From Diabetes Care to Heart Failure Management: A Potential Therapeutic Approach Combining SGLT2 Inhibitors and Plant Extracts
Abstract
:1. Introduction
2. SGLT2 Inhibitors and Molecular Mechanisms of Cardioprotection
2.1. SGLT2 Inhibitors and Modulation of Metabolism in Heart Tissue
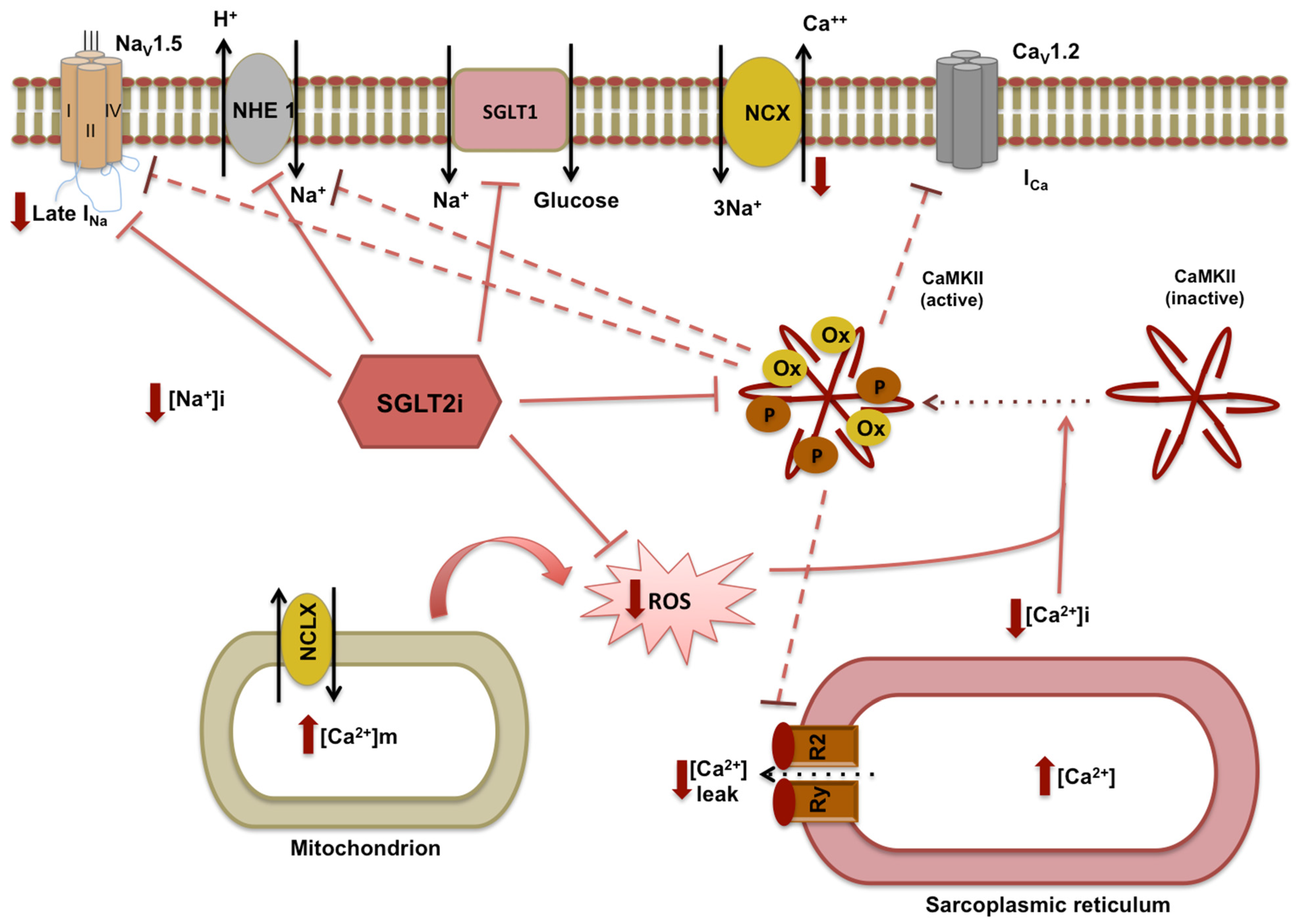
2.2. SGLT2i-Mediated Anti-Inflammatory, Antifibrotic, and Antioxidant Effects on the Heart
3. SGLT2i and Cardiometabolic Risk Factors
3.1. SGLT2i and Modulation of Lipid Metabolism
3.2. Haemodynamic and Vascular Effects
4. DCM and Cardiometabolic Risk: Can Plant Extract Supplementation Support SGLT2i Action?
4.1. Insulin Resistance
4.2. Antioxidant and Anti-Inflammatory Effects
4.3. Lipid Metabolism
4.4. Pleiotropic Effects of Plant Extracts in Counteracting Cardiometabolic Risk Factors and Heart Failure: Berberia Vulgaris and Citrus Bergamia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S17–S38. [Google Scholar] [CrossRef]
- Reid, J.B. Re: Registry. Can we talk. Radiol. Technol. 1990, 61, 307–308. [Google Scholar] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. S1), S83–S96. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Tendal, B.; Mustafa, R.A.; Vandvik, P.O.; Li, S.; Hao, Q.; Tunnicliffe, D.; Ruospo, M.; Natale, P.; Saglimbene, V.; et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2021, 372, m4573. [Google Scholar] [CrossRef] [PubMed]
- Larkin, H.D. FDA Expands Empagliflozin Heart Failure Indication. JAMA 2022, 327, 1219. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Thorvaldsen, T.; Ferrannini, G.; Mellbin, L.; Benson, L.; Cosentino, F.; McMurray, J.; Dahlström, U.; Lund, L.H.; Savarese, G. Eligibility for Dapagliflozin and Empagliflozin in a Real-world Heart Failure Population. J. Card. Fail. 2022, 28, 1050–1062. [Google Scholar] [CrossRef]
- Prattichizzo, F.; de Candia, P.; Ceriello, A. Diabetes and kidney disease: Emphasis on treatment with SGLT-2 inhibitors and GLP-1 receptor agonists. Metab. Clin. Exp. 2021, 120, 154799. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Butler, J.; Filippatos, G.; Januzzi, J.L.; Sumin, M.; Zwick, M.; Saadati, M.; Pocock, S.J.; Sattar, N.; et al. Effect of Empagliflozin on Circulating Proteomics in Heart Failure: Mechanistic Insights from the EMPEROR Program. Eur. Heart J. 2022; ehac495, Advance online publication. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, P.; Fu, L.; Sun, L.; Shen, W.; Wu, Q. Comparative Cardiovascular Outcomes of SGLT2 Inhibitors in Type 2 Diabetes Mellitus: A Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 802992. [Google Scholar] [CrossRef] [PubMed]
- Hoong, C.; Chua, M. SGLT2 Inhibitors as Calorie Restriction Mimetics: Insights on Longevity Pathways and Age-Related Diseases. Endocrinology 2021, 162, bqab079. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hirai, T.; Koya, D.; Kitada, M. Effects of SGLT2 Inhibitors on Atherosclerosis: Lessons from Cardiovascular Clinical Outcomes in Type 2 Diabetic Patients and Basic Researches. J. Clin. Med. 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Fragasso, G.; Petrie, M.; Mullens, W.; Ferrari, R.; Thum, T.; Bauersachs, J.; Anker, S.D.; Ray, R.; Çavuşoğlu, Y.; et al. Sodium-glucose co-transporter 2 inhibitors in heart failure: Beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Booij, H.G.; Koning, A.M.; van Goor, H.; de Boer, R.A.; Westenbrink, B.D. Selecting heart failure patients for metabolic interventions. Expert Rev. Mol. Diagn 2017, 17, 141–152. [Google Scholar] [CrossRef]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef]
- Bedi, K.C.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef]
- Yurista, S.R.; Silljé, H.H.W.; Oberdorf-Maass, S.U.; Schouten, E.M.; Pavez Giani, M.G.; Hillebrands, J.L.; van Goor, H.; van Veldhuisen, D.J.; de Boer, R.A.; Westenbrink, B.D. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur. J. Heart Fail. 2019, 21, 862–873. [Google Scholar] [CrossRef]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; et al. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl. Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Burhans, M.S.; Hagman, D.K.; Kuzma, J.N.; Schmidt, K.A.; Kratz, M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr. Physiol. 2018, 9, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Sukhanov, S.; Higashi, Y.; Yoshida, T.; Mummidi, S.; Aroor, A.R.; Jeffrey Russell, J.; Bender, S.B.; DeMarco, V.G.; Chandrasekar, B. The SGLT2 inhibitor Empagliflozin attenuates interleukin-17A-induced human aortic smooth muscle cell proliferation and migration by targeting TRAF3IP2/ROS/NLRP3/Caspase-1-dependent IL-1β and IL-18 secretion. Cell. Signal. 2021, 77, 109825. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Lee, S.G.; Kim, S.H.; Kim, J.H.; Choi, E.; Cho, W.; Rim, J.H.; Hwang, I.; Lee, C.J.; Lee, M.; et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat. Commun. 2020, 11, 2127. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.; Tilley, D.G. The Role of Leukocytes in Diabetic Cardiomyopathy. Front. Physiol. 2018, 9, 1547. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Santulli, G.; Gallinoro, E.; Cesaro, A.; Gragnano, F.; Sardu, C.; Mileva, N.; Foà, A.; Armillotta, M.; et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: A multicenter international registry. Cardiovasc. Diabetol. 2022, 21, 77. [Google Scholar] [CrossRef]
- Packer, M. Activation and Inhibition of Sodium-Hydrogen Exchanger Is a Mechanism That Links the Pathophysiology and Treatment of Diabetes Mellitus With That of Heart Failure. Circulation 2017, 136, 1548–1559. [Google Scholar] [CrossRef]
- Chen, S.; Coronel, R.; Hollmann, M.W.; Weber, N.C.; Zuurbier, C.J. Direct cardiac effects of SGLT2 inhibitors. Cardiovasc. Diabetol. 2022, 21, 45. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wüst, R.C.; Fiolet, J.W.; Stienen, G.J.; Coronel, R.; Zuurbier, C.J. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 2017, 60, 568–573. [Google Scholar] [CrossRef]
- Habibi, J.; Aroor, A.R.; Sowers, J.R.; Jia, G.; Hayden, M.R.; Garro, M.; Barron, B.; Mayoux, E.; Rector, R.S.; Whaley-Connell, A.; et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc. Diabetol. 2017, 16, 9. [Google Scholar] [CrossRef] [Green Version]
- Hammoudi, N.; Jeong, D.; Singh, R.; Farhat, A.; Komajda, M.; Mayoux, E.; Hajjar, R.; Lebeche, D. Empagliflozin Improves Left Ventricular Diastolic Dysfunction in a Genetic Model of Type 2 Diabetes. Cardiovasc. Drugs 2017, 31, 233–246. [Google Scholar] [CrossRef]
- Escudero, D.S.; Pérez, N.G.; Díaz, R.G. Myocardial Impact of NHE1 Regulation by Sildenafil. Front. Cardiovasc. Med. 2021, 8, 617519. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, C.R.; Chu, W.W.; Pu, J.; Foell, J.D.; Haworth, R.A.; Wolff, M.R.; Kamp, T.J.; Makielski, J.C. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J. Mol. Cell. Cardiol. 2005, 38, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Trum, M.; Riechel, J.; Wagner, S. Cardioprotection by SGLT2 Inhibitors-Does It All Come Down to Na+. Int. J. Mol. Sci. 2021, 22, 7976. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Cinti, F.; Moffa, S.; Impronta, F.; Cefalo, C.M.; Sun, V.A.; Sorice, G.P.; Mezza, T.; Giaccari, A. Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: Evidence to date. Drug Des. Devel. 2017, 11, 2905–2919. [Google Scholar] [CrossRef]
- Zinman, B.; Inzucchi, S.E.; Lachin, J.M.; Wanner, C.; Ferrari, R.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Kempthorne-Rawson, J.; Newman, J.; et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME™). Cardiovasc. Diabetol. 2014, 13, 102. [Google Scholar] [CrossRef]
- Szekeres, Z.; Toth, K.; Szabados, E. The Effects of SGLT2 Inhibitors on Lipid Metabolism. Metabolites 2021, 11, 87. [Google Scholar] [CrossRef]
- Hayashi, T.; Fukui, T.; Nakanishi, N.; Yamamoto, S.; Tomoyasu, M.; Osamura, A.; Ohara, M.; Yamamoto, T.; Ito, Y.; Hirano, T. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: Comparison with sitagliptin. Cardiovasc. Diabetol. 2017, 16, 8. [Google Scholar] [CrossRef]
- Kamijo, Y.; Ishii, H.; Yamamoto, T.; Kobayashi, K.; Asano, H.; Miake, S.; Kanda, E.; Urata, H.; Yoshida, M. Potential Impact on Lipoprotein Subfractions in Type 2 Diabetes. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419866811. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.H.; Li, H.; Shen, L.; Fu, J.J.; Ma, J.; Gu, Z.C.; Lin, H.W. High-dose sodium-glucose co-transporter-2 inhibitors are superior in type 2 diabetes: A meta-analysis of randomized clinical trials. Diabetes Obes. Metab. 2021, 23, 2125–2136. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Huang, W.; Han, Y.; Tan, H.; An, M.; Xiang, Q.; Zhou, R.; Yang, L.; Cheng, Y. Dapagliflozin Alleviates Hepatic Steatosis by Restoring Autophagy via the AMPK-mTOR Pathway. Front. Pharm. 2021, 12, 589273. [Google Scholar] [CrossRef]
- Ji, W.; Zhao, M.; Wang, M.; Yan, W.; Liu, Y.; Ren, S.; Lu, J.; Wang, B.; Chen, L. Effects of canagliflozin on weight loss in high-fat diet-induced obese mice. PLoS ONE 2017, 12, e0179960. [Google Scholar] [CrossRef]
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A.T.; Neve, B.; Torra, I.P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001, 7, 53–58. [Google Scholar] [CrossRef]
- Cai, X.; Yang, W.; Gao, X.; Chen, Y.; Zhou, L.; Zhang, S.; Han, X.; Ji, L. The Association Between the Dosage of SGLT2 Inhibitor and Weight Reduction in Type 2 Diabetes Patients: A Meta-Analysis. Obesity 2018, 26, 70–80. [Google Scholar] [CrossRef]
- Clamp, L.D.; Hume, D.J.; Lambert, E.V.; Kroff, J. Enhanced insulin sensitivity in successful, long-term weight loss maintainers compared with matched controls with no weight loss history. Nutr. Diabetes 2017, 7, e282. [Google Scholar] [CrossRef]
- Xu, L.; Ota, T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: Focus on fat browning and macrophage polarization. Adipocyte 2018, 7, 121–128. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Koike, Y.; Shirabe, S.I.; Maeda, H.; Yoshimoto, A.; Arai, K.; Kumakura, A.; Hirao, K.; Terauchi, Y. Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pr. 2019, 149, 140–146. [Google Scholar] [CrossRef]
- Singh, A.K.; Unnikrishnan, A.G.; Zargar, A.H.; Kumar, A.; Das, A.K.; Saboo, B.; Sinha, B.; Gangopadhyay, K.K.; Talwalkar, P.G.; Ghosal, S.; et al. Evidence-Based Consensus on Positioning of SGLT2i in Type 2 Diabetes Mellitus in Indians. Diabetes 2019, 10, 393–428. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, A.; Yokokawa, H.; Sanada, H.; Naito, T. Changes in Levels of Biomarkers Associated with Adipocyte Function and Insulin and Glucagon Kinetics During Treatment with Dapagliflozin Among Obese Type 2 Diabetes Mellitus Patients. Drugs R D 2016, 16, 255–261. [Google Scholar] [CrossRef]
- Sano, M. Sodium glucose cotransporter (SGLT)-2 inhibitors alleviate the renal stress responsible for sympathetic activation. Adv. Cardiovasc. Dis. 2020, 14, 1753944720939383. [Google Scholar] [CrossRef]
- Sasaki, T.; Sugawara, M.; Fukuda, M. Sodium-glucose cotransporter 2 inhibitor-induced changes in body composition and simultaneous changes in metabolic profile: 52-week prospective LIGHT (Luseogliflozin: The Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J. Diabetes Investig. 2019, 10, 108–117. [Google Scholar] [CrossRef]
- Cemerikić, D.; Wilcox, C.S.; Giebisch, G. Intracellular potential and K+ activity in rat kidney proximal tubular cells in acidosis and K+ depletion. J. Membr. Biol. 1982, 69, 159–165. [Google Scholar] [CrossRef]
- Layton, A.T.; Vallon, V.; Edwards, A. Predicted consequences of diabetes and SGLT inhibition on transport and oxygen consumption along a rat nephron. Am. J. Physiol. Ren. Physiol. 2016, 310, F1269–F1283. [Google Scholar] [CrossRef]
- Wilcox, C.S. Antihypertensive and Renal Mechanisms of SGLT2 (Sodium-Glucose Linked Transporter 2) Inhibitors. Hypertension 2020, 75, 894–901. [Google Scholar] [CrossRef]
- Varzideh, F.; Kansakar, U.; Santulli, G. SGLT2 inhibitors in cardiovascular medicine. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, e67–e68. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Cooper, M.E.; Tikkanen, I.; Pfarr, E.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Lund, S.S. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018, 93, 231–244. [Google Scholar] [CrossRef]
- Thiele, K.; Rau, M.; Hartmann, N.K.; Möller, M.; Möllmann, J.; Jankowski, J.; Keszei, A.P.; Böhm, M.; Floege, J.; Marx, N.; et al. Empagliflozin reduces markers of acute kidney injury in patients with acute decompensated heart failure. ESC Heart Fail. 2022, 9, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Fu, Y.; Patel, R.; Darshi, M.; Crespo-Masip, M.; Huang, W.; Song, P.; Freeman, B.; Kim, Y.C.; Soleimani, M.; et al. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am. J. Physiol.-Ren. Physiol. 2020, 319, F712–F728. [Google Scholar] [CrossRef] [PubMed]
- Lipkowitz, M.S. Regulation of uric acid excretion by the kidney. Curr. Rheumatol. Rep. 2012, 14, 179–188. [Google Scholar] [CrossRef]
- Gisler, S.M.; Madjdpour, C.; Bacic, D.; Pribanic, S.; Taylor, S.S.; Biber, J.; Murer, H. PDZK1: II. an anchoring site for the PKA-binding protein D-AKAP2 in renal proximal tubular cells. Kidney Int. 2003, 64, 1746–1754. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Shen, W.; Boulton, D.W.; Leslie, B.R.; Griffen, S.C. Interaction Between the Sodium-Glucose-Linked Transporter 2 Inhibitor Dapagliflozin and the Loop Diuretic Bumetanide in Normal Human Subjects. J. Am. Heart Assoc. 2018, 7, e007046. [Google Scholar] [CrossRef]
- Cristóbal-García, M.; García-Arroyo, F.E.; Tapia, E.; Osorio, H.; Arellano-Buendía, A.S.; Madero, M.; Rodríguez-Iturbe, B.; Pedraza-Chaverrí, J.; Correa, F.; Zazueta, C.; et al. Renal oxidative stress induced by long-term hyperuricemia alters mitochondrial function and maintains systemic hypertension. Oxid. Med. Cell. Longev. 2015, 2015, 535686. [Google Scholar] [CrossRef]
- Rahman, A.; Fujisawa, Y.; Nakano, D.; Hitomi, H.; Nishiyama, A. Effect of a selective SGLT2 inhibitor, luseogliflozin, on circadian rhythm of sympathetic nervous function and locomotor activities in metabolic syndrome rats. Clin. Exp. Pharm. Physiol. 2017, 44, 522–525. [Google Scholar] [CrossRef]
- Li, C.Y.; Wang, L.X.; Dong, S.S.; Hong, Y.; Zhou, X.H.; Zheng, W.W.; Zheng, C. Phlorizin Exerts Direct Protective Effects on Palmitic Acid (PA)-Induced Endothelial Dysfunction by Activating the PI3K/AKT/eNOS Signaling Pathway and Increasing the Levels of Nitric Oxide (NO). Med. Sci. Monit. Basic. Res. 2018, 24, 1–9. [Google Scholar] [CrossRef]
- Aroor, A.R.; Das, N.A.; Carpenter, A.J.; Habibi, J.; Jia, G.; Ramirez-Perez, F.I.; Martinez-Lemus, L.; Manrique-Acevedo, C.M.; Hayden, M.R.; Duta, C.; et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc. Diabetol. 2018, 17, 108. [Google Scholar] [CrossRef]
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Har, R.; Fagan, N.; Johansen, O.E.; Woerle, H.J.; von Eynatten, M.; Broedl, U.C. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc. Diabetol. 2014, 13, 28. [Google Scholar] [CrossRef]
- Wei, R.; Wang, W.; Pan, Q.; Guo, L. Effects of SGLT-2 Inhibitors on Vascular Endothelial Function and Arterial Stiffness in Subjects With Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 826604. [Google Scholar] [CrossRef]
- Sposito, A.C.; Breder, I.; Soares, A.; Kimura-Medorima, S.T.; Munhoz, D.B.; Cintra, R.; Bonilha, I.; Oliveira, D.C.; Breder, J.C.; Cavalcante, P.; et al. Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: A randomized active-controlled trial. Cardiovasc. Diabetol. 2021, 20, 74. [Google Scholar] [CrossRef]
- Park, S.H.; Belcastro, E.; Hasan, H.; Matsushita, K.; Marchandot, B.; Abbas, M.; Toti, F.; Auger, C.; Jesel, L.; Ohlmann, P.; et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: Protective effect of gliflozins. Cardiovasc. Diabetol. 2021, 20, 65. [Google Scholar] [CrossRef]
- Mone, P.; Lombardi, A.; Kansakar, U.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; De Gennaro, S.; Famiglietti, M.; Macina, G.; Frullone, S.; et al. Empagliflozin improves the microRNA signature of endothelial dysfunction in patients with HFpEF and diabetes. J. Pharmacol. Exp. Ther. 2022, 382, JPET-AR-2022-001251. [Google Scholar] [CrossRef]
- Mone, P.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Lombardi, A.; Frullone, S.; Santulli, G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights From Frail Hypertensive and Diabetic Patients. Hypertension 2022, 79, 1633–1643. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Zhao, Y.B.; Li, C.M.; Chen, D.M.; Wang, G.P.; Chang, R.F.; Shu, H.R. Potential polyphenol markers of phase change in apple (Malus domestica). J. Plant Physiol. 2007, 164, 574–580. [Google Scholar] [CrossRef]
- Mollace, V.; Rosano, G.M.C.; Anker, S.D.; Coats, A.J.S.; Seferovic, P.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; et al. Pathophysiological Basis for Nutraceutical Supplementation in Heart Failure: A Comprehensive Review. Nutrients 2021, 13, 257. [Google Scholar] [CrossRef]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant Extracts for Type 2 Diabetes: From Traditional Medicine to Modern Drug Discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Cho, H.; Thorvaldsen, J.L.; Chu, Q.; Feng, F.; Birnbaum, M.J. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 2001, 276, 38349–38352. [Google Scholar] [CrossRef]
- Dong, X.C.; Copps, K.D.; Guo, S.; Li, Y.; Kollipara, R.; DePinho, R.A.; White, M.F. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008, 8, 65–76. [Google Scholar] [CrossRef]
- Schmoll, D.; Walker, K.S.; Alessi, D.R.; Grempler, R.; Burchell, A.; Guo, S.; Walther, R.; Unterman, T.G. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J. Biol. Chem. 2000, 275, 36324–36333. [Google Scholar] [CrossRef]
- Cook, J.R.; Langlet, F.; Kido, Y.; Accili, D. Pathogenesis of selective insulin resistance in isolated hepatocytes. J. Biol. Chem. 2015, 290, 13972–13980. [Google Scholar] [CrossRef] [Green Version]
- Nakae, J.; Kitamura, T.; Silver, D.L.; Accili, D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Invest. 2001, 108, 1359–1367. [Google Scholar] [CrossRef]
- He, L.; Li, Y.; Zeng, N.; Stiles, B.L. Regulation of basal expression of hepatic PEPCK and G6Pase by AKT2. Biochem. J. 2020, 477, 1021–1031. [Google Scholar] [CrossRef]
- De Las Heras, N.; Valero-Muñoz, M.; Martín-Fernández, B.; Ballesteros, S.; López-Farré, A.; Ruiz-Roso, B.; Lahera, V. Molecular factors involved in the hypolipidemic- and insulin-sensitizing effects of a ginger (Zingiber officinale Roscoe) extract in rats fed a high-fat diet. Appl. Physiol. Nutr. Metab. 2017, 42, 209–215. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Makboul, R.M.; Al-Mokhtar, M.A.; Nicola, M.A. Polyphenol-rich Boswellia serrata gum prevents cognitive impairment and insulin resistance of diabetic rats through inhibition of GSK3β activity, oxidative stress and pro-inflammatory cytokines. Biomed. Pharm. 2019, 109, 281–292. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; El Ashry, F.E.; El Maraghy, N.N.; Fahmy, A. Studies on the antidiabetic activities of Momordica charantia fruit juice in streptozotocin-induced diabetic rats. Pharm. Biol. 2017, 55, 758–765. [Google Scholar] [CrossRef]
- Ngueguim, F.T.; Esse, E.C.; Dzeufiet, P.D.; Gounoue, R.K.; Bilanda, D.C.; Kamtchouing, P.; Dimo, T. Oxidised palm oil and sucrose induced hyperglycemia in normal rats: Effects of Sclerocarya birrea stem barks aqueous extract. BMC Complement. Altern. Med. 2016, 16, 47. [Google Scholar] [CrossRef]
- Mousum, S.A.; Ahmed, S.; Gawali, B.; Kwatra, M.; Ahmed, A.; Lahkar, M. Nyctanthes arbor-tristis leaf extract ameliorates hyperlipidemia- and hyperglycemia-associated nephrotoxicity by improving anti-oxidant and anti-inflammatory status in high-fat diet-streptozotocin-induced diabetic rats. Inflammopharmacology 2018, 26, 1415–1428. [Google Scholar] [CrossRef]
- Shim, D.W.; Han, J.W.; Ji, Y.E.; Shin, W.Y.; Koppula, S.; Kim, M.K.; Kim, T.K.; Park, P.J.; Kang, T.B.; Lee, K.H. Cichorium intybus Linn. Extract Prevents Type 2 Diabetes Through Inhibition of NLRP3 Inflammasome Activation. J. Med. Food 2016, 19, 310–317. [Google Scholar] [CrossRef]
- Pellegrini, C.; Fornai, M.; Antonioli, L.; Blandizzi, C.; Calderone, V. Phytochemicals as Novel Therapeutic Strategies for NLRP3 Inflammasome-Related Neurological, Metabolic, and Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 2876. [Google Scholar] [CrossRef]
- Lee, H.; Choi, J.; Shin, S.S.; Yoon, M. Effects of Korean red ginseng (Panax ginseng) on obesity and adipose inflammation in ovariectomized mice. J. Ethnopharmacol. 2016, 178, 229–237. [Google Scholar] [CrossRef]
- Kozics, K.; Klusová, V.; Srančíková, A.; Mučaji, P.; Slameňová, D.; Hunáková, L.; Kusznierewicz, B.; Horváthová, E. Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 2013, 141, 2198–2206. [Google Scholar] [CrossRef]
- Govindaraj, J.; Sorimuthu Pillai, S. Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: Streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2015, 404, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Fernandes, D.; Silva, I.; Mateus, V. Potential Anti-Inflammatory Effect of Rosmarinus officinalis in Preclinical In Vivo Models of Inflammation. Molecules 2022, 27, 609. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pathak, S.; Gupta, G.; Sharma, S.K.; Singh, L.; Sharma, R.K.; Mishra, A.; Dua, K. Pharmacological evaluation of aqueous extract of syzigium cumini for its antihyperglycemic and antidyslipidemic properties in diabetic rats fed a high cholesterol diet-Role of PPARγ and PPARα. Biomed. Pharm. 2017, 89, 447–453. [Google Scholar] [CrossRef]
- Arayne, M.S.; Sultana, N.; Bahadur, S.S. The berberis story: Berberis vulgaris in therapeutics. Pak. J. Pharm. Sci. 2007, 20, 83–92. [Google Scholar]
- Tomosaka, H.; Chin, Y.W.; Salim, A.A.; Keller, W.J.; Chai, H.; Kinghorn, A.D. Antioxidant and cytoprotective compounds from Berberis vulgaris (barberry). Phytother. Res. 2008, 22, 979–981. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Qaredashi, R.; Hashemzaei, M.; Hosseinzadeh, H. Inhibitory Effect of Berberis vulgaris Aqueous Extract on Acquisition and Reinstatement Effects of Morphine in Conditioned Place Preferences (CPP) in Mice. Jundishapur. J. Nat. Pharm. Prod. 2014, 9, e16145. [Google Scholar] [CrossRef]
- Grundy, S.M.; Vega, G.L.; Yuan, Z.; Battisti, W.P.; Brady, W.E.; Palmisano, J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial). Am. J. Cardiol. 2005, 95, 462–468. [Google Scholar] [CrossRef]
- Vergès, B. Fenofibrate therapy and cardiovascular protection in diabetes: Recommendations after FIELD. Curr. Opin. Lipidol. 2006, 17, 653–658. [Google Scholar] [CrossRef]
- Lin, J.; Cai, Q.; Liang, B.; Wu, L.; Zhuang, Y.; He, Y.; Lin, W. Berberine, a Traditional Chinese Medicine, Reduces Inflammation in Adipose Tissue, Polarizes M2 Macrophages, and Increases Energy Expenditure in Mice Fed a High-Fat Diet. Med. Sci. Monit. 2019, 25, 87–97. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yu, R.Y.; Liu, Y.H.; Tu, X.Y.; Tu, J.; Wang, Y.S.; Xu, G.L. Interaction of baicalin with berberine for glucose uptake in 3T3-L1 adipocytes and HepG2 hepatocytes. J. Ethnopharmacol. 2014, 151, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Gardana, C.; Nalin, F.; Simonetti, P. Evaluation of flavonoids and furanocoumarins from Citrus bergamia (Bergamot) juice and identification of new compounds. Molecules 2008, 13, 2220–2228. [Google Scholar] [CrossRef] [PubMed]
- Salerno, R.; Casale, F.; Calandruccio, C.; Procopio, A. Characterization of flavonoids in Citrus bergamia (Bergamot) polyphenolic fraction by liquid chromatography–high resolution mass spectrometry (LC/HRMS). Pharma Nutr. 2016, 4, S1–S7. [Google Scholar] [CrossRef]
- Mollace, V.; Ragusa, S.; Sacco, I.; Muscoli, C.; Sculco, F.; Visalli, V.; Palma, E.; Muscoli, S.; Mondello, L.; Dugo, P.; et al. The protective effect of bergamot oil extract on lecitine-like oxyLDL receptor-1 expression in balloon injury-related neointima formation. J. Cardiovasc. Pharm. 2008, 13, 120–129. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Gliozzi, M.; Carresi, C.; Musolino, V.; Palma, E.; Muscoli, C.; Vitale, C.; Gratteri, S.; Muscianisi, G.; Janda, E.; Muscoli, S.; et al. The effect of bergamot-derived polyphenolic fraction on LDL small dense particles and non-alcoholic fatty liver disease in patients with metabolic syndrome. Adv. Biol. Chem. 2014, 4, 129–137. [Google Scholar] [CrossRef]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Scicchitano, M.; Scarano, F.; Macrì, R.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.C.; Mollace, R.; Guarnieri, L.; et al. The Potential Properties of Natural Compounds in Cardiac Stem Cell Activation: Their Role in Myocardial Regeneration. Nutrients 2021, 13, 275. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gliozzi, M.; Macrì, R.; Coppoletta, A.R.; Musolino, V.; Carresi, C.; Scicchitano, M.; Bosco, F.; Guarnieri, L.; Cardamone, A.; Ruga, S.; et al. From Diabetes Care to Heart Failure Management: A Potential Therapeutic Approach Combining SGLT2 Inhibitors and Plant Extracts. Nutrients 2022, 14, 3737. https://doi.org/10.3390/nu14183737
Gliozzi M, Macrì R, Coppoletta AR, Musolino V, Carresi C, Scicchitano M, Bosco F, Guarnieri L, Cardamone A, Ruga S, et al. From Diabetes Care to Heart Failure Management: A Potential Therapeutic Approach Combining SGLT2 Inhibitors and Plant Extracts. Nutrients. 2022; 14(18):3737. https://doi.org/10.3390/nu14183737
Chicago/Turabian StyleGliozzi, Micaela, Roberta Macrì, Anna Rita Coppoletta, Vincenzo Musolino, Cristina Carresi, Miriam Scicchitano, Francesca Bosco, Lorenza Guarnieri, Antonio Cardamone, Stefano Ruga, and et al. 2022. "From Diabetes Care to Heart Failure Management: A Potential Therapeutic Approach Combining SGLT2 Inhibitors and Plant Extracts" Nutrients 14, no. 18: 3737. https://doi.org/10.3390/nu14183737







