Flammulina velutipes Mycorrhizae Attenuate High Fat Diet-Induced Lipid Disorder, Oxidative Stress and Inflammation in the Liver and Perirenal Adipose Tissue of Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition Analysis
2.2. Preparation of Polysaccharide
2.3. Analysis of Monosaccharide Compositions
2.4. SEC-MALLS-RI Measurement
2.5. Fourier-Transform Infrared Spectra (FT-IR)
2.6. Animal Experiments
2.7. Histopathological Examination
2.8. Triglyceride (TG), Cholesterol (TC) and Transaminase Determination
2.9. Oxidative Stress Parameters Determination
2.10. Cytokines Determination
2.11. Untargeted Metabolomics
2.12. Targeted Metabolomics
2.13. RNA Isolation, cDNA Synthesis and Real-Time PCR
2.14. Statistical Analysis
3. Results
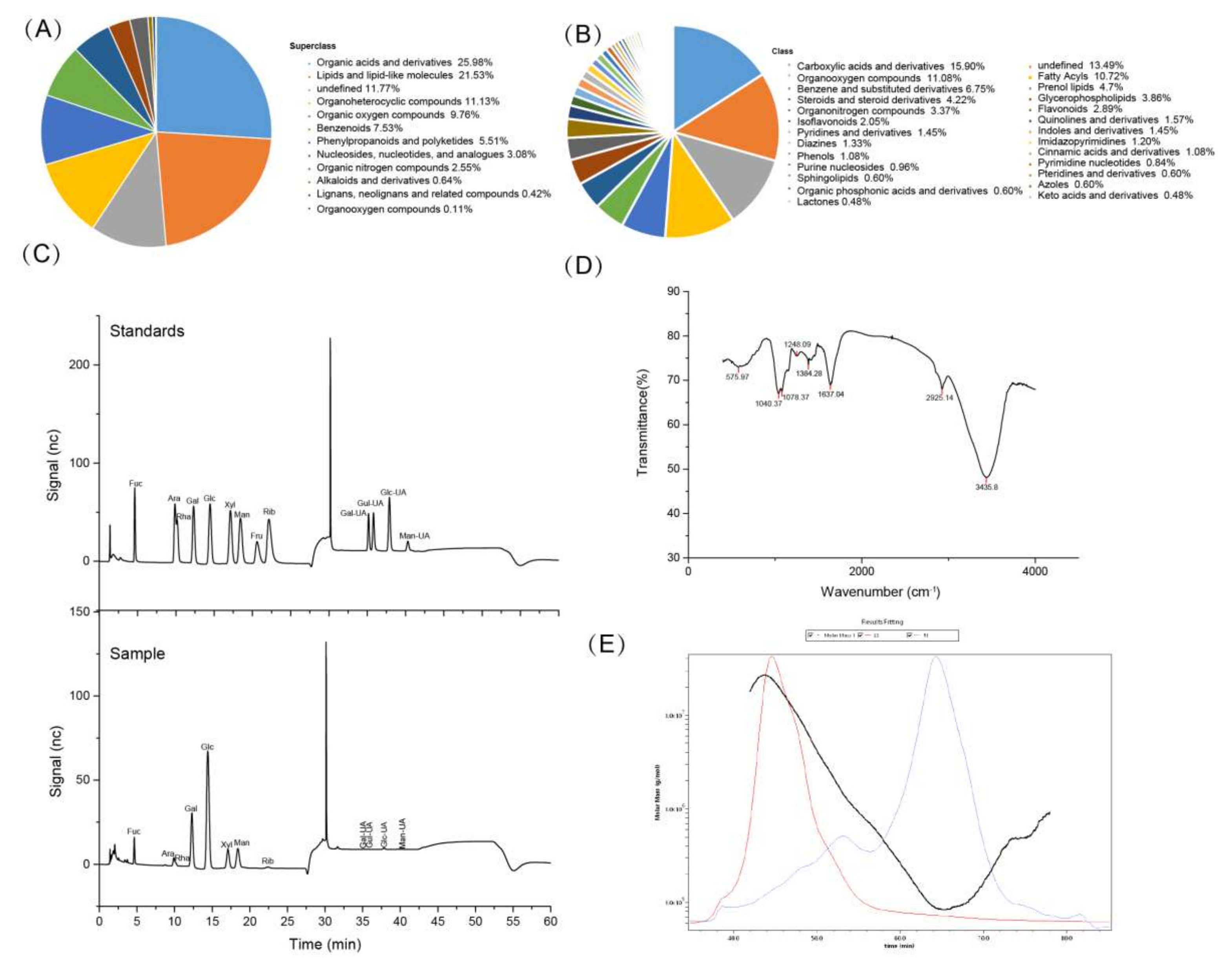
3.1. Compositional Analysis of FV Mycorrhizae
3.2. Effect of FV Mycorrhizae on Mice Body Weight, Food Intake, Organs Weight and Plasma Lipid Disorders
3.3. Effect of FV Mycorrhizae on Lipid Metabolism and Oxidative Stress Parameters in the Liver and PAT
3.4. Effect of FV Mycorrhizae on Gene Expression of Lipid Metabolism and Mitochondrial Biogenesis in the Liver and PAT
3.5. Effect of FV Mycorrhizae on Inflammation in the Liver and PAT
3.6. Effect of FV Mycorrhizae on Differential Metabolites in Plasma
3.7. Effect of FV Mycorrhizae on Medium Long Chain Fatty Acids in the Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology 2020, 158, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e6. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, F.; Zeigerer, A.; Kory, N.; Krahmer, N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin. Cell Dev. Biol. 2020, 108, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Bindua, S.; Mazumderb, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Datta, A.; Flynn, N.R.; Barnette, D.A.; Woeltje, K.F.; Miller, G.P.; Swamidass, S.J. Machine learning liver-injuring drug interactions with non-steroidal anti-inflammatory drugs (NSAIDs) from a retrospective electronic health record (EHR) cohort. PLoS Comput. Biol. 2021, 17, e1009053. [Google Scholar] [CrossRef]
- Wang, H.-N.; Xiang, J.-Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit. Rev. Food Sci. Nutr. 2022, 62, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Tang, C.; Hoo, P.C.-X.; Tan, L.T.-H.; Pusparajah, P.; Mehmood Khan, T.; Lee, L.-H.; Goh, B.-H.; Chan, K.-G. Golden needle mushroom: A culinary medicine with evidenced-based biological activities and health promoting properties. Front. Pharmacol. 2016, 7, 474. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Liu, Y. Characterization and antioxidant activities of polysaccharides from thirteen boletus mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef]
- Sande, D.; Oliveira, G.P.D.; Moura, M.A.F.; Martins, B.D.A.; Matheus, L.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-F.; Liu, N.-X.; Mao, X.-X.; Li, Y.; Li, C.-T. Activation effects of polysaccharides of Flammulina velutipes mycorrhizae on the T lymphocyte immune function. J. Immunol. Res. 2014, 2014, 285421. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, S.; Song, S.; Zhang, J.; Jia, F. Flammulina velutipes mycorrhizae dietary fiber improves lipid metabolism disorders in obese mice through activating AMPK signaling pathway mediated by gut microbiota. Food Biosci. 2021, 43, 101246. [Google Scholar] [CrossRef]
- Liu, B.-X.; Sun, W.; Kong, X.-Q. Perirenal fat: A unique fat pad and potential target for cardiovascular disease. Angiology 2019, 70, 584–593. [Google Scholar] [CrossRef]
- Grigoraș, A.; Balan, R.A.; Căruntu, I.-D.; Giușcă, S.E.; Lozneanu, L.; Avadanei, R.E.; Rusu, A.; Riscanu, L.A.; Amalinei, C. Perirenal adipose tissue—current knowledge and future opportunities. J. Clin. Med. 2021, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Yang, S. Feed Analysis and Feed Quality Testing Technology; Beijing Agricultural University Press: Beijing, China, 1993. [Google Scholar]
- Luo, Z.; Gao, Q.; Zhang, H.; Zhang, Y.; Zhou, S.; Zhang, J.; Xu, W.; Xu, J. Microbe-derived antioxidants attenuate cobalt chloride-induced mitochondrial function, autophagy and BNIP3-dependent mitophagy pathways in BRL3A cells. Ecotox. Environ. Safe 2022, 232, 113219. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, B.; Xiao, J.; Huang, Q.; Li, C.; Fu, X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chem. 2018, 249, 127–135. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, X.; Zhao, S.; Sho, T.; Luo, W.; Zhang, J.; Xu, W.; Hon, K.; Xu, J. Inclusion of microbe-derived antioxidant during pregnancy and lactation attenuates high-fat diet-induced hepatic oxidative stress, lipid disorders, and NLRP3 inflammasome in mother rats and offspring. Food Nutr. Res. 2019, 63, 1–11. [Google Scholar] [CrossRef]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets. Oxidative Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Y.; Liu, N.; Zhang, J.; Tan, G.; Kannan, B.; Liu, X.; Bell, A.E. Rheological properties of polysaccharides from Dioscorea opposita Thunb. Food Chem. 2017, 227, 64–72. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, I.T.; Cortez-Pinto, H.; Fidalgo, G.; Rodrigues, D.; Camilo, M.E. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin. Nutr. 2002, 21, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Grams, M.E.; Tin, A.; Rebholz, C.M.; Shafi, T.; Köttgen, A.; Perrone, R.D.; Sarnak, M.J.; Inker, L.A.; Levey, A.S.; Coresh, J. Metabolomic alterations associated with cause of CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1787. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016, 125, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Shou, Q.; Lu, Y.; Wang, G.; Qiu, J.; Wang, J.; He, L.; Chen, J.; Jiao, J.; Zhang, Y. Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. BBA-Mol. Basis Dis. 2017, 1863, 2715–2726. [Google Scholar] [CrossRef]
- Yoo, W.; Gjuka, D.; Stevenson, H.L.; Song, X.; Shen, H.; Yoo, S.Y.; Wang, J.; Fallon, M.; Ioannou, G.N.; Harrison, S.A.; et al. Fatty acids in non-alcoholic steatohepatitis: Focus on pentadecanoic acid. PLoS ONE 2017, 12, e0189965. [Google Scholar] [CrossRef]
- Rioux, V.; Pédrono, F.; Legrand, P. Regulation of mammalian desaturases by myristic acid: N-terminal myristoylation and other modulations. BBA Mol. Cell Biol. Lipids 2011, 1811, 1–8. [Google Scholar] [CrossRef]
- Zhang, F.; Song, M.; Chen, L.; Yang, X.; Li, F.; Yang, Q.; Duan, C.; Ling, M.; Lai, X.; Zhu, X.; et al. Dietary supplementation of lauric acid alleviates the irregular estrous cycle and the impaired metabolism and thermogenesis in female mice fed with high-fat diet (HFD). J. Agr. Food Chem. 2020, 68, 12631–12640. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Siswanta, D.; Solikhah, E.N. Isolation and antibacterial activity test of lauric acid from crude coconut oil (Cocos nucifera L.). Procedia Chem. 2016, 18, 132–140. [Google Scholar] [CrossRef]
- Keppley, L.J.W.; Walker, S.J.; Gademsey, A.N.; Smith, J.P.; Keller, S.R.; Kester, M.; Fox, T.E. Nervonic acid limits weight gain in a mouse model of diet-induced obesity. FASEB J. 2020, 34, 15314–15326. [Google Scholar] [CrossRef]
- Oda, E.; Hatada, K.; Kimura, J.; Aizawa, Y.; Thanikachalam, P.V.; Watanabe, K. Relationships between serum unsaturated fatty acids and coronary risk factors negative relations between nervonic acid and obesity-related risk factors. Int. Heart J. 2005, 46, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Y.; Liang, Y.; Tian, X.; Peng, C.; Ma, K.Y.; Liu, J.; Huang, Y.; Chen, Z.Y. DPA n-3, DPA n-6 and DHA improve lipoprotein profiles and aortic function in hamsters fed a high cholesterol diet. Atherosclerosis 2012, 221, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Horas, H.; Nababan, S.; Nishiumi, S.; Kobayashi, T.; Yoshida, M.; Azuma, T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch. Biochem. Biophys. 2017, 623–624, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Gui, T.; Li, C.; Zhang, Q.; Feng, H.; Li, Y.; Wu, J.; Gai, Z. Recent progress on lipid intake and chronic kidney disease. BioMed Res. Int. 2020, 2020, 3680397. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Claude, T.; Trauner, M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrin. Met. 2014, 25, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont, J.-P.; Djouadi, F.; Prip-Buus, C.; Gobin, S.; Munnich, A.; Bastin, J. Carnitine palmitoyltransferases 1 and 2: Biochemical, molecular and medical aspects. Mol. Aspects Med. 2004, 25, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Abo, A.O.; Lopaschuk, G.D. Role of CoA and acetyl-CoA in regulating cardiac fatty acid and glucose oxidation. Biochem. Soc. Trans. 2014, 42, 1043–1051. [Google Scholar]
- Colbert, C.L.; Kim, C.-W.; Moon, Y.-A.; Henry, L.; Palnitkar, M.; McKean, W.B.; Fitzgerald, K.; Deisenhofer, J.; Horton, J.D.; Kwon, H.J. Crystal structure of Spot 14, a modulator of fatty acid synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18820–18825. [Google Scholar] [CrossRef]
- You, M.; Crabb, D.W. Recent advances in alcoholic liver disease II. minireview: Molecular mechanisms of alcoholic fatty liver. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 287, G1–G6. [Google Scholar] [CrossRef]
- Zhao, R.; Ji, Y.; Chen, X.; Hu, Q.; Zhao, L. Polysaccharide from Flammulina velutipes attenuates markers of metabolic syndrome by modulating the gut microbiota and lipid metabolism in high fat diet-fed mice. Food Funct. 2021, 12, 6964–6980. [Google Scholar] [CrossRef]
- Yeh, M.-Y.; Ko, W.-C.; Lin, L.-Y. Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). BioMed Res. Int. 2014, 2014, 352385. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Mori, K.; Ouchi, K.; Kushida, M.; Tsuduki, T. Effects of dietary intake of Japanese mushrooms on visceral fat accumulation and gut microbiota in mice. Nutrients 2018, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radical Bio. Med. 2008, 45, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Bio. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Hammoud, S.H.; AlZaim, I.; Al-Dhaheri, Y.; Eid, A.H.; El-Yazbi, A.F. Perirenal adipose tissue inflammation: Novel insights linking metabolic dysfunction to renal diseases. Front. Endocrinol. 2021, 12, 942. [Google Scholar] [CrossRef]
- Martel, J.; Ko, Y.-F.; Ojcius, D.M.; Lu, C.-C.; Chang, C.-J.; Lin, C.-S.; Laisin, H.C.; Young, J.D. Immunomodulatory properties of plants and mushrooms. Trends Pharmacol. Sci. 2017, 38, 967–981. [Google Scholar] [CrossRef]
- Duwaerts, C.C.; Maher, J.J. Macronutrients and the adipose-liver axis in obesity and fatty liver. Cell. Mol. Gastroenter. 2019, 7, 749–761. [Google Scholar] [CrossRef]
- Naidu, P.B.; Uddandrao, V.V.S.; Naik, R.R.; Suresh, P.; Meriga, B.; Begum, M.S.; Pandiyan, R.; Saravanan, G. Ameliorative potential of gingerol: Promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol. Cell. Endocrinol. 2016, 419, 139–147. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Chen, W.; Xu, S.; Feng, X.; Zhang, L. Natural products: The role and mechanism in low-density lipoprotein oxidation and atherosclerosis. Phytother. Res. 2021, 35, 2945–2967. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Luo, M.; Chen, N.; Deng, X.; He, J.; Zhang, L.; Luo, P.; Wu, J. Inhibition of PAI-1 attenuates perirenal fat inflammation and the associated nephropathy in high-fat diet-induced obese mice. Am. J. Physiol.-Endocrinol. Metab. 2019, 316, E260–E267. [Google Scholar] [CrossRef]





| Item | Results |
|---|---|
| Sugar composition (%) | |
| Glc | 46.02 |
| Gal | 20.67 |
| Man | 11.3 |
| Xyl | 7.38 |
| Fuc | 6.95 |
| Ara | 2.83 |
| Rib | 1.1 |
| Rha | 1.09 |
| Fru | 0 |
| Gul-UA | 1.11 |
| Man-UA | 1.02 |
| Gal-UA | 0.32 |
| Glu-UA | 0.22 |
| Molecular characteristics | |
| Mn (g/mol) | 1.632 × 105 |
| Mw (g/mol) | 1.809 × 106 |
| Mz (g/mol) | 1.427 × 107 |
| Polydispersity (Mw/Mn) | 11.085 |
| Rn (nm) | 45.3 |
| Rw (nm) | 42.6 |
| Rz (nm) | 37.2 |
| Adduct | Name | VIP | FC | p-Value | m/z |
|---|---|---|---|---|---|
| Negative | |||||
| [M-H]- | [6]-gingerol | 5.634407 | 1.422392 | 0.000207 | 293.1763 |
| [M-H-C6H12O]- | 8,11-tridecadienoic acid, 13-(3-pentyl-2-oxiranyl)-, (8z,11z)- | 1.649861 | 1.3444 | 0.00024 | 221.1545 |
| [M-H]- | Tetradec-5-ynoic acid | 1.299375 | 0.357147 | 0.00122 | 223.1702 |
| [M-H-C17H27SO]- | Probucol | 2.159909 | 1.65893 | 0.003397 | 236.1054 |
| [M-H]- | Cis-.delta.2-11-methyldodecenoic acid | 1.599766 | 0.186622 | 0.004097 | 211.1701 |
| [M-H]- | 12-hydroxydodecanoic acid | 1.252276 | 1.765689 | 0.006782 | 215.1654 |
| [M-H-CO2]- | 3,5-di-tert-butyl-4-hydroxybenzoic acid | 1.876308 | 0.365134 | 0.021764 | 205.1596 |
| [M-H-C18H34O2]- | 1,2-dioleoyl-sn-glycero-3-phosphate | 1.975814 | 2.621357 | 0.022762 | 417.2414 |
| [M-H]- | 1h-indole-1-pentanoic acid, 3-(1-naphthalenylcarbonyl)- | 3.057796 | 0.318523 | 0.023438 | 370.1697 |
| [M+Cl]- | 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine | 1.347016 | 0.665036 | 0.031371 | 816.5336 |
| [M-H]- | Linoleic acid | 14.5538 | 0.618988 | 0.033811 | 279.2333 |
| [M-H]- | 2′,2′-difluoro-2′-deoxyuridine | 2.68574 | 0.656394 | 0.03705 | 263.0445 |
| [M-H]- | Deoxythymidine 5′-phosphate (dTMP) | 2.468689 | 3.258614 | 0.040277 | 321.0441 |
| [M-H]- | 3-hydroxycapric acid | 1.544758 | 1.462081 | 0.041071 | 187.1337 |
| Positive | |||||
| [M+H]+ | Lauryldimethylamine oxide | 4.245431 | 0.489801 | 0.000229 | 230.2479 |
| [M+H]+ | N-acetyl-o-fluoro-dl-phenylalanine | 3.723481 | 1.206781 | 0.003209 | 226.084 |
| [M+H]+ | Coumarin | 1.934626 | 0.525499 | 0.00427 | 147.0554 |
| [M+H-NH3]+ | Porphobilinogen | 3.582239 | 1.384773 | 0.005902 | 210.0796 |
| (M+H)+ | L-Proline | 1.783547 | 0.545155 | 0.005949 | 116.0709 |
| [M+Na]+ | Stavudine | 1.570389 | 1.749773 | 0.009195 | 247.0607 |
| [M+H-H2O]+ | Prostaglandin e3 | 1.374113 | 0.454302 | 0.012679 | 333.2059 |
| [M+H]+ | Pro-Trp | 1.402203 | 0.572081 | 0.01372 | 302.3052 |
| [M+H]+ | Tetraethylene glycol monomethyl ether | 5.241345 | 0.431367 | 0.013853 | 209.1385 |
| [M+H]+ | Fenpropimorph | 1.4829 | 1.619531 | 0.014177 | 304.2845 |
| [M+H]+ | Pentapropylene glycol | 2.740352 | 0.51482 | 0.016788 | 309.227 |
| [M+H-H2O]+ | Fingolimod | 1.43912 | 0.595578 | 0.016981 | 290.2688 |
| [M+H]+ | 3,4-dimethylmethcathinone | 1.876348 | 0.319043 | 0.020052 | 192.1596 |
| [M+H]+ | Triethylene glycol monobutyl ether | 3.491736 | 1.698806 | 0.021376 | 207.1592 |
| [M+H]+ | Tributyl phosphate | 1.08038 | 1.705361 | 0.022941 | 267.1719 |
| [M+H]+ | Decanoyl m-nitroaniline | 1.016153 | 0.557516 | 0.023725 | 293.211 |
| (M-H+2Na)+ | 1-Stearoyl-sn-glycerol 3-phosphocholine | 13.17318 | 1.343167 | 0.024106 | 568.3402 |
| [M+H]+ | .gamma.-nonalactone | 1.413858 | 1.068891 | 0.030097 | 157.1337 |
| [M+H]+ | Lpc 18:1 | 20.32652 | 1.257069 | 0.03399 | 522.3565 |
| [M+NH4]+ | Desferrioxamine d2 | 2.815889 | 4.933469 | 0.034191 | 604.3541 |
| [M+NH4]+ | 1,2-dilinoleoylglycerol | 1.123168 | 0.244778 | 0.036202 | 634.5408 |
| [M+H]+ | 2-fluoroamphetamine | 1.286169 | 1.631088 | 0.039465 | 154.0863 |
| [M+H-CH2O2]+ | 4-hydroxynonenal alkyne | 1.267965 | 3.974206 | 0.042399 | 107.0859 |
| [M+Na]+ | Anisomycin | 1.322298 | 3.083059 | 0.04541 | 288.144 |
| [M+H]+ | Fenpropidin | 9.809554 | 0.31689 | 0.049266 | 274.2742 |
| Item | HFD | HFDFV | p | |
|---|---|---|---|---|
| SFA | 2973.287 ± 356.423 | 2865.914 ± 262.981 | 0.813 | |
| Octanoic acid | C8:0 | 0.259 ± 0.033 | 0.22 ± 0.019 | 0.335 |
| Decanoic acid | C10:0 | 0.489 ± 0.095 | 0.468 ± 0.039 | 0.838 |
| Lauric acid | C12:0 | 0.247 ± 0.018 | 0.337 ± 0.036 | 0.047 |
| Tridecanoic acid | C13:0 | 0.368 ± 0.026 | 0.361 ± 0.018 | 0.827 |
| Myristic acid | C14:0 | 3.577 ± 0.551 | 5.19 ± 0.922 | 0.164 |
| Pentadecanoic Acid | C15:0 | 1.907 ± 0.225 | 2.522 ± 0.178 | 0.057 |
| Palmitic acid | C16:0 | 299.472 ± 32.524 | 308.18 ± 18.933 | 0.822 |
| Margaric Acid | C17:0 | 5.693 ± 0.699 | 6.715 ± 0.698 | 0.325 |
| Stearic acid | C18:0 | 2641.364 ± 320.93 | 2524.592 ± 244.726 | 0.778 |
| Arachidic Acid | C20:0 | 5.342 ± 0.661 | 3.793 ± 0.547 | 0.101 |
| Docosanoic acid | C22:0 | 10.2 ± 1.475 | 8.68 ± 1.272 | 0.453 |
| Tricosanoic Acid | C23:0 | 1.262 ± 0.162 | 1.581 ± 0.17 | 0.204 |
| Lignoceric Acid | C24:0 | 3.107 ± 0.526 | 3.276 ± 0.584 | 0.834 |
| MUFA | 39.561 ± 3.541 | 40.531 ± 1.791 | 0.812 | |
| Myristelaidic Acid | C14:1 | 0.608 ± 0.029 | 0.571 ± 0.038 | 0.450 |
| 10-Pentadecenoic Acid | C15:1 | 4.307 ± 0.912 | 4.265 ± 0.632 | 0.970 |
| Palmitoleic Acid | C16:1 | 5.177 ± 0.607 | 5.23 ± 0.36 | 0.941 |
| Heptadecanoic acid (cis-10) | C17:1 | 3.704 ± 0.45 | 4.069 ± 0.321 | 0.524 |
| Oleic acid | C18:1 | 0.261 ± 0.109 | 0.625 ± 0.162 | 0.092 |
| cis-11-Eicosenoic acid | C20:1 | 22.144 ± 2.478 | 20.347 ± 1.561 | 0.553 |
| Nervonic acid | C24:1 | 3.36 ± 0.503 | 5.425 ± 0.629 | 0.028 |
| TFA | 131.714 ± 19.462 | 208.164 ± 13.972 | 0.010 | |
| Hexadecanoic acid (trans-9) | C16:1T | 10.689 ± 2.988 | 22.393 ± 5.15 | 0.078 |
| Trans-10-HeptadecenoicAcid(C17:1T) | C17:1T | 1.611 ± 0.214 | 1.944 ± 0.214 | 0.297 |
| Trans-10-Nonadecenoic acid | C19:1T | 2.193 ± 0.307 | 1.77 ± 0.215 | 0.286 |
| Trans-11-Eicosenoic Acid | C20:1T | 1.653 ± 0.217 | 1.982 ± 0.262 | 0.356 |
| Linoelaidic Acid | C18:2TT | 115.569 ± 16.333 | 180.075 ± 11.339 | 0.009 |
| PUFA | 1420.182 ± 231.802 | 1401.189 ± 153.088 | 0.947 | |
| γ-Linolenic acid | C18:3 | 14.133 ± 2.116 | 16.241 ± 1.172 | 0.404 |
| α-Linolenic acid | C18:3 | 296.486 ± 38.875 | 234.847 ± 14.542 | 0.168 |
| Eicosapentaenoate | C20:5 | 358.805 ± 40.702 | 377.603 ± 32.96 | 0.727 |
| Arachidonic acid | C20:4 | 443.149 ± 148.283 | 415.357 ± 134.038 | 0.892 |
| Cis-11,14,17-Eicosatrienoic Acid | C20:3 | 54.141 ± 6.682 | 48.17 ± 5.572 | 0.508 |
| 11C,14C-Eicosadienoic Acid | C20:2 | 4.505 ± 0.593 | 2.011 ± 0.379 | 0.005 |
| Docosapentaenoate (C22:5n-6) | C22:5n-6 | 72.843 ± 7.426 | 39.97 ± 3.493 | 0.002 |
| Docosapentaenoate (C22:5n-3) | C22:5n-3 | 158.709 ± 20.87 | 254.869 ± 16.861 | 0.005 |
| Adrenic Acid | C22:4 | 15.307 ± 1.763 | 10.138 ± 1.475 | 0.048 |
| 13C,16C-Docosadienoic Acid | C22:2 | 2.103 ± 0.362 | 1.985 ± 0.51 | 0.854 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Gao, Q.; Li, Y.; Bai, Y.; Zhang, J.; Xu, W.; Xu, J. Flammulina velutipes Mycorrhizae Attenuate High Fat Diet-Induced Lipid Disorder, Oxidative Stress and Inflammation in the Liver and Perirenal Adipose Tissue of Mice. Nutrients 2022, 14, 3830. https://doi.org/10.3390/nu14183830
Luo Z, Gao Q, Li Y, Bai Y, Zhang J, Xu W, Xu J. Flammulina velutipes Mycorrhizae Attenuate High Fat Diet-Induced Lipid Disorder, Oxidative Stress and Inflammation in the Liver and Perirenal Adipose Tissue of Mice. Nutrients. 2022; 14(18):3830. https://doi.org/10.3390/nu14183830
Chicago/Turabian StyleLuo, Zhen, Qingying Gao, Yuanfei Li, Yifei Bai, Jing Zhang, Weina Xu, and Jianxiong Xu. 2022. "Flammulina velutipes Mycorrhizae Attenuate High Fat Diet-Induced Lipid Disorder, Oxidative Stress and Inflammation in the Liver and Perirenal Adipose Tissue of Mice" Nutrients 14, no. 18: 3830. https://doi.org/10.3390/nu14183830
APA StyleLuo, Z., Gao, Q., Li, Y., Bai, Y., Zhang, J., Xu, W., & Xu, J. (2022). Flammulina velutipes Mycorrhizae Attenuate High Fat Diet-Induced Lipid Disorder, Oxidative Stress and Inflammation in the Liver and Perirenal Adipose Tissue of Mice. Nutrients, 14(18), 3830. https://doi.org/10.3390/nu14183830





