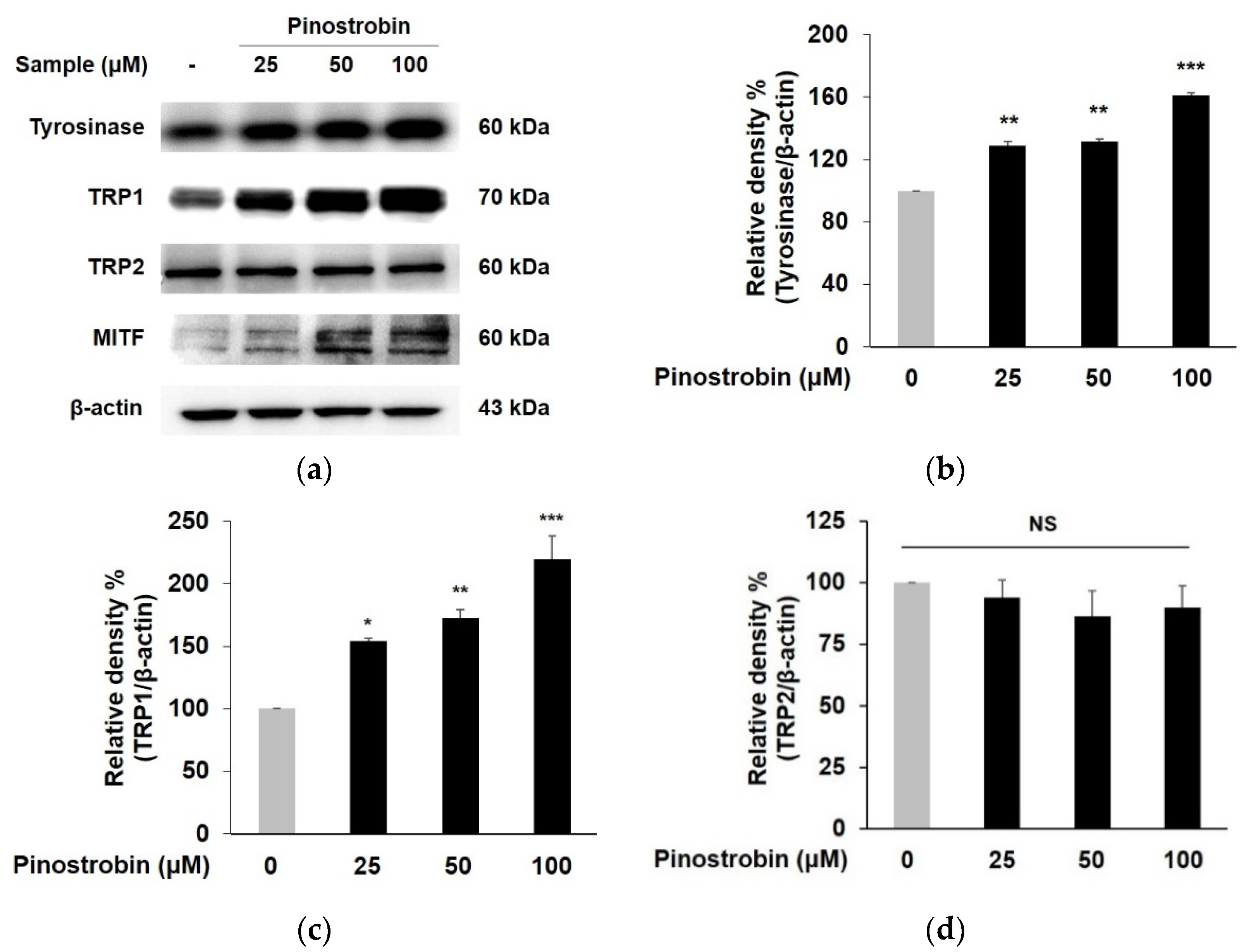
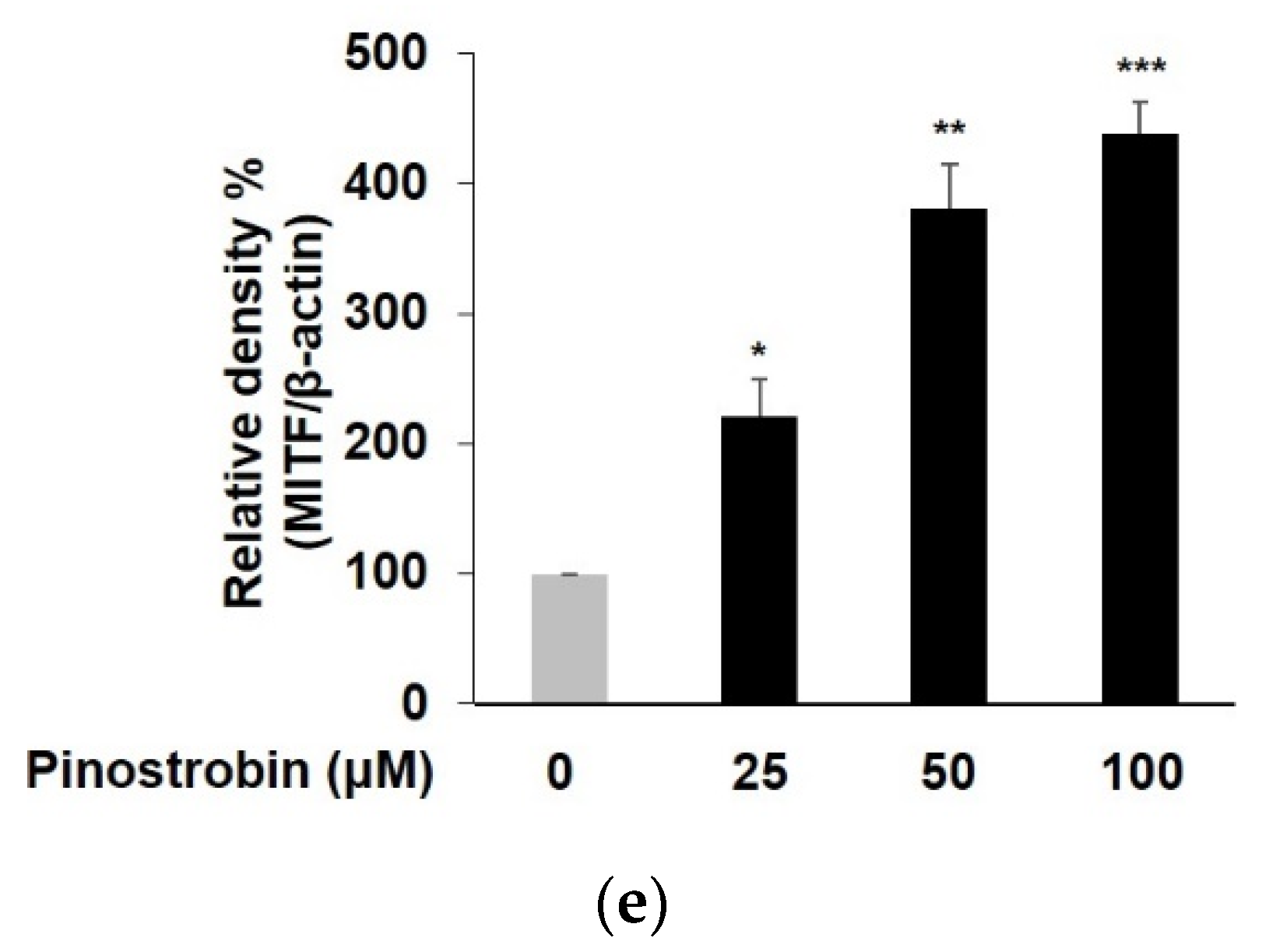
3.2. Pinostrobin Regulated the Protein Expression of Melanogenic Enzymes and MITF
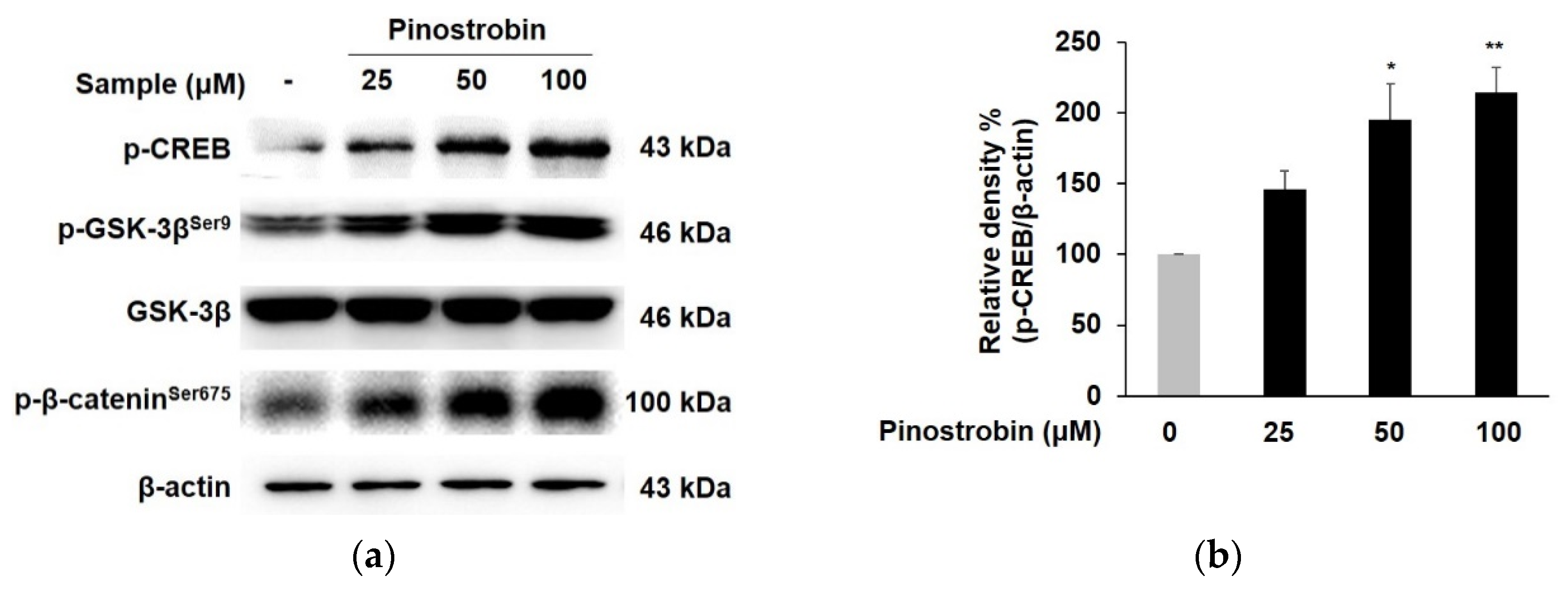
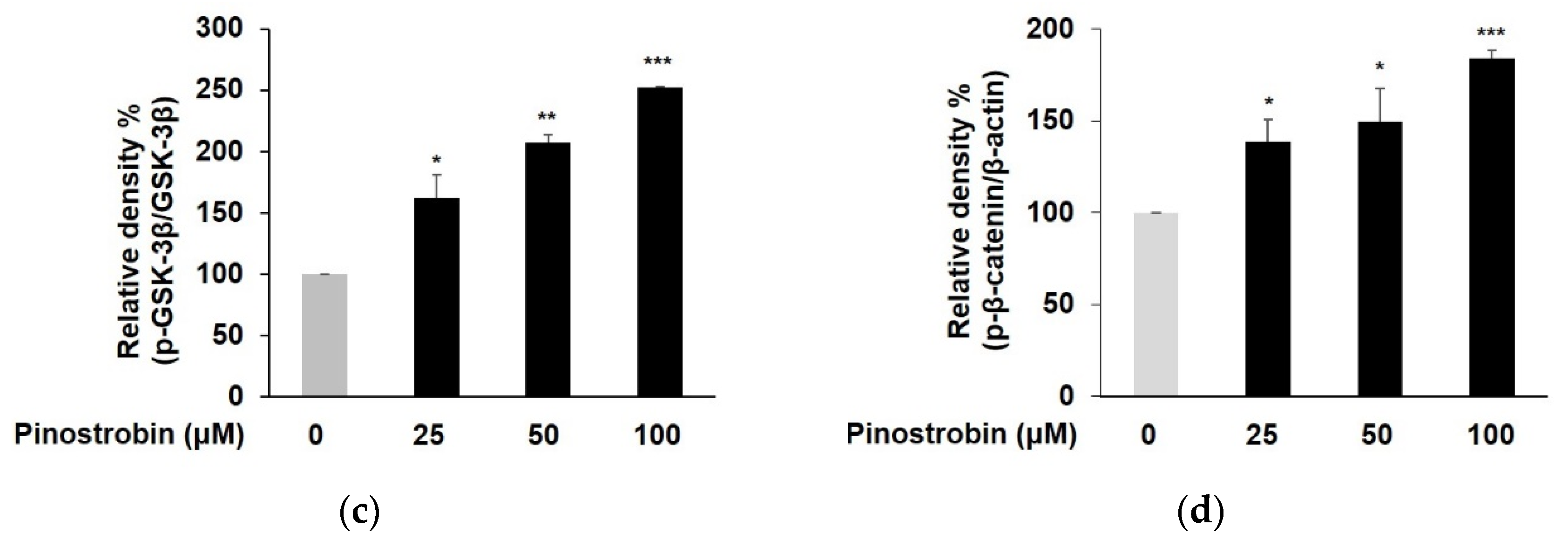
3.3. Pinostrobin Mediated Melanogenesis by Upregulating CREB, GSK-3β and β-Catenin
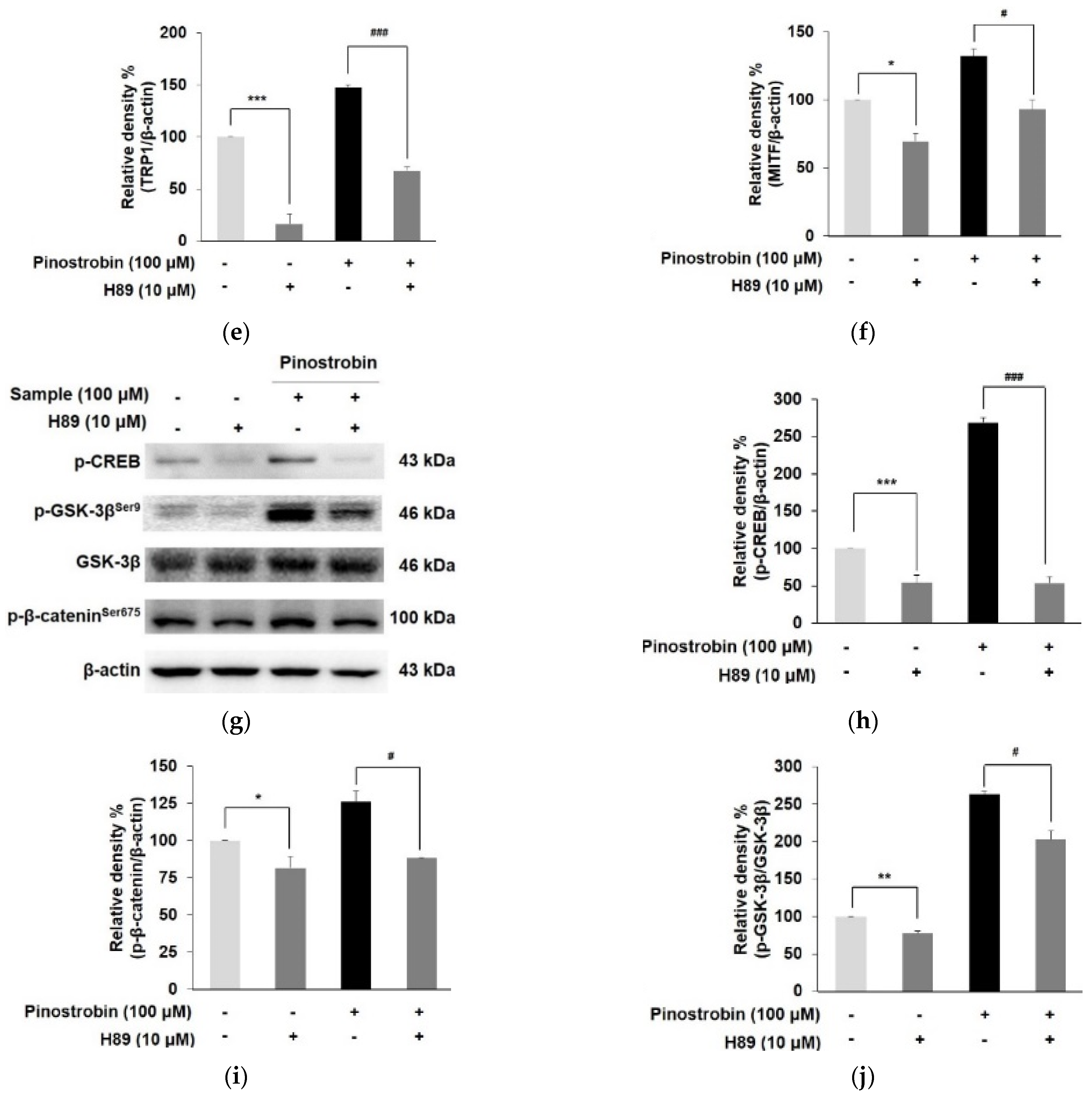
3.4. Pinostrobin Controlled the Melanogenesis via PKA Dependent Signaling Pathway
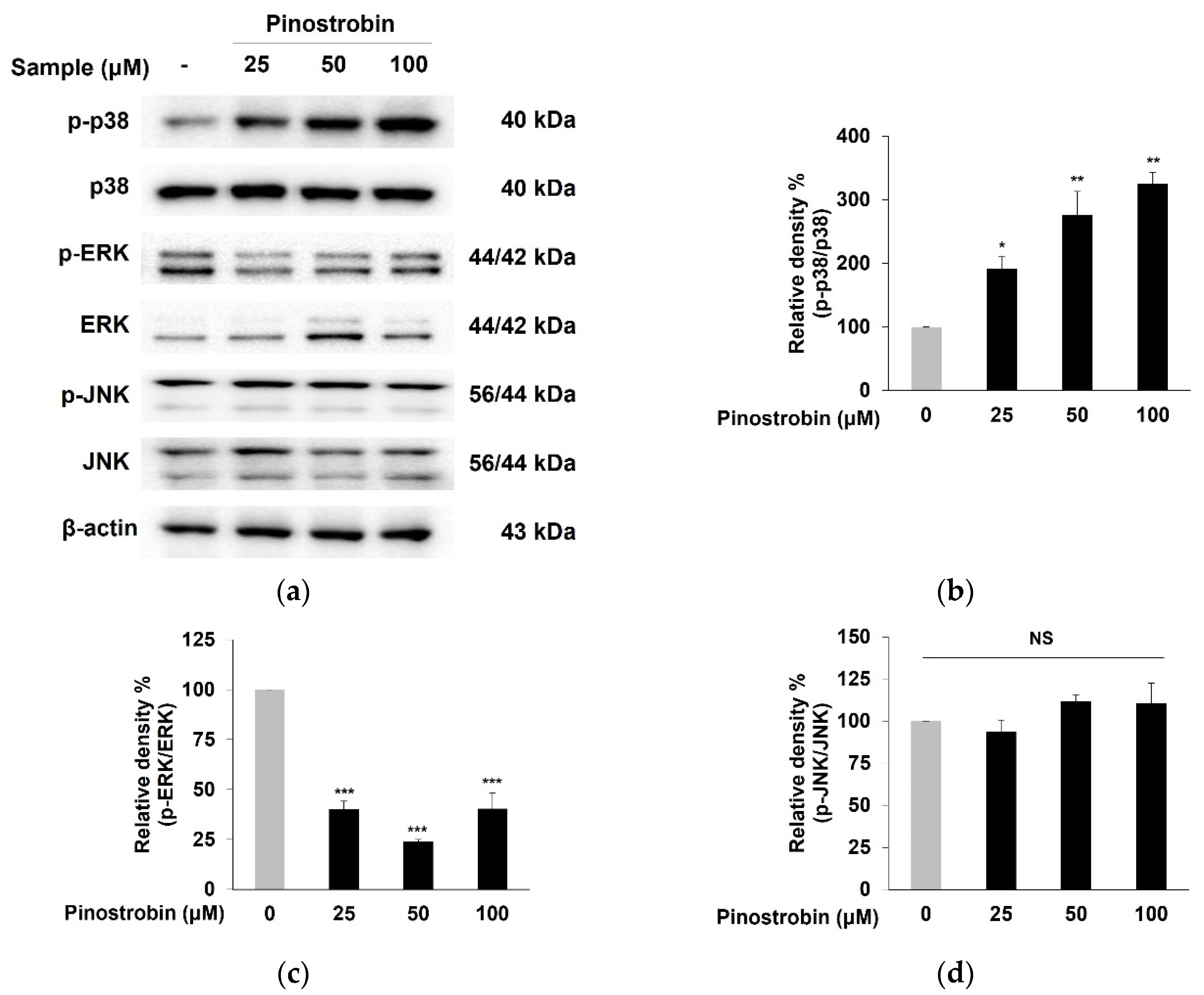
3.5. Pinostrobin Regulated p38 and ERK Signaling Pathway
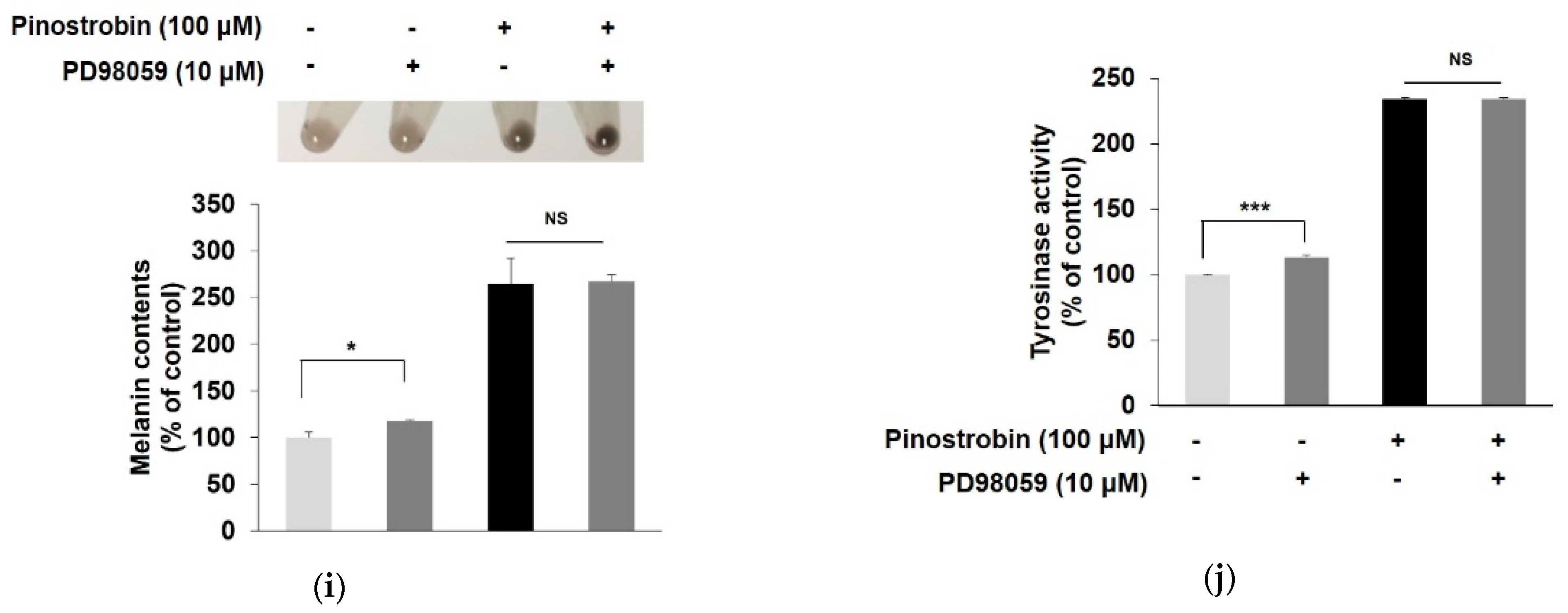
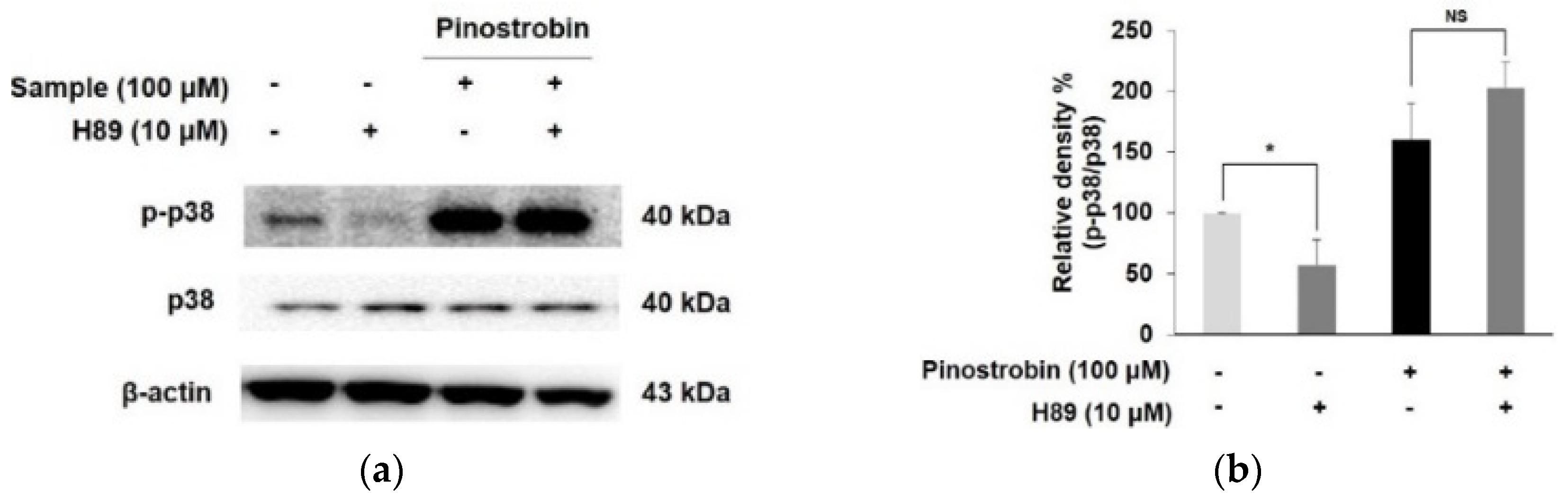
3.6. Pinostrobin Stimulated Melanogenesis via p38 Signaling Pathway
3.7. Molecular Docking Simulation of Pinostrobin
3.8. In Silico SwissADME Profile of Pinostrobin
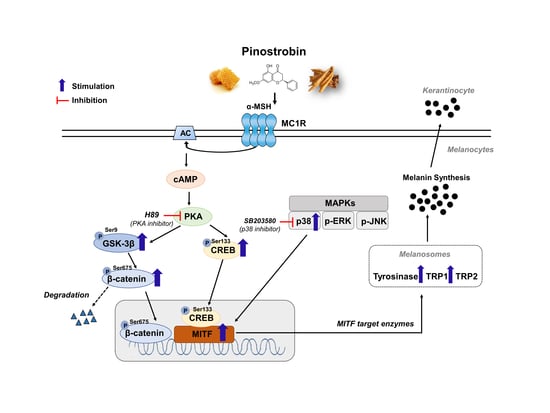
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madronich, S.; McKenzie, R.L.; Björn, L.O.; Caldwell, M.M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J. Photochem. Photobiol. B 1998, 46, 5–19. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Invest. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Aisa, H.A. Upregulation of melanogenesis and tyrosinase activity: Potential agents for vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [PubMed]
- del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Naish-Byfield, S.; Riley, P.A. Tyrosinase autoactivation and the problem of the lag period. Pigment Cell Res. 1998, 11, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, C.; Buscà, R.; Abbe, P.; Ortonne, J.P.; Ballotti, R. Microphthalmia-associated transcription factor (MITF) is required but is not sufficient to induce the expression of melanogenic genes. Pigment Cell Res. 2003, 16, 374–382. [Google Scholar] [CrossRef]
- Millington, G.W. Proopiomelanocortin (POMC): The cutaneous roles of its melanocortin products and receptors. Clin. Exp. Dermatol. 2006, 31, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.P.; Ballotti, R. Microphtalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef]
- Bellei, B.; Pitisci, A.; Catricala, C.; Larue, L.; Picardo, M. Wnt/beta-catenin signaling is stimulated by alpha-melanocyte-stimulating hormone in melanoma and melanocyte cells: Implication in cell differentiation. Pigment. Cell. Melanoma Res. 2011, 24, 309–325. [Google Scholar] [CrossRef]
- Hino, S.; Tanji, C.; Nakayama, K.I.; Kikuchi, A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol. Cell. Biol. 2005, 25, 9063–9072. [Google Scholar] [CrossRef] [Green Version]
- Tsang, T.F.; Chan, B.; Tai, W.C.; Huang, G.; Wang, J.; Li, X.; Jiang, Z.H.; Hsiao, W.L.W. Gynostemma pentaphyllum saponins induce melanogenesis and activate cAMP/PKA and Wnt/beta-catenin signaling pathways. Phytomedicine 2019, 60, 153008. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Kim, M.S.; Choi, Y.H.; Oren, M.; Park, E.K.; Kim, G.Y. Flumequine-Mediated Upregulation of p38 MAPK and JNK Results in Melanogenesis in B16F10 Cells and Zebrafish Larvae. Biomolecules 2019, 9, 596. [Google Scholar] [CrossRef]
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef]
- Wang, Y.C.; Haung, X.Y.; Chiu, C.C.; Lin, M.Y.; Lin, W.H.; Chang, W.T.; Tseng, C.C.; Wang, H.M.D. Inhibitions of melanogenesis via Phyllanthus emblica fruit extract powder in B16F10 cells. Food Biosci. 2019, 28, 177–182. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Wong, Z.C.; Wang, C.; Fung, A.H.; Wong, E.O.Y.; Chan, G.K.L.; Dong, T.T.X.; Chen, Y.; Tsim, K.W.K. Isoorientin derived from Gentiana veitchiorum Hemsl. flowers inhibits melanogenesis by down-regulating MITF-induced tyrosinase expression. Phytomedicine 2019, 57, 129–136. [Google Scholar] [CrossRef]
- Jia, C.Y.; Li, J.Y.; Hao, G.F.; Yang, G.F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. Today 2020, 25, 248–258. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Hartman, M.L.; Czyz, M. MITF in melanoma: Mechanisms behind its expression and activity. Cell. Mol. Life Sci. 2015, 72, 1249–1260. [Google Scholar] [CrossRef] [Green Version]
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Primers 2015, 1, 15011. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Hassan, R. Concise review of recent studies in vitiligo. Qatar Med. J. 2013, 2, 10. [Google Scholar]
- Spritz, R.A. The genetics of generalized vitiligo and associated autoimmune diseases. J. Dermatol. Sci. 2006, 41, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Migayron, L.; Boniface, K.; Seneschal, J. Vitiligo, From Physiopathology to Emerging Treatments: A Review. Dermatol. Ther. 2020, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Xie, H.; Aisa, H.A. Janus Kinase Inhibitors: A Review of Their Application in the Vitiligo. Mini Rev. Med. Chem. 2021, 21, 3203–3218. [Google Scholar] [CrossRef]
- Niu, C.; Zang, D.; Aisa, H.A. Study of Novel Furocoumarin Derivatives on Anti-Vitiligo Activity, Molecular Docking and Mechanism of Action. Int. J. Mol. Sci. 2022, 23, 7959. [Google Scholar] [CrossRef]
- Gauthier, Y.; Cario-Andre, M.; Lepreux, S.; Pain, C.; Taieb, A. Melanocyte detachment after skin friction in non lesional skin of patients with generalized vitiligo. Br. J. Dermatol. 2003, 148, 95–101. [Google Scholar] [CrossRef]
- Fahey, J.F.; Stephenson, K.K. Pinostrobin from honey and Thai ginger (Boesenbergia pandurata): A potent flavonoid inducer of mammalian phase 2 chemoprotective and antioxidant enzymes. J. Agric. Food Chem. 2002, 50, 7472–7476. [Google Scholar] [CrossRef]
- Youn, K.; Jun, M. Biological evaluation and docking analysis of potent BACE1 inhibitors from Boesenbergia rotunda. Nutrients 2019, 11, 662. [Google Scholar] [CrossRef]
- Miller, K.A.; Tsukamoto, S.; Williams, R.M. Asymmetric total syntheses of (+)- and (-)-versicolamide B and biosynthetic implications. Nat. Chem. 2009, 1, 63–68. [Google Scholar] [CrossRef]
- Finefield, J.M.; Sherman, D.H.; Kreitman, M.; Williams, R.M. Enantiomeric natural products: Occurrence and biogenesis. Angew. Chem. Int. Ed. Engl. 2012, 51, 4802–4836. [Google Scholar] [CrossRef] [Green Version]
- Patel, N.K.; Jaiswal, G.; Bhutani, K.K. A review on biological sources, chemistry and pharmacological activities of pinostrobin. Nat. Prod. Res. 2016, 30, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Pobłocka-Olech, L.; Inkielewicz-Stepniak, I.; Krauze-Baranowska, M. Anti-inflammatory and antioxidative effects of the buds from different species of Populus in human gingival fibroblast cells: Role of bioflavanones. Phytomedicine 2019, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, H.D.; Mendyk, E.; Bogucka-Kocka, A.; Kockic, J. Pinostrobin-An anti-leukemic flavonoid from Polygonum lapathifolium L. ssp nodosum (Pers.) Dans. Z. Naturforsch. C J. Biosci. 2006, 61, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.F.; Ip, S.P.; Lin, Z.X.; Mao, Q.Q.; Su, Z.R.; Lai, X.P. Protective effects of pinostrobin on beta-amyloid-induced neurotoxicity in PC12 cells. Cell. Mol. Neurobiol. 2012, 32, 1223–1230. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in reponse to stress. FASEB J. 2007, 27, 976–994. [Google Scholar] [CrossRef]
- Suzuki, A.; Ozono, K.; Kubota, H.; Tachikawa, K.; Michigami, T. PTH/cAMP/PKA signaling facilitates canonical Wnt signaling via inactivation of glycogen synthase kinase-3beta in osteoblastic Saos-2 cells. J. Cell. Biochem. 2008, 104, 304–317. [Google Scholar] [CrossRef]
- Kang, S.J.; Choi, B.R.; Lee, E.K.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Song, C.H.; Lee, Y.J.; Ku, S.K. Inhibitory Effect of Dried Pomegranate Concentration Powder on Melanogenesis in B16F10 Melanoma Cells; Involvement of p38 and PKA Signaling Pathways. Int. J. Mol. Sci. 2015, 16, 24219–24242. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, I.S.; Dong, Y.; Lee, I.S.; Kim, J.S.; Kim, J.S.; Woo, J.T.; Cha, B.Y. Melanogenesis-inducing effect of cirsimaritin through increases in microphthalmia-associated transcription factor and tyrosinase expression. Int. J. Mol. Sci. 2015, 16, 8772–8788. [Google Scholar] [CrossRef]
- Ohguchi, K.; Akao, Y.; Nozawa, Y. Stimulation of melanogenesis by the citrus flavonoid naringenin in mouse B16 melanoma cells. Biosci. Biotechnol. Biochem. 2006, 70, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Chiou, Y. Citrus flavanone naringenin enhances melanogenesis through the activation of Wnt/β-catenin signalling in mouse melanoma cells. Phytomedicine 2011, 18, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L. Melanogenesis of murine melanoma cells induced by hesperetin, a Citrus hydrolysate-derived flavonoid. Food Chem. Toxicol. 2012, 50, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, W.J.; Chang, S.E.; Lee, G.Y. Hesperidin, a popular antioxidant inhibits melanogenesis via Erk1/2 mediated MITF degradation. Int. J. Mol. Sci. 2015, 16, 18384–18395. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, X.; Chen, S. The Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Pinostrobin in Rats: Ultra-High-Performance Liquid Chromatography Coupled with Linear Trap Quadrupole Orbitrap Mass Spectrometry Studies. Front. Pharmacol. 2020, 11, 574638. [Google Scholar] [CrossRef]
- Sayre, C.L.; Alrushaid, S.; Martinez, S.E.; Anderson, H.D.; Davies, N.M. Pre-clinical pharmacokinetic and pharmacodynamic characterization of selected chiral flavonoids: Pinocembrin and pinostrobin. J. Pharm. Pharmaceut. Sci. 2015, 18, 368–396. [Google Scholar] [CrossRef]
- Charoensin, S.; Punvittayagul, C.; Pompimon, W.; Mevatee, U.; Wongpoomchai, R. Toxicological and clastogenic evaluation of pinocembrin and pinostrobin isolated from Boesenbergia pandurata in Wistar rats. Thai J. Toxicol. 2010, 25, 29–40. [Google Scholar]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Ogu, C.C.; Maxa, J.L. Drug interactions due to cytochrome P450. In Baylor University Medical Center Proceedings; Taylor & Francis: Abingdon, UK, 2000; Volume 13, pp. 421–423. [Google Scholar]




| Target Protein | Binding Energy (kcal/mol) | No. of H- Bonds | H-Bonding Residues | H-Bond Length (Å) | van Der Waals Residues |
|---|---|---|---|---|---|
| PKA | –7.3 | 2 | Asp214, Gln377 | 3.15 2.84 | Asp149, Leu151, Tyr213, Arg216, Gly217, Asp309, Asp375 |
| p38 | –7.6 | 2 | Ser56, His64 | 2.82 3.03 | Ala34, Tyr35, Arg67, Thr68, Glu71, Asp168 |
| Properties | Predicted Value | Standard Value |
|---|---|---|
| Permeability of Caco-2 (log Papp in 10−6 cm/s) | 1.3 | Caco-2 permeability >0.90 (high) |
| Human intestinal absorption (%) | 93.8 | <30% (poorly absorbed) |
| Skin Permeability (log Kp) | –2.8 | Log Kp >–2.5 (low) |
| CYP3A4 inhibitor | No | |
| CYP1A2 inhibitor | Yes | |
| CYP2C19 inhibitor | Yes | |
| CYP2C9 inhibitor | No | |
| CYP2D6 inhibitor | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.-H.; Youn, K.; Jun, M. Discovery of Pinostrobin as a Melanogenic Agent in cAMP/PKA and p38 MAPK Signaling Pathway. Nutrients 2022, 14, 3713. https://doi.org/10.3390/nu14183713
Yoon J-H, Youn K, Jun M. Discovery of Pinostrobin as a Melanogenic Agent in cAMP/PKA and p38 MAPK Signaling Pathway. Nutrients. 2022; 14(18):3713. https://doi.org/10.3390/nu14183713
Chicago/Turabian StyleYoon, Jeong-Hyun, Kumju Youn, and Mira Jun. 2022. "Discovery of Pinostrobin as a Melanogenic Agent in cAMP/PKA and p38 MAPK Signaling Pathway" Nutrients 14, no. 18: 3713. https://doi.org/10.3390/nu14183713
APA StyleYoon, J.-H., Youn, K., & Jun, M. (2022). Discovery of Pinostrobin as a Melanogenic Agent in cAMP/PKA and p38 MAPK Signaling Pathway. Nutrients, 14(18), 3713. https://doi.org/10.3390/nu14183713