Nova1 or Bim Deficiency in Pancreatic β-Cells Does Not Alter Multiple Low-Dose Streptozotocin-Induced Diabetes and Diet-Induced Obesity in Mice
Abstract
1. Introduction
2. Materials and Methods
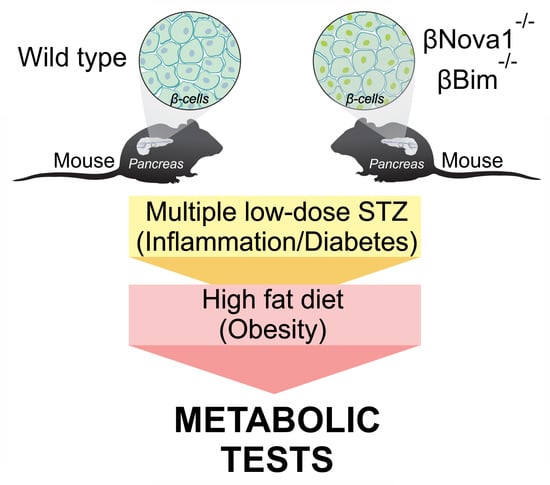
2.1. Animal Models and In Vivo Procedures
2.2. Diabetes Induction and High Fat Diet Treatment
2.3. Isolation of Mouse Islet and Fluorescence-Activated Cell Sorting (FACS) of β-Cells
2.4. Glucose Stimulated Insulin Secretion
2.5. Western Blotting
2.6. Histological Studies and Immunohistochemical Staining
2.7. Pancreatic Insulin Content
2.8. Intraperitoneal Glucose and Insulin Tolerance Tests (IPGTT and ITT)
2.9. Statistical Analysis
3. Results
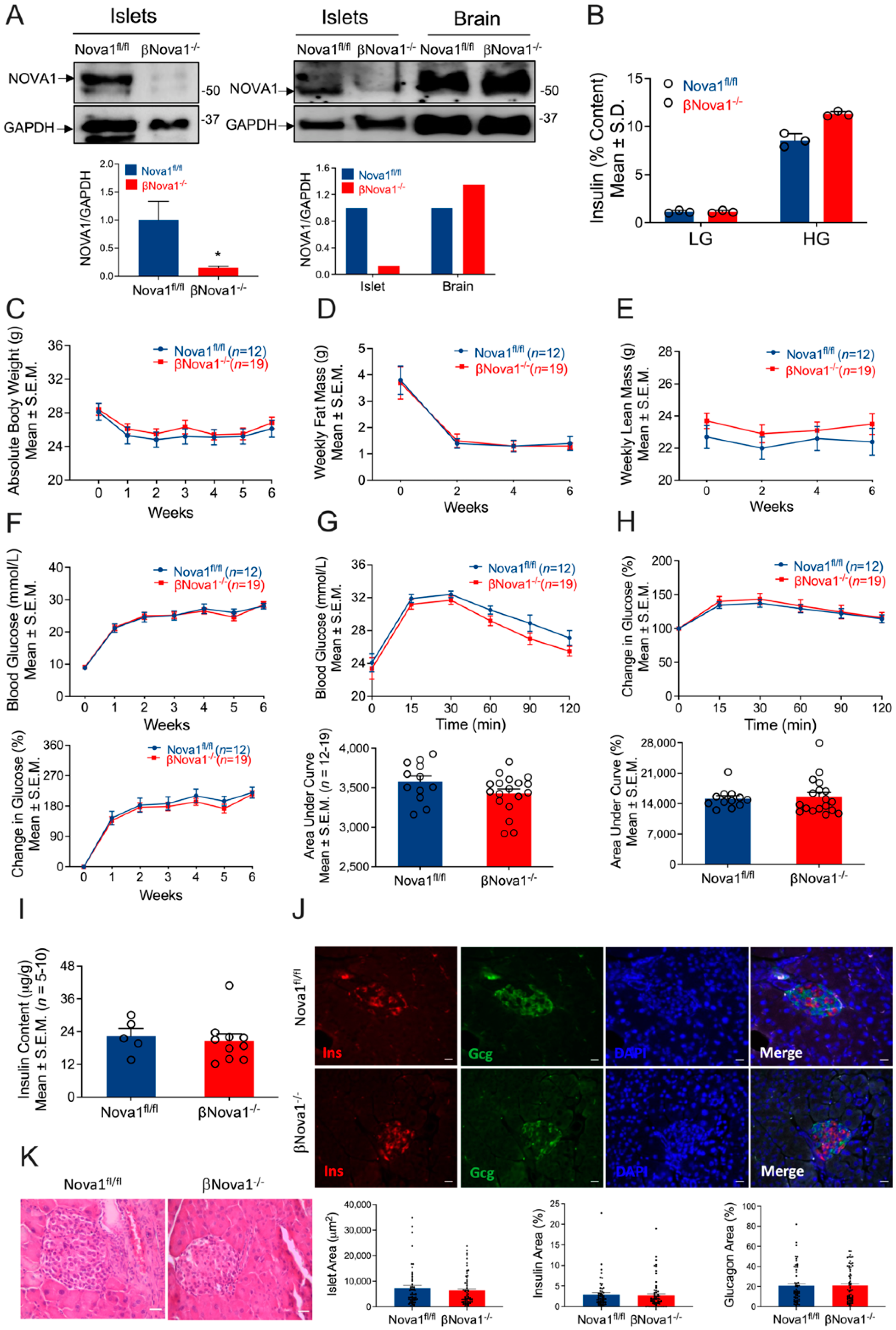
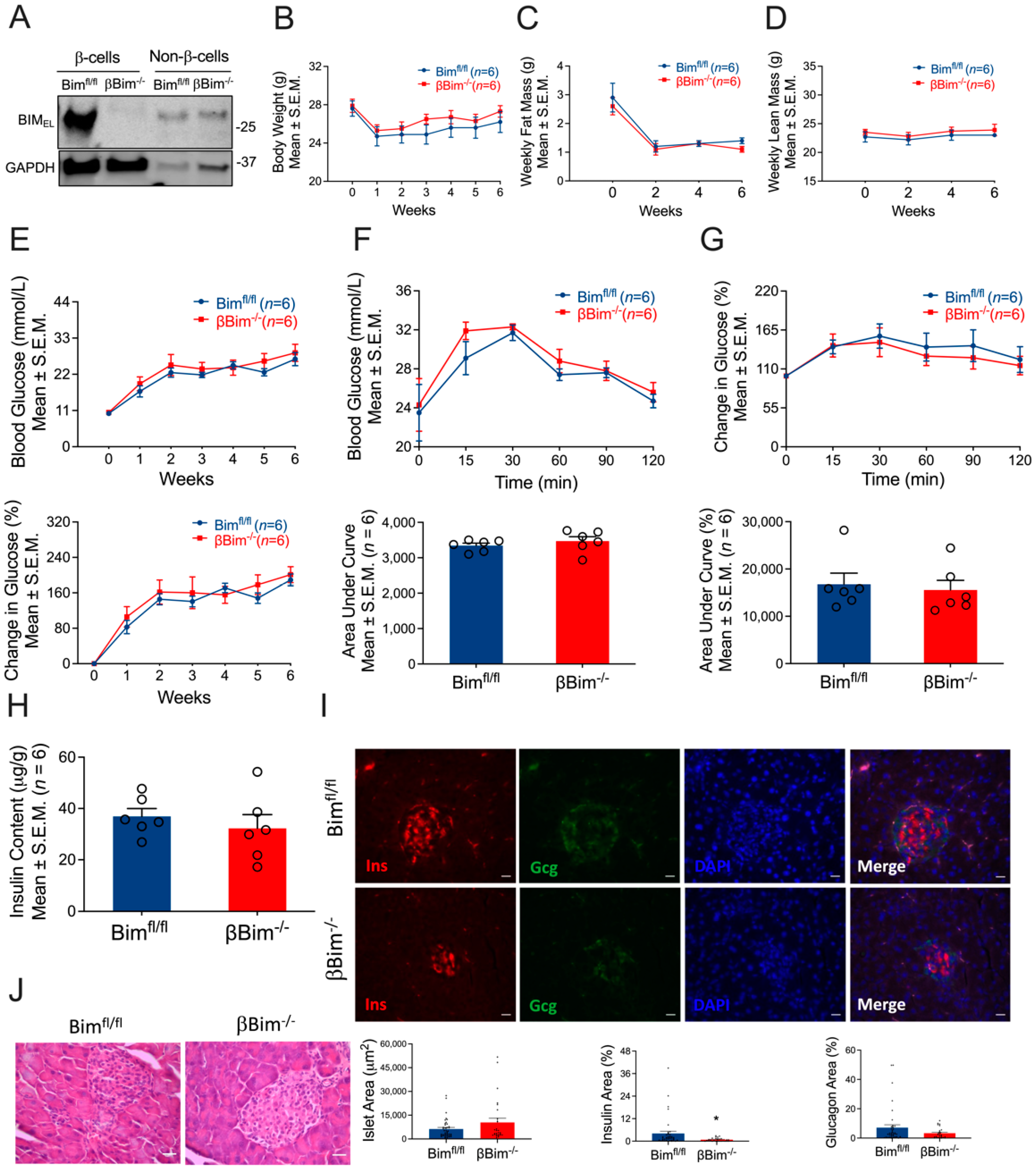
3.1. Generation of Mice with β-Cell-Specific Loss of Nova1 (βNova1−/−) or Bim (βBim−/−)
3.2. In Vivo Glucose Homeostasis Was Unchanged in βNova1−/− Mice in Response to MLD-STZ-Induced Diabetes
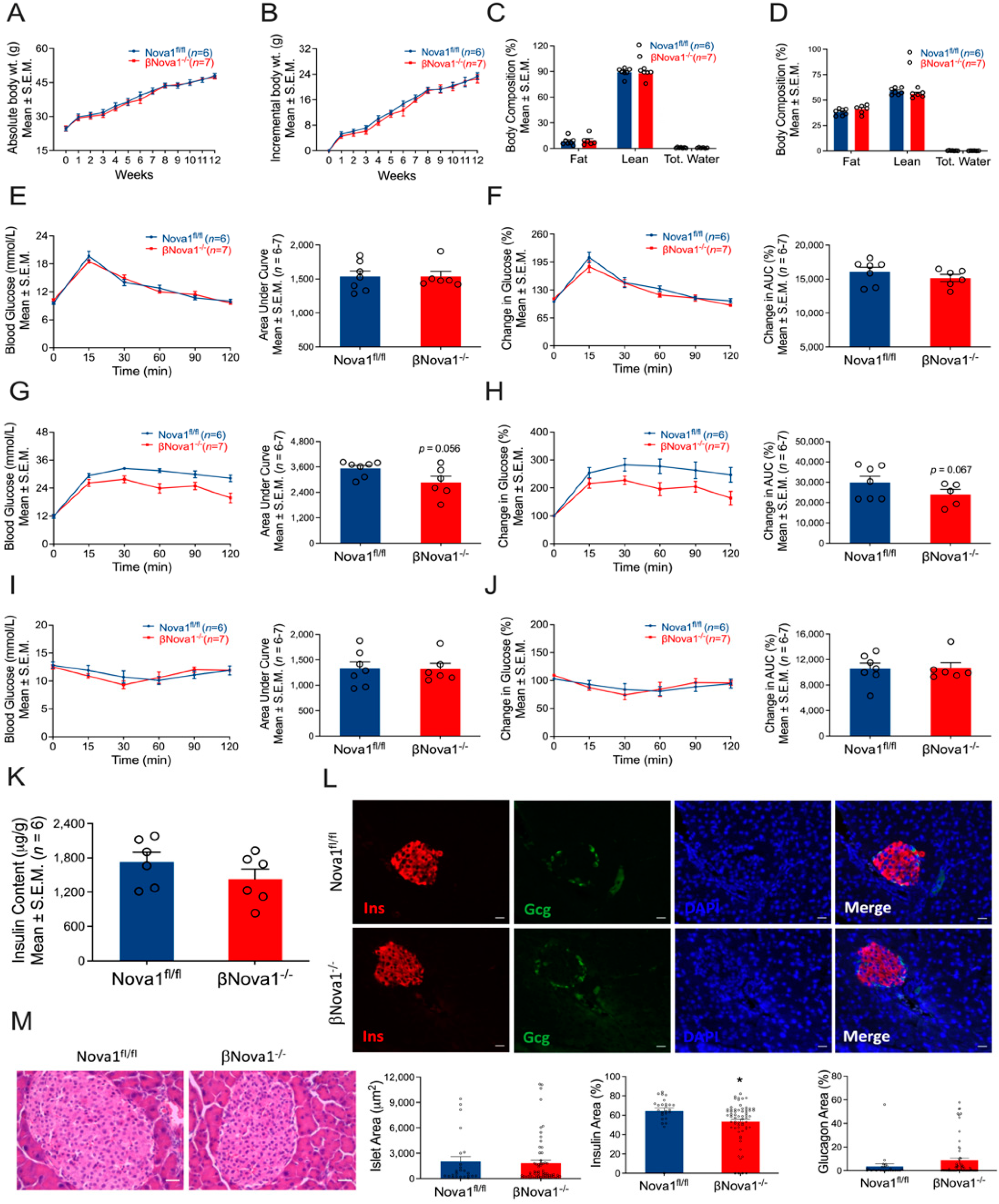
3.3. In Vivo Glucose Homeostasis Was Unchanged in βNova1−/− Mice un Response to High-Fat Diet
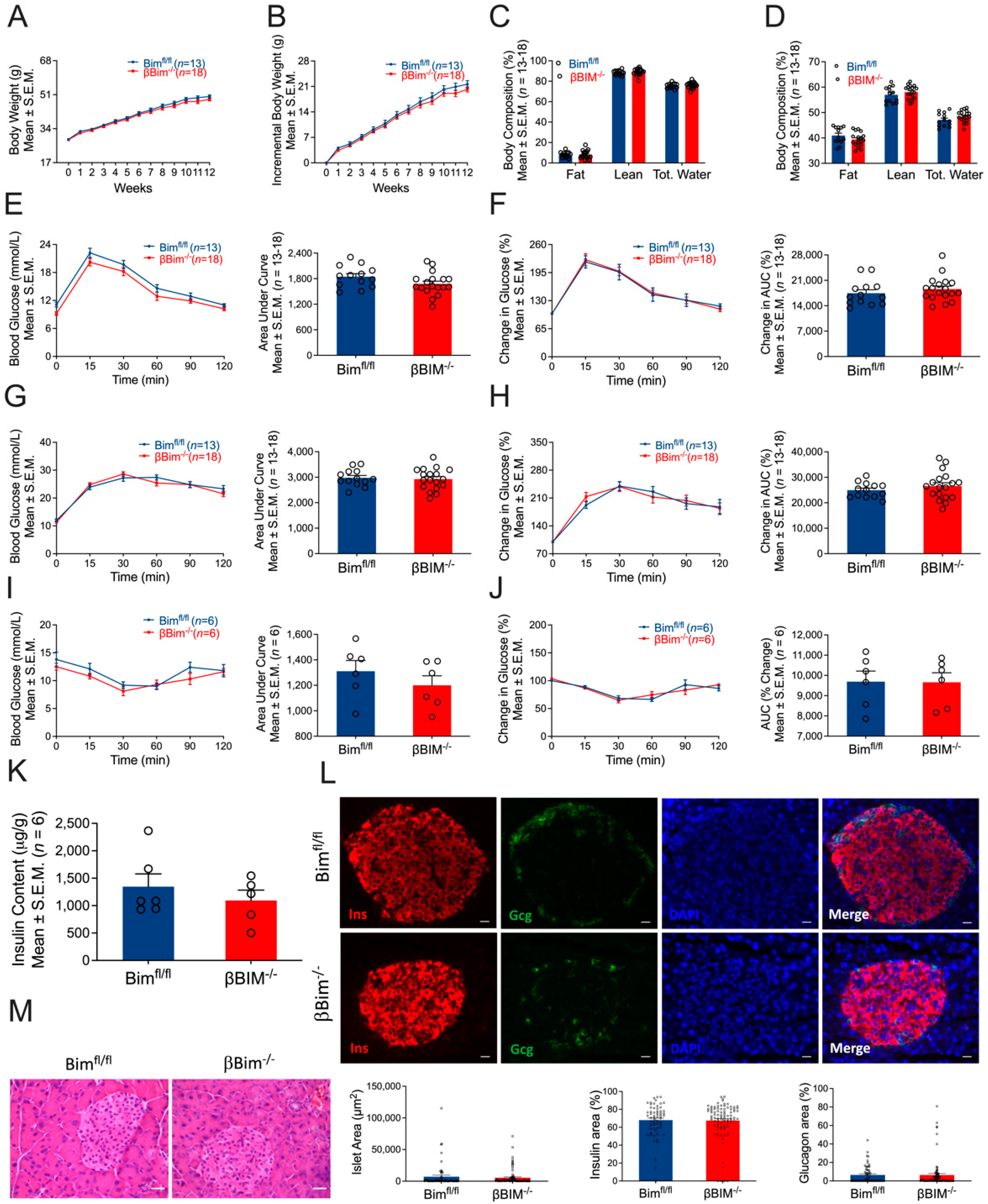
3.4. Bim Deletion in β-Cells Did Not Alter In Vivo Glucose Homeostasis in Response to MLD-STZ-Induced T1D or High-Fat Diet-Induced Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurzov, E.N.; Ke, P.C.; Ahlgren, U.; Garcia Ribeiro, R.S.; Gotthardt, M. Novel Strategies to Protect and Visualize Pancreatic beta Cells in Diabetes. Trends Endocrinol. Metab. 2020, 31, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Gurzov, E.N.; Eizirik, D.L. Bcl-2 proteins in diabetes: Mitochondrial pathways of beta-cell death and dysfunction. Trends Cell Biol. 2011, 21, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Storling, J.; Pociot, F. Type 1 Diabetes Candidate Genes Linked to Pancreatic Islet Cell Inflammation and Beta-Cell Apoptosis. Genes 2017, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Licatalosi, D.D.; Darnell, R.B. RNA processing and its regulation: Global insights into biological networks. Nat. Rev. Genet. 2010, 11, 75–87. [Google Scholar] [CrossRef]
- Wong, C.M.; Xu, L.; Yau, M.Y. Alternative mRNA Splicing in the Pathogenesis of Obesity. Int. J. Mol. Sci. 2018, 19, 632. [Google Scholar] [CrossRef]
- Webster, N.J.G. Alternative RNA Splicing in the Pathogenesis of Liver Disease. Front. Endocrinol. 2017, 8, 133. [Google Scholar] [CrossRef]
- Lipscombe, D.; Lopez Soto, E.J. Alternative splicing of neuronal genes: New mechanisms and new therapies. Curr. Opin. Neurobiol. 2019, 57, 26–31. [Google Scholar] [CrossRef]
- Alvelos, M.I.; Bruggemann, M.; Sutandy, F.R.; Juan-Mateu, J.; Colli, M.L.; Busch, A.; Lopes, M.; Castela, A.; Aartsma-Rus, A.; Konig, J.; et al. The RNA-binding profile of the splicing factor SRSF6 in immortalized human pancreatic beta-cells. Life Sci. Alliance 2021, 4. [Google Scholar] [CrossRef]
- Marcheva, B.; Perelis, M.; Weidemann, B.J.; Taguchi, A.; Lin, H.; Omura, C.; Kobayashi, Y.; Newman, M.V.; Wyatt, E.J.; McNally, E.M.; et al. A role for alternative splicing in circadian control of exocytosis and glucose homeostasis. Genes Dev. 2020, 34, 1089–1105. [Google Scholar] [CrossRef]
- Yau, B.; Blood, Z.; An, Y.; Su, Z.; Kebede, M.A. Type 2 diabetes-associated single nucleotide polymorphism in Sorcs1 gene results in alternative processing of the Sorcs1 protein in INS1 beta-cells. Sci. Rep. 2019, 9, 19466. [Google Scholar] [CrossRef]
- Cnop, M.; Abdulkarim, B.; Bottu, G.; Cunha, D.A.; Igoillo-Esteve, M.; Masini, M.; Turatsinze, J.V.; Griebel, T.; Villate, O.; Santin, I.; et al. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes 2014, 63, 1978–1993. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, T.C.; Paula, F.M.; Villate, O.; Colli, M.L.; Moura, R.F.; Cunha, D.A.; Marselli, L.; Marchetti, P.; Cnop, M.; Julier, C.; et al. GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet. 2013, 9, e1003532. [Google Scholar] [CrossRef] [PubMed]
- Nutter, C.A.; Kuyumcu-Martinez, M.N. Emerging roles of RNA-binding proteins in diabetes and their therapeutic potential in diabetic complications. Wiley Interdiscip. Rev. RNA 2018, 9, e1459. [Google Scholar] [CrossRef] [PubMed]
- Juan-Mateu, J.; Alvelos, M.I.; Turatsinze, J.V.; Villate, O.; Lizarraga-Mollinedo, E.; Grieco, F.A.; Marroqui, L.; Bugliani, M.; Marchetti, P.; Eizirik, D.L. SRp55 Regulates a Splicing Network That Controls Human Pancreatic beta-Cell Function and Survival. Diabetes 2018, 67, 423–436. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Sammeth, M.; Bouckenooghe, T.; Bottu, G.; Sisino, G.; Igoillo-Esteve, M.; Ortis, F.; Santin, I.; Colli, M.L.; Barthson, J.; et al. The human pancreatic islet transcriptome: Expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012, 8, e1002552. [Google Scholar] [CrossRef]
- Juan-Mateu, J.; Rech, T.H.; Villate, O.; Lizarraga-Mollinedo, E.; Wendt, A.; Turatsinze, J.V.; Brondani, L.A.; Nardelli, T.R.; Nogueira, T.C.; Esguerra, J.L.S.; et al. Neuron-enriched RNA-binding Proteins Regulate Pancreatic Beta Cell Function and Survival. J. Biol. Chem. 2017, 292, 3466–3480. [Google Scholar] [CrossRef]
- Meldolesi, J. Alternative Splicing by NOVA Factors: From Gene Expression to Cell Physiology and Pathology. Int. J. Mol. Sci. 2020, 21, 3941. [Google Scholar] [CrossRef]
- Villate, O.; Turatsinze, J.V.; Mascali, L.G.; Grieco, F.A.; Nogueira, T.C.; Cunha, D.A.; Nardelli, T.R.; Sammeth, M.; Salunkhe, V.A.; Esguerra, J.L.; et al. Nova1 is a master regulator of alternative splicing in pancreatic beta cells. Nucleic Acids Res. 2014, 42, 11818–11830. [Google Scholar] [CrossRef]
- Barthson, J.; Germano, C.M.; Moore, F.; Maida, A.; Drucker, D.J.; Marchetti, P.; Gysemans, C.; Mathieu, C.; Nunez, G.; Jurisicova, A.; et al. Cytokines tumor necrosis factor-alpha and interferon-gamma induce pancreatic beta-cell apoptosis through STAT1-mediated Bim protein activation. J. Biol. Chem. 2011, 286, 39632–39643. [Google Scholar] [CrossRef]
- Santin, I.; Moore, F.; Colli, M.L.; Gurzov, E.N.; Marselli, L.; Marchetti, P.; Eizirik, D.L. PTPN2, a candidate gene for type 1 diabetes, modulates pancreatic beta-cell apoptosis via regulation of the BH3-only protein Bim. Diabetes 2011, 60, 3279–3288. [Google Scholar] [CrossRef]
- Wali, J.A.; Rondas, D.; McKenzie, M.D.; Zhao, Y.; Elkerbout, L.; Fynch, S.; Gurzov, E.N.; Akira, S.; Mathieu, C.; Kay, T.W.; et al. The proapoptotic BH3-only proteins Bim and Puma are downstream of endoplasmic reticulum and mitochondrial oxidative stress in pancreatic islets in response to glucotoxicity. Cell Death Dis. 2014, 5, e1124. [Google Scholar] [CrossRef] [PubMed]
- Miani, M.; Elvira, B.; Gurzov, E.N. Sweet Killing in Obesity and Diabetes: The Metabolic Role of the BH3-only Protein BIM. J. Mol. Biol. 2018, 430, 3041–3050. [Google Scholar] [CrossRef] [PubMed]
- Vernia, S.; Edwards, Y.J.; Han, M.S.; Cavanagh-Kyros, J.; Barrett, T.; Kim, J.K.; Davis, R.J. An alternative splicing program promotes adipose tissue thermogenesis. eLife 2016, 5, e17672. [Google Scholar] [CrossRef] [PubMed]
- Raynor, J.; Sholl, A.; Plas, D.R.; Bouillet, P.; Chougnet, C.A.; Hildeman, D.A. IL-15 Fosters Age-Driven Regulatory T Cell Accrual in the Face of Declining IL-2 Levels. Front. Immunol. 2013, 4, 161. [Google Scholar] [CrossRef]
- Litwak, S.A.; Pang, L.; Galic, S.; Igoillo-Esteve, M.; Stanley, W.J.; Turatsinze, J.V.; Loh, K.; Thomas, H.E.; Sharma, A.; Trepo, E.; et al. JNK Activation of BIM Promotes Hepatic Oxidative Stress, Steatosis, and Insulin Resistance in Obesity. Diabetes 2017, 66, 2973–2986. [Google Scholar] [CrossRef]
- Pipeleers, D.G.; In’t Veld, P.A.; Van de Winkel, M.; Maes, E.; Schuit, F.C.; Gepts, W. A new in vitro model for the study of pancreatic A and B cells. Endocrinology 1985, 117, 806–816. [Google Scholar] [CrossRef]
- Schaschkow, A.; Pang, L.; Vandenbempt, V.; Elvira, B.; Litwak, S.A.; Vekeriotaite, B.; Maillard, E.; Vermeersch, M.; Paula, F.M.M.; Pinget, M.; et al. STAT3 Regulates Mitochondrial Gene Expression in Pancreatic beta-Cells and Its Deficiency Induces Glucose Intolerance in Obesity. Diabetes 2021, 70, 2026–2041. [Google Scholar] [CrossRef]
- Litwak, S.A.; Loh, K.; Stanley, W.J.; Pappas, E.G.; Wali, J.A.; Selck, C.; Strasser, A.; Thomas, H.E.; Gurzov, E.N. p53-upregulated-modulator-of-apoptosis (PUMA) deficiency affects food intake but does not impact on body weight or glucose homeostasis in diet-induced obesity. Sci. Rep. 2016, 6, 23802. [Google Scholar] [CrossRef]
- Litwak, S.A.; Wali, J.A.; Pappas, E.G.; Saadi, H.; Stanley, W.J.; Varanasi, L.C.; Kay, T.W.; Thomas, H.E.; Gurzov, E.N. Lipotoxic Stress Induces Pancreatic beta-Cell Apoptosis through Modulation of Bcl-2 Proteins by the Ubiquitin-Proteasome System. J. Diabetes Res. 2015, 2015, 280615. [Google Scholar] [CrossRef]
- Magnuson, M.A.; Osipovich, A.B. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013, 18, 9–20. [Google Scholar] [CrossRef]
- Estall, J.L.; Screaton, R.A. Of Mice and Men, Redux: Modern Challenges in beta Cell Gene Targeting. Endocrinology 2020, 161, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Oropeza, D.; Jouvet, N.; Budry, L.; Campbell, J.E.; Bouyakdan, K.; Lacombe, J.; Perron, G.; Bergeron, V.; Neuman, J.C.; Brar, H.K.; et al. Phenotypic Characterization of MIP-CreERT1Lphi Mice With Transgene-Driven Islet Expression of Human Growth Hormone. Diabetes 2015, 64, 3798–3807. [Google Scholar] [CrossRef] [PubMed]
- Gurzov, E.N.; Tran, M.; Fernandez-Rojo, M.A.; Merry, T.L.; Zhang, X.; Xu, Y.; Fukushima, A.; Waters, M.J.; Watt, M.J.; Andrikopoulos, S.; et al. Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metab. 2014, 20, 85–102. [Google Scholar] [CrossRef]
- Kutlu, B.; Burdick, D.; Baxter, D.; Rasschaert, J.; Flamez, D.; Eizirik, D.L.; Welsh, N.; Goodman, N.; Hood, L. Detailed transcriptome atlas of the pancreatic beta cell. BMC Med. Genom. 2009, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Dredge, B.K.; Stefani, G.; Zhong, R.; Buckanovich, R.J.; Okano, H.J.; Yang, Y.Y.; Darnell, R.B. Nova-1 regulates neuron-specific alternative splicing and is essential for neuronal viability. Neuron 2000, 25, 359–371. [Google Scholar] [CrossRef]
- Ruggiu, M.; Herbst, R.; Kim, N.; Jevsek, M.; Fak, J.J.; Mann, M.A.; Fischbach, G.; Burden, S.J.; Darnell, R.B. Rescuing Z+ agrin splicing in Nova null mice restores synapse formation and unmasks a physiologic defect in motor neuron firing. Proc. Natl. Acad. Sci. USA 2009, 106, 3513–3518. [Google Scholar] [CrossRef]
- Mosser, R.E.; Maulis, M.F.; Moullé, V.S.; Dunn, J.C.; Carboneau, B.A.; Arasi, K.; Pappan, K.; Poitout, V.; Gannon, M. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E573–E582. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Sun, J.; Wang, C.; Ye, H.; Mao, L.; Cheng, E.H.; Bell, G.I.; Polonsky, K.S. Role of BH3-only molecules Bim and Puma in β-cell death in Pdx1 deficiency. Diabetes 2014, 63, 2744–2750. [Google Scholar] [CrossRef]
- Ren, D.; Sun, J.; Mao, L.; Ye, H.; Polonsky, K.S. BH3-only molecule Bim mediates β-cell death in IRS2 deficiency. Diabetes 2014, 63, 3378–3387. [Google Scholar] [CrossRef]
- Shansky, R.M. Are hormones a “female problem” for animal research? Science 2019, 364, 825–826. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brahma, M.K.; Xiao, P.; Popa, M.; Negueruela, J.; Vandenbempt, V.; Demine, S.; Cardozo, A.K.; Gurzov, E.N. Nova1 or Bim Deficiency in Pancreatic β-Cells Does Not Alter Multiple Low-Dose Streptozotocin-Induced Diabetes and Diet-Induced Obesity in Mice. Nutrients 2022, 14, 3866. https://doi.org/10.3390/nu14183866
Brahma MK, Xiao P, Popa M, Negueruela J, Vandenbempt V, Demine S, Cardozo AK, Gurzov EN. Nova1 or Bim Deficiency in Pancreatic β-Cells Does Not Alter Multiple Low-Dose Streptozotocin-Induced Diabetes and Diet-Induced Obesity in Mice. Nutrients. 2022; 14(18):3866. https://doi.org/10.3390/nu14183866
Chicago/Turabian StyleBrahma, Manoja K., Peng Xiao, Madalina Popa, Javier Negueruela, Valerie Vandenbempt, Stéphane Demine, Alessandra K. Cardozo, and Esteban N. Gurzov. 2022. "Nova1 or Bim Deficiency in Pancreatic β-Cells Does Not Alter Multiple Low-Dose Streptozotocin-Induced Diabetes and Diet-Induced Obesity in Mice" Nutrients 14, no. 18: 3866. https://doi.org/10.3390/nu14183866
APA StyleBrahma, M. K., Xiao, P., Popa, M., Negueruela, J., Vandenbempt, V., Demine, S., Cardozo, A. K., & Gurzov, E. N. (2022). Nova1 or Bim Deficiency in Pancreatic β-Cells Does Not Alter Multiple Low-Dose Streptozotocin-Induced Diabetes and Diet-Induced Obesity in Mice. Nutrients, 14(18), 3866. https://doi.org/10.3390/nu14183866