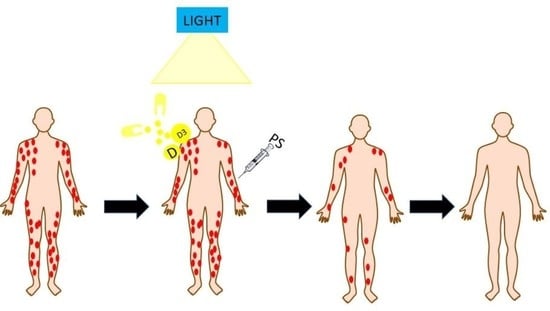
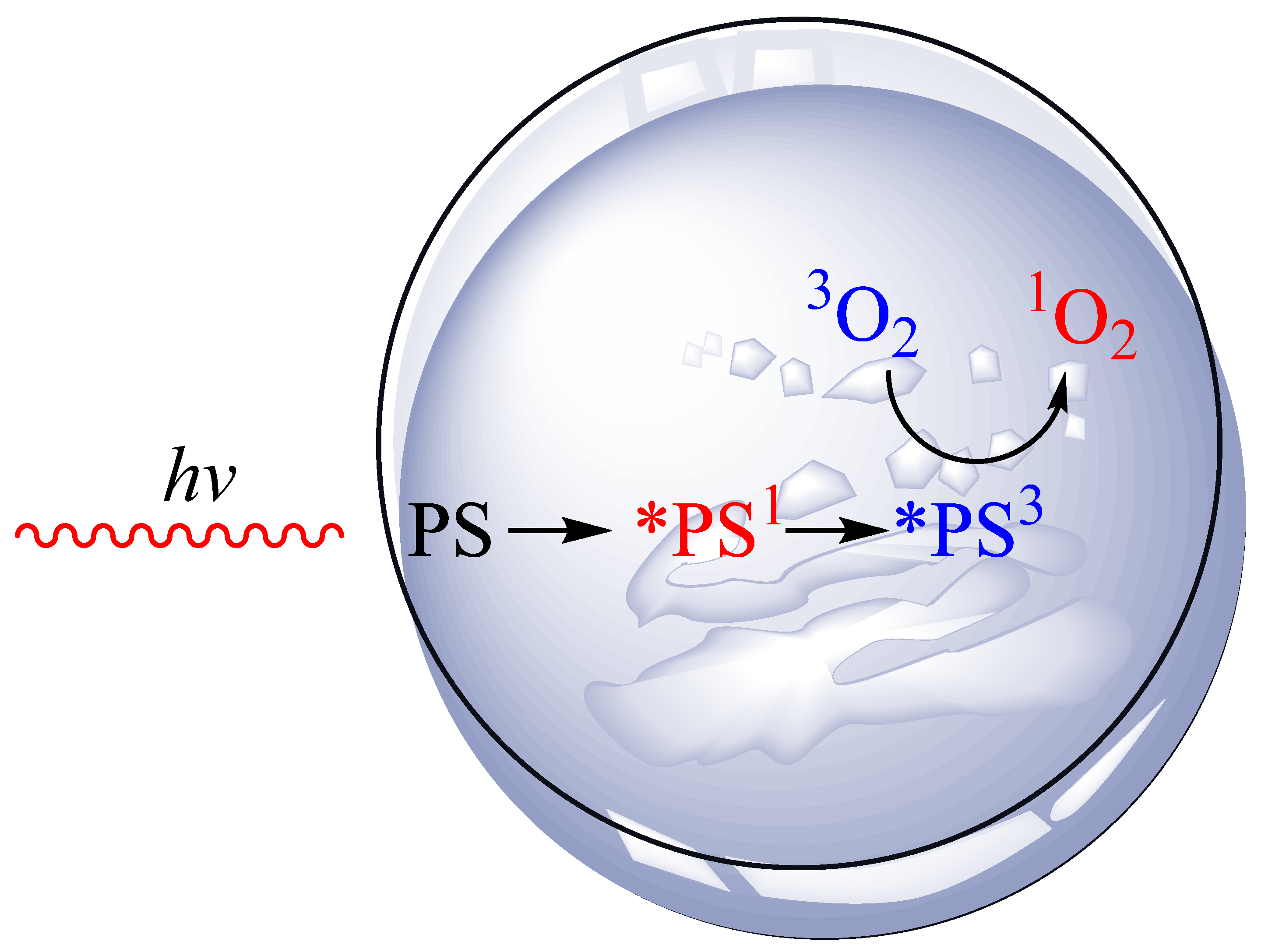
Vitamin D and Vitamin D3 Supplementation during Photodynamic Therapy: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Select Criteria
- vitamin D supplemented or administered during PDT treatment;
- analysis of the effect of synthetic vitamin D supplementation on the effectiveness of PDT;
- clinical and experimental studies of the effect of vitamin D supplementation on the effectiveness of PDT in animal studies or in in vitro studies on cell lines;
- dermatological diseases, cancer treatment, and treatment of internal organ tumors
- no analysis of the relationship between vitamin D supplementation and PDT effectiveness
- conducting photochemotherapy
2.2. Data Extraction
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Results of Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitiz-ers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, I.O.; Nunes, J.; Figueiró Longo, J.P.; Muehlmann, L.A.; Azevedo, R.B. Photodynamic therapy in super-ficial basal cell carcinoma treatment. Photodiagn. Photodyn. Ther. 2019, 27, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, S.R.; Brianti, P.; Dattola, A.; Bennardo, L.; Silvestri, M.; Schipani, G.; Nisticò, S.P. CO2 laser and photodynamic therapy: Study of efficacy in periocular BCC. Dermatol. Ther. 2018, 31, e12616. [Google Scholar] [CrossRef] [PubMed]
- Fusano, M.; Zane, C.; Calzavara-Pinton, P.; Bencini, P.L. Photodynamic therapy for actinic keratosis in vegan and omnivore patients: The role of diet on skin healing. J. Dermatolog. Treat. 2021, 32, 78–83. [Google Scholar] [CrossRef]
- Calin, M.A.; Diaconeasa, A.; Savastru, D.; Tautan, M. Photosensitizers and light sources for photodynamic therapy of the Bow-en’s disease. Arch. Dermatol. Res. 2011, 303, 145–151. [Google Scholar] [CrossRef]
- Umegaki, N.; Moritsugu, R.; Katoh, S.; Harada, K.; Nakano, H.; Tamai, K.; Hanada, K.; Tanaka, M. Photodynamic therapy may be useful in debulking cutaneous lymphoma prior to radiotherapy. Clin. Exp. Dermatol. 2004, 29, 42–45. [Google Scholar] [CrossRef]
- Fonda-Pascual, P.; Fernandez-Gonzalez, P.; Sanchez-Los Arcos, L.; Alcantara-Nicolas, F.; Lopez-Galan, C.; Canseco-Martin, M.; Vidal-Asensi, S. Treatment of cutaneous Kaposi sarcoma with methylaminolevulinate photodynamic therapy: A case series. Photodermatol. Photoimmunol. Photomed. 2020, 36, 392–395. [Google Scholar] [CrossRef]
- Osuchowski, M.; Osuchowski, F.; Latos, W.; Kawczyk-Krupka, A. The Use of Upconversion Nanoparticles in Prostate Cancer Photodynamic Therapy. Life 2021, 11, 360. [Google Scholar] [CrossRef]
- Ikeda, N.; Usuda, J.; Maehara, S. Photodynamic therapy for central-type early-stage lung cancer. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 679–683. [Google Scholar] [CrossRef]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The potential of photodynamic therapy in current breast cancer treatment methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Ożóg, Ł.; Domka, W.; Aebisher, D. Rose Bengal and Future Directions in Larynx Tumor Photodynamic Therapy. Photochem. Photobiol. 2021, 97, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Railkar, R.; Agarwal, P.K. Photodynamic Therapy in the Treatment of Bladder Cancer: Past Challenges and Current Innova-tions. Eur. Urol. Focus. 2018, 4, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Adelzadeh, L.; Wu, J.J. Photodynamic therapy for psoriasis. J Dermatolog. Treat. 2015, 26, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Boen, M.; Brownell, J.; Patel, P.; Tsoukas, M.M. The Role of Photodynamic Therapy in Acne: An Evidence-Based Review. Am. J. Clin. Dermatol. 2017, 18, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Del Duca, E.; Manfredini, M.; Petrini, N.; Farnetani, F.; Chester, J.; Bennardo, L.; Schipani, G.; Tamburi, F.; Sannino, M.; Canna-rozzo, G.; et al. Daylight photodynamic therapy with 5-aminolevulinic acid 5% gel for the treatment of mild-to-moderate inflammatory acne. Ital. J. Dermatol. Venerol. 2021, 156, 46–50. [Google Scholar] [CrossRef]
- Marotti, J.; Aranha, A.C.; Eduardo Cde, P.; Ribeiro, M.S. Photodynamic therapy can be effective as a treatment for herpes sim-plex labialis. Photomed. Laser Surg. 2009, 27, 357–363. [Google Scholar] [CrossRef]
- Akilov, O.E.; Kosaka, S.; O’Riordan, K.; Hasan, T. Parasiticidal effect of delta-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp. Dermatol. 2007, 16, 651–660. [Google Scholar] [CrossRef]
- Exadaktylou, D.; Kurwa, H.A.; Calonje, E.; Barlow, R.J. Treatment of Darier’s disease with photodynamic therapy. Br. J. Derma-tol. 2003, 149, 606–610. [Google Scholar] [CrossRef]
- Zhao, Y.; Tu, P.; Zhou, G.; Zhou, Z.; Lin, X.; Yang, H.; Lu, Z.; Gao, T.; Tu, Y.; Xie, H.; et al. Hemo-porfin Photodynamic Therapy for Port-Wine Stain: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0156219. [Google Scholar] [CrossRef]
- Acedo, P.; Stockert, J.C.; Cañete, M.; Villanueva, A. Two combined photosensitizers: A goal for more effective photodynamic therapy of cancer. Cell Death Dis. 2014, 5, e1122. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.; Oliveira, T.C.; Gomes, V.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene blue photodynamic therapy induces selective and massive cell death in human breast can-cer cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kes-sel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Marcus, S.L. Photodynamic therapy. Eur. J. Cancer 1992, 28A, 1734–1742. [Google Scholar] [CrossRef]
- Czarnecka-Czapczyńska, M.; Aebisher, D.; Oleś, P.; Sosna, B.; Krupka-Olek, M.; Dynarowicz, K.; Latos, W.; Cieślar, G.; Kaw-czyk-Krupka, A. The role of photodynamic therapy in breast cancer-A review of in vitro research. Biomed. Pharmacother. 2021, 144, 112342. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Braathen, L.R.; Morton, C.A.; Basset-Seguin, N.; Bissonnette, R.; Gerritsen, M.J.; Gilaberte, Y.; Calzavara-Pinton, P.; Sidoroff, A.; Wulf, H.C.; Szeimies, R.M. Photodynamic therapy for skin field cancerization: An international consensus. International Soci-ety for Photodynamic Therapy in Dermatology. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1063–1066. [Google Scholar] [CrossRef]
- Collier, N.J.; Haylett, A.K.; Wong, T.H.; Morton, C.A.; Ibbotson, S.H.; McKenna, K.E.; Mallipeddi, R.; Moseley, H.; Seukeran, D.; Ward, K.A.; et al. Conventional and combination topical photodynamic therapy for basal cell carcinoma: Systematic review and meta-analysis. Br. J. Dermatol. 2018, 179, 1277–1296. [Google Scholar] [CrossRef]
- O’Connell, K.A.; Okhovat, J.P.; Zeitouni, N.C. Photodynamic therapy for Bowen’s Disease (squamous cell carcinoma in situ) current review and update. Photodiagnosis Photodyn. Ther. 2018, 24, 109–114. [Google Scholar] [CrossRef]
- Ming, L.; Cheng, K.; Chen, Y.; Yang, R.; Chen, D. Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Med. 2021, 10, 257–268. [Google Scholar] [CrossRef]
- Kubrak, T.; Karakuła, M.; Czop, M.; Kawczyk-Krupka, A.; Aebisher, D. Advances in Management of Bladder Cancer-The Role of Photodynamic Therapy. Molecules 2022, 27, 731. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhang, Y.; He, Y.; Xiong, M.; Huang, H.; Pei, S.; Liao, J.; Wang, Y.; Shao, D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf. B Biointerfaces 2019, 180, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Sun, W.; Fan, J.; Du, J.; Peng, X. An estrogen receptor targeted ruthenium complex as a two-photon photody-namic therapy agent for breast cancer cells. Chem. Commun. 2018, 54, 7038–7041. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, X.; Dong, X.; Li, B. Nano-photosensitizers for enhanced photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 36, 102597. [Google Scholar] [CrossRef]
- Zhu, T.; Shi, L.; Yu, C.; Dong, Y.; Qiu, F.; Shen, L.; Qian, Q.; Zhou, G.; Zhu, X. Ferroptosis Promotes Photodynamic Therapy: Supramolecular Photosensitizer-Inducer Nanodrug for Enhanced Cancer Treatment. Theranostics 2019, 9, 3293–3307. [Google Scholar] [CrossRef]
- Maytin, E.V.; Honari, G.; Khachemoune, A.; Taylor, C.R.; Ortel, B.; Pogue, B.W.; Sznycer-Taub, N.; Hasan, T. Vitamin D Com-bined with Aminolevulinate (ALA)-Mediated Photodynamic Therapy (PDT) for Human Psoriasis: A Proof-of-Principle Study. Isr. J. Chem. 2012, 52, 767–775. [Google Scholar] [CrossRef]
- Bullock, T.A.; Negrey, J.; Hu, B.; Warren, C.B.; Hasan, T.; Maytin, E.V. Significant improvement of facial actinic keratoses after blue light photodynamic therapy with oral vitamin D pretreatment: An interventional cohort-controlled trial. J. Am. Acad. Der-matol. 2022, 87, 80–86. [Google Scholar] [CrossRef]
- Moreno, R.; Nájera, L.; Mascaraque, M.; Juarranz, Á.; González, S.; Gilaberte, Y. Influence of Serum Vitamin D Level in the Response of Actinic Keratosis to Photodynamic Therapy with Methylaminolevulinate. J. Clin. Med. 2020, 9, 398. [Google Scholar] [CrossRef]
- Anand, S.; Wilson, C.; Hasan, T.; Maytin, E.V. Vitamin D3 enhances the apoptotic response of epithelial tumors to ami-nolevulinate-based photodynamic therapy. Cancer Res. 2011, 71, 6040–6050. [Google Scholar] [CrossRef]
- Matsuyama, A.; Nakano, H.; Harada, K.; Yamazaki, T.; Kanno, T.; Wakui, M.; Hanada, K. Enhancement of photodynamic effect in normal rat keratinocytes by treatment with 1,25 dihydroxy vitamin D3. Photodermatol. Photoimmunol. Photomed. 2003, 19, 303–308. [Google Scholar] [CrossRef]
- Gerstmeier, J.; Possmayer, A.L.; Bozkurt, S.; Hoffmann, M.E.; Dikic, I.; Herold-Mende, C.; Burger, M.C.; Munch, C.; Kogel, D.; Linder, B. Calcitriol promotes differentiation of glioma stem-like cells and increases their susceptibility to Temozolomide. Cancers 2021, 13, 3577. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, R.; Li, Y.; Zhang, Q.; Cai, X.; Li, L. Calcitriol enhances the effect of photodynamic therapy in human breast can-cer. Off. J. Balk. Union Oncol. 2016, 21, 1068–1075. [Google Scholar]
- Chen, X.; Wang, C.; Teng, L.; Liu, Y.; Chen, X.; Yang, G.; Wang, L.; Liu, H.; Liu, Z.; Zhang, D.; et al. Calcitriol enhances 5-aminolevulinic acid-induced fluorescence and the effect of photodynamic therapy in human glioma. Acta Oncol. 2014, 53, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Parry, P.V.; Engh, J.A. Calcitriol enhances 5-aminolevulinic acid-induced fluorescence and the effect of photodynamic therapy in human glioma. Neurosurgery 2014, 74, N8–N9. [Google Scholar] [CrossRef]
- Cicarma, E.; Tuorkey, M.; Juzeniene, A.; Ma, L.W.; Moan, J. Calcitriol treatment improves methyl aminolaevulinate-based photodynamic therapy in human squamous cell carcinoma A431 cells. Br. J. Dermatol. 2009, 161, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ortel, B.; Sharlin, D.; O’Donnell, D.; Sinha, A.K.; Maytin, E.V.; Hasan, T. Differentiation enhances aminolevulinic ac-id-dependent photodynamic treatment of LNCaP prostate cancer cells. Br. J. Cancer 2002, 87, 1321–1327. [Google Scholar] [CrossRef]
- Anand, S.; Rollakanti, K.R.; Horst, R.L.; Hasan, T.; Maytin, E.V. Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma. Photochem. Photobiol. 2014, 90, 1126–1135. [Google Scholar] [CrossRef]
- Rollakanti, K.R.; Anand, S.; Maytin, E.V. Vitamin D enhances the efficacy of photodynamic therapy in a murine model of breast cancer. Cancer Med. 2015, 4, 633–642. [Google Scholar] [CrossRef]
- Yang, D.F.; Chen, J.H.; Chiang, C.P.; Huang, Z.; Lee, J.W.; Liu, C.J.; Chang, J.L.; Hsu, Y.C. Improve efficacy of topical ALA-PDT by calcipotriol through up-regulation of coproporphyrinogen oxidase. Photodiagn. Photodyn. Ther. 2014, 11, 331–341. [Google Scholar] [CrossRef]
- Rollakanti, K.; Anand, S.; Maytin, E.V. Topical calcitriol prior to photodynamic therapy enhances treatment efficacy in non-melanoma skin cancer mouse models. Proc. SPIE Int. Soc. Opt. Eng. 2015, 9308, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Galimberti, G.N. Calcipotriol as pretreatment prior to daylight-mediated photodynamic therapy in patients with actinic keratosis: A case series. Photodiagn. Photodyn. Ther. 2018, 21, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Borgia, F.; Riso, G.; Catalano, F.; Coppola, M.; Giuffrida, R.; Cannavò, S.P. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: An intra-patient randomized study. Photodiagn. Photodyn. Ther. 2020, 31, 101803. [Google Scholar] [CrossRef]
- Giuffrida, R.; Borgia, F.; Marafioti, I.; Riso, G.; Cannavò, S.P. Combination of tacalcitol ointment and photodynamic therapy for the treatment of follicular mucinosis of the scalp. Photodiagn. Photodyn. Ther. 2019, 27, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Song, K.H. Topical calcipotriol before ablative fractional laser-assisted photodynamic therapy enhances treatment outcomes for actinic keratosis in Fitzpatrick grades III-V skin: A prospective randomized clinical trial. J. Am. Acad. Dermatol. 2018, 78, 795–797. [Google Scholar] [CrossRef]
- Piaserico, S.; Piccioni, A.; Gutiérrez Garcìa-Rodrigo, C.; Sacco, G.; Pellegrini, C.; Fargnoli, M.C. Sequential treatment with calcitriol and methyl aminolevulinate-daylight photodynamic therapy for patients with multiple actinic keratoses of the upper extremities. Photodiagn. Photodyn. Ther. 2021, 34, 102325. [Google Scholar] [CrossRef]
- Torezan, L.; Grinblat, B.; Haedersdal, M.; Festa-Neto, C.; Szeimies, R.M. A 12-month follow-up split-scalp study comparing calcipotriol-assisted MAL-PDT with conventional MAL-PDT for the treatment of actinic keratosis: A randomized controlled trial. Eur. J. Dermatol. 2021, 31, 638–644. [Google Scholar] [CrossRef]
- Torezan, L.; Grinblat, B.; Haedersdal, M.; Valente, N.; Festa-Neto, C.; Szeimies, R.M. A randomized split-scalp study compar-ing calcipotriol-assisted methyl aminolaevulinate photodynamic therapy (MAL-PDT) with conventional MAL-PDT for the treatment of actinic keratosis. Br. J. Dermatol. 2018, 179, 829–835. [Google Scholar] [CrossRef]
- Albahrani, A.A.; Greaves, R.F. Fat-Soluble Vitamins: Clinical Indications and Current Challenges for Chromatographic Measurement. Clin. Biochem. Rev. 2016, 37, 27–47. [Google Scholar]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Walicka, M.; Tałałaj, M.; Horst-Sikorska, W.; Ignaszak-Szczepaniak, M.; Sewerynek, E. Vitamin D supplementation in adults-guidelines. Endokrynol. Pol. 2010, 61, 723–729. [Google Scholar] [PubMed]
- Karczmarewicz, E.; Łukaszkiewicz, J.; Lorenc, R. Vitamin D-metabolism, action, requirements and treatment strategies. Stand. Med. 2007, 4, 137–142. [Google Scholar]
- Jones, G. Expanding role for vitamin D in chronic kidney disease: Importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3). Semin. Dial. 2007, 20, 316–324. [Google Scholar] [CrossRef]
- Kechichian, E.; Ezzedine, K. Vitamin D and the Skin: An Update for Dermatologists. Am. J. Clin. Dermatol. 2018, 19, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psori-asis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Maytin, E.V.; Hasan, T. Vitamin D and Other Differentiation-promoting Agents as Neoadjuvants for Photodynamic Therapy of Cancer. Photochem. Photobiol. 2020, 96, 529–538. [Google Scholar] [CrossRef]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef]
- Ortel, B.; Jabeen, S.; Greer, A. Adjuvants that Empower the Action of Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 725–727. [Google Scholar] [CrossRef]
- Anand, S.; Ortel, B.J.; Pereira, S.P.; Hasan, T.; Maytin, E.V. Biomodulatory approaches to photodynamic therapy for solid tu-mors. Cancer Lett. 2012, 326, 8–16. [Google Scholar] [CrossRef]
- Hasan, T. Using cellular mechanisms to develop effective combinations of photodynamic therapy and targeted therapies. J. Natl. Compr. Canc. Netw. 2012, 10, S23–S26. [Google Scholar] [CrossRef]
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity-A Clini-cal Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.; Khurshid, A.; Yousaf, M.S.; Aalam, M.; Salman, M.; Ikram, M. Effect of vitamin A as a neoadjuvant agent in chemotherapy and photodynamic therapy of Rhabdomyosarcoma cells. Photodiagn. Photodyn. Ther. 2020, 32, 102088. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, V.O.; Bezdetnaya, L.N.; Brault, D.; Potapenko, A.Y.; Guillemin, F. Enhancement of meta-tetrahydroxyphenylchlorin-sensitized photodynamic treatment on human tumor xenografts using a water-soluble vitamin E analogue, Trolox. Int. J. Cancer 2000, 88, 798–803. [Google Scholar] [CrossRef]
- Mahmood, R.; Khurshid, A.; Khan, J.A.; Rafi, M.; Yousaf, M.S.; Maqsood, M.; Aalam, M.; Salman, M.; Ikram, M. Enhanced efficacy of chemo-photodynamic therapy of rhabdomyosarcoma cells by using vitamin K3 as a neoadjuvant agent. Laser Phys. 2018, 29, 015603. [Google Scholar] [CrossRef]
- Grimm, S.; Mvondo, D.; Grune, T.; Breusing, N. The outcome of 5-ALA-mediated photodynamic treatment in melanoma cells is influenced by vitamin C and heme oxygenase-1. Biofactors 2011, 37, 17–24. [Google Scholar] [CrossRef]

| Type of Case | Type of Application | Form of Vitamin D | Stage of Administration | Type of Photosensitizer | The Source of Light | Effect on PDT Efficacy (Comments) | Reference |
|---|---|---|---|---|---|---|---|
| in vitro studies: germ-free fetal rat keratinocytes | application of calcitriol to the cell solution | medium containing calcitriol, final concentration | before and after phototherapy | tetrasulfonate (AlPcTs) | 500 W halogen lamp (SX-UI 500 JH; Ushio, Tokyo, Japan) | The addition of calcitriol enhanced DNA fragmentation of cells, thus improving the effectiveness of PDT. | [40] |
| in vitro studies: human breast cancer cell lines MCF7 and MDA-MB-231 | application of calcitriol to the cell solution | calcitriol | before therapy | hematoporphyrin derivatives (HPD) | diode laser with a power density of 7.5 J/cm2 (XD635AB; Xingda, Guilin, China) | Calcitriol improved the efficacy of PDT by increasing the PpIX levels in cells. | [43] |
| in vitro studies: human glioma cell lines U87 and T98 | application of calcitriol to the cell solution | calcitriol | before therapy | 5-aminolevulinic acid (ALA) | laser with a power density of 30 mW/cm2 (XD-635AB; Xingda, Guilin, China) | Calcitriol treatment of glioblastoma cells selectively increased PpIX levels and increased ALA-induced phototoxicity. Additionally, the administered calcitriol significantly increased the number of tumor cells killed after ALA-PDT treatment. | [44,45] |
| in vitro studies: human squamous cell carcinoma A431 cells | application of calcitriol to the cell solution | calcitriol | 96 h before therapy | methyl aminolevulinate (MAL) | fluorescent lamps (Model 3026; Applied Photophysics, London, UK) in the wavelength range 370–450 nm | Calcitriol enhanced PDT. | [46] |
| in vitro studies: LNCaP prostate cancer cells | application of calcitriol to the cell solution | calcitriol and its analogues (R0-25-9022 and R0-26-2198) | 96 h before therapy | 5-aminolevulinic acid (ALA) | argon laser with a wavelength of 514 nm (Coherent, Inc., Santa Clara, CA, USA) | Calcitriol and its analogues significantly increased ALA–PpIX accumulation in cells | [47] |
| animal studies: human squamous cell carcinoma cell line | diet or systemic administration | one of three forms: • D3: cholecalciferol • monohydroxy D3: calcidiol(25(OH) D3) • dihydroxy D3: calcitriol, (1,25(OH)2 D3) | diet: 10 days before phototherapy systemic administration: 3 days before | 5-aminolevulinic acid (ALA) | 633 nm noncoherent light source (LumaCare Products, Newport Beach, CA, USA) | Tumor cells treated with D3 and monohydroxy D3 showed an approximately 2.5- and 3-fold increase in PpIX (protoporphyrin IX) levels compared to vehicle control. Tumors treated with dihydroxy D3 showed an approximately 3.5-fold increase in PpIX (protoporphyrin IX) levels compared to vehicle control. Research showed a clear pattern of increase in cell death induced by ALA-PDT (5-aminolevulinic acid-photodynamic therapy) with vitamin D3 pretreatment. | [48] |
| animal studies: murine model of breast cancer | intraperitoneally | calcitriol | 3 days before | 5-aminolevulinic acid (ALA) | 633 nm noncoherent light source (LumaCare USA, Newport Beach, CA, USA) | Increased cell death was observed in tumors injected with calcitriol prior to ALA-PDT compared to ALA-PDT alone. ALA with calcitriol treatment induced 3.3 ± 0.5-fold increase in intracellular PpIX levels. | [49] |
| animal studies: squamous cell skin cancers | topically/Intraperitoneally | calcipotriene 0.005% deep tumors: | 3 days before | 5-aminolevulinic acid (ALA) | 633 nm non-coherent light source (LumaCare Products, Newport Beach, CA, USA) | There was a 10-fold increase in the accumulation of ALA protoporphyrin-IX (PpIX) in neoplastic cells due to changes in the expression of porphyrin synthesis enzymes. | [39] |
| animal studies: precancerous lesions in the buccal | ointment | calcipotriol 0.005% [100 µL] | every 24 h 3 times | 5-aminolevulinic acid (ALA) | LED with 640 nm wavelength | Pre-conditioning of precancerous lesions with calcipotriol affects the amount of PpIX, which may improve the efficacy of PDT. | [50] |
| animal studies: non-melanoma skin cancer mouse models | topical for the skin | calcitriol | 3 days before | 5-aminolevulinic acid (ALA) | 633 nm noncoherent light source (LumaCare USA, Newport Beach, CA, USA) | Histological examination of tumor tissues from combination therapy (calcitriol + ALA-PDT) showed pyknotic/shrunken testes, reduction of collagen, and growth of dead areas. | [51] |
| human studies: human psoriasis | cream or ointment | calcipotriol | 6 days before | 5-aminolevulinic acid (ALA) | 635 nm diode laser (HPD 7401, High Power Devices, Inc., North Brunswick, NJ, USA) | In a combination of ALA-PDT therapy with calcipotriol, there was an improvement in the clinical response in psoriatic plaques. | [36] |
| human studies: actinic keratoses | ointment | calcipotriol | 15 days before | methyl aminolevulinate (MAL) | daylight-mediated photodynamic therapy (DL-PDT) | There was a 15% increase in overall response to treatment with DL-PDT in combination with calcipotriol compared to DL-PDT alone. | [52] |
| human studies: actinic keratoses | ointment | tacalcitol | 15 days before | 5-aminolevulinic acid (ALA) | 630 nm diode (S630, AlphaStrumenti, Milan, Italy) | The combination of PDT with tacalcitol was more effective than the practiced PDT alone. The percentage reduction in the total number of lesions was 44.4%. | [53] |
| human studies: follicular mucinosis of the scalp | ointment | tacalcitol | 1 month before and continued throughout the treatment period | 5-aminolevulinic acid (ALA) | ded diode with a wavelength of 630 nm | Applied PDT with tacalcitol effectively reduced inflammation and increased the penetration of 5-ALA into the skin. | [54] |
| human studies: actinic keratoses | oral | cholecalciferol 10,000 IU | before therapy | 5-aminolevulinic acid (ALA) | blue light (10 mW/cm2, Blu-U, Sun/DUSA Pharmaceuticals) | Oral vitamin D3 therapy before PDT led to an 18% increase in response to treatment. An increase in the effectiveness of the therapy (by 11%) in removing lesions was also observed. | [37] |
| human studies: actinic keratosis | cream | calcipotriol 0.005% | 2 times a day for 2 weeks before therapy | methyl aminolevulinate (MAL) | red diode lamp (dose 37 J/cm2) | Topical therapy with calcipotriol before PDT enhances cell differentiation and apoptosis, thereby increasing the effectiveness of treatment. | [55] |
| human studies: actinic keratoses | ointment | calcitriol | 14 days before | methyl aminolevulinate (MAL) | daylight-mediated photodynamic therapy (DL-PDT) | The effectiveness of the therapy with calcitriol was higher by 6.11% compared to the therapy without calcitriol. | [56] |
| human studies: actinic keratoses | ointment | calcipotriol | 15 days before | methyl aminolevulinate (MAL) | - | After 12 months, PDT in combination with calcipotriol was safer and more effective (by approximately 27%) compared to conventional PDT. | [57] |
| human studies: actinic keratosis | ointment | calcipotriol | 15 days before | methyl aminolevulinate (MAL) | red light-emitting diode (LED) (Aktilite; PhotoCure, Oslo, Norway) | The use of PDT with calcipotriol doubled the number of actinic keratoses compared to untreated PDT. | [58] |
| Type of Study | Number | Type of Case | Number | Method of Delivery of Synthetic Vitamin D or D3 * | Number | Type of Photosensitizer | Number |
|---|---|---|---|---|---|---|---|
| human studies | 9 | actinic keratoses | 5 | ointment/cream | 11 | 5-ALA | 11 |
| in vitro cell line | 5 | squamous cell skin cancers | 3 | application to the cell solution | 5 | MAL | 6 |
| animal studies | 5 | breast cancer cell line | 2 | systemic/intraperitoneally | 3 | Other | 2 |
| glioma cell lines | 1 | oral/diet | 2 | ||||
| psoriasis | 1 | ||||||
| other (individual cases) | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, A.; Koziorowska, K.; Dynarowicz, K.; Aebisher, D.; Bartusik-Aebisher, D. Vitamin D and Vitamin D3 Supplementation during Photodynamic Therapy: A Review. Nutrients 2022, 14, 3805. https://doi.org/10.3390/nu14183805
Mazur A, Koziorowska K, Dynarowicz K, Aebisher D, Bartusik-Aebisher D. Vitamin D and Vitamin D3 Supplementation during Photodynamic Therapy: A Review. Nutrients. 2022; 14(18):3805. https://doi.org/10.3390/nu14183805
Chicago/Turabian StyleMazur, Anna, Katarzyna Koziorowska, Klaudia Dynarowicz, David Aebisher, and Dorota Bartusik-Aebisher. 2022. "Vitamin D and Vitamin D3 Supplementation during Photodynamic Therapy: A Review" Nutrients 14, no. 18: 3805. https://doi.org/10.3390/nu14183805
APA StyleMazur, A., Koziorowska, K., Dynarowicz, K., Aebisher, D., & Bartusik-Aebisher, D. (2022). Vitamin D and Vitamin D3 Supplementation during Photodynamic Therapy: A Review. Nutrients, 14(18), 3805. https://doi.org/10.3390/nu14183805