The Effects of Alpha-Glycerylphosphorylcholine on Heart Rate Variability and Hemodynamic Variables Following Sprint Interval Exercise in Overweight and Obese Women
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
2.2. Experimental Design
2.3. Heart Rate Variability
2.4. Blood Pressure Measurements
2.5. A-GPC Supplementation
2.6. Sprint Interval Exercise (SIE)
2.7. Statistical Analyses
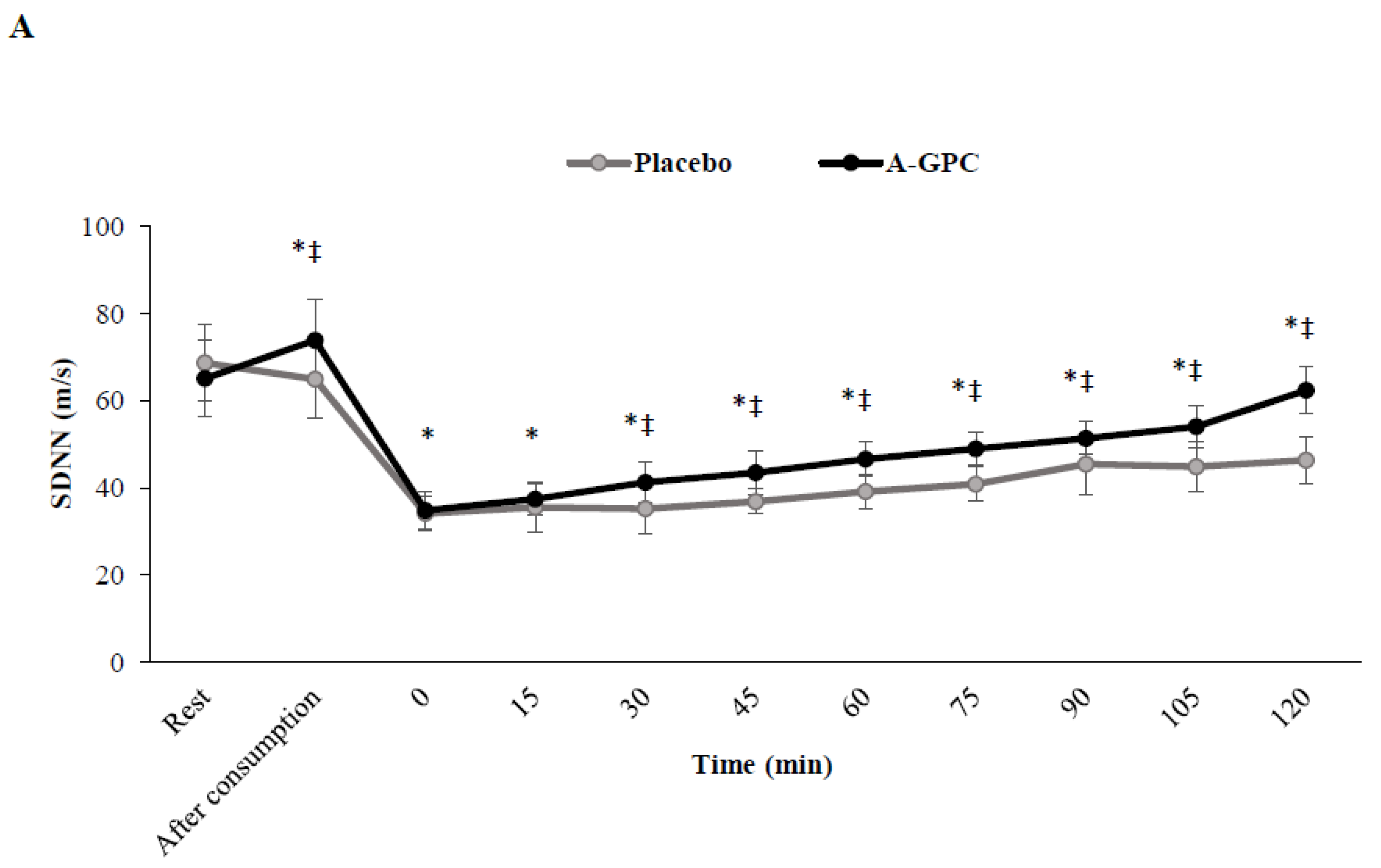
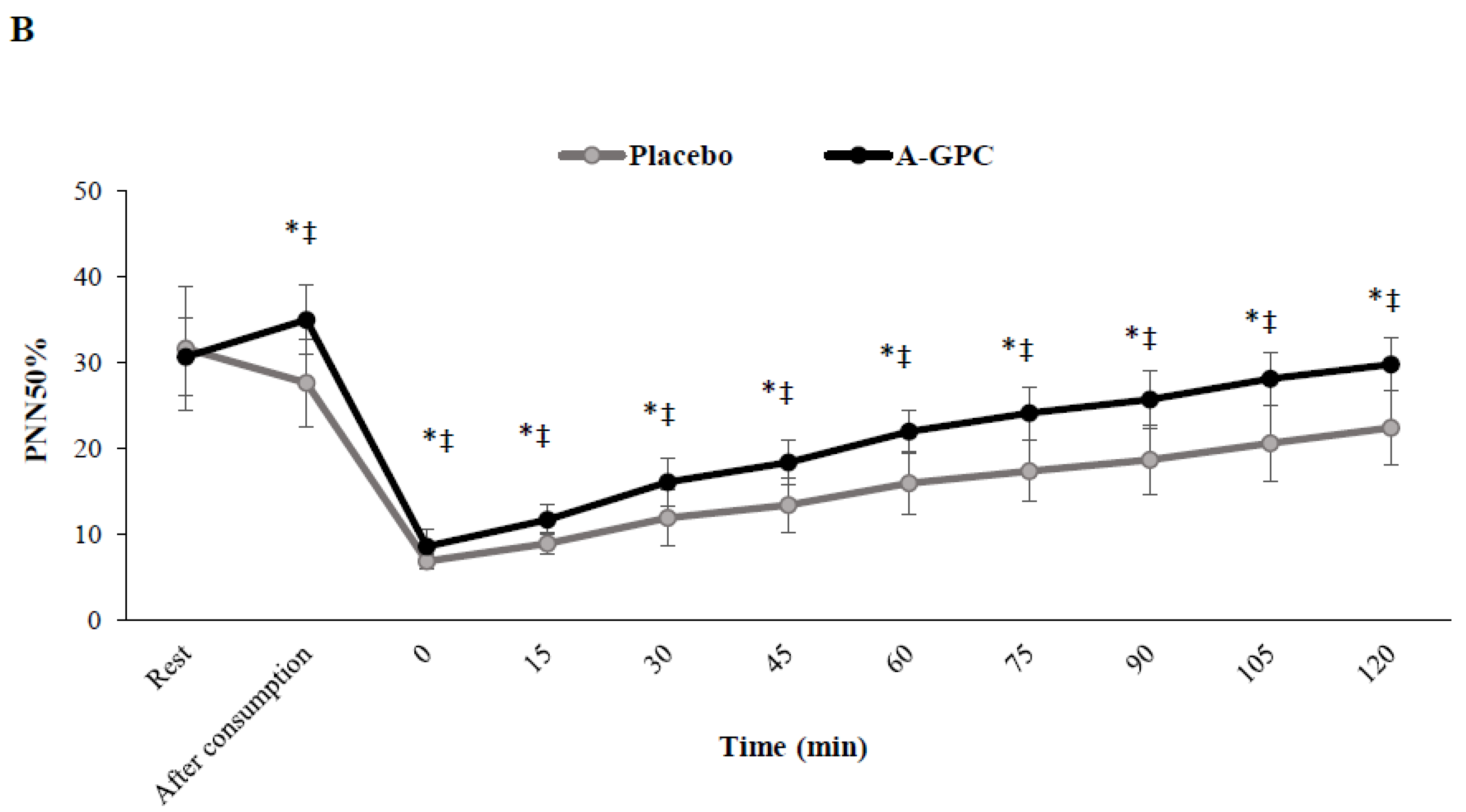
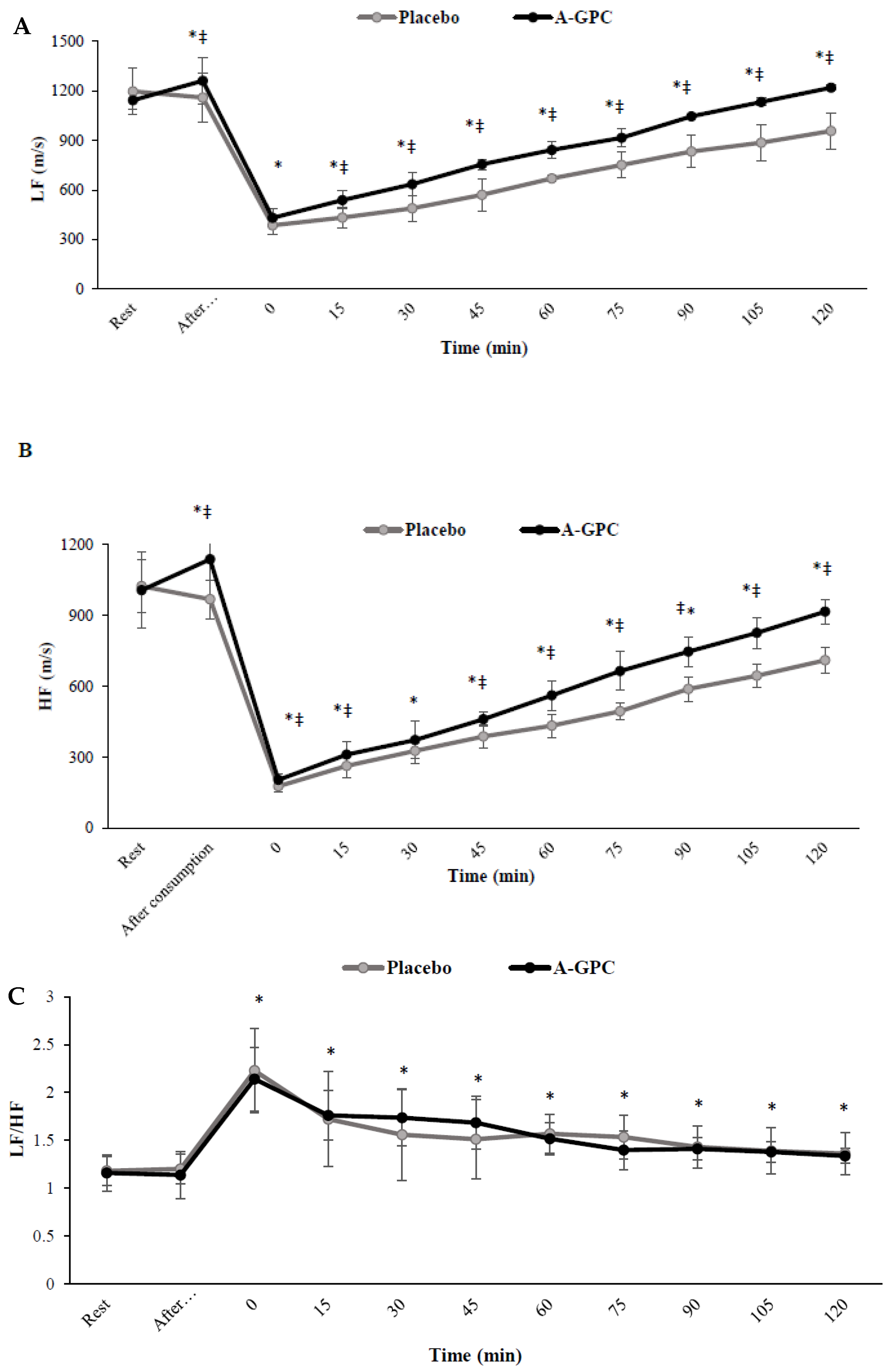
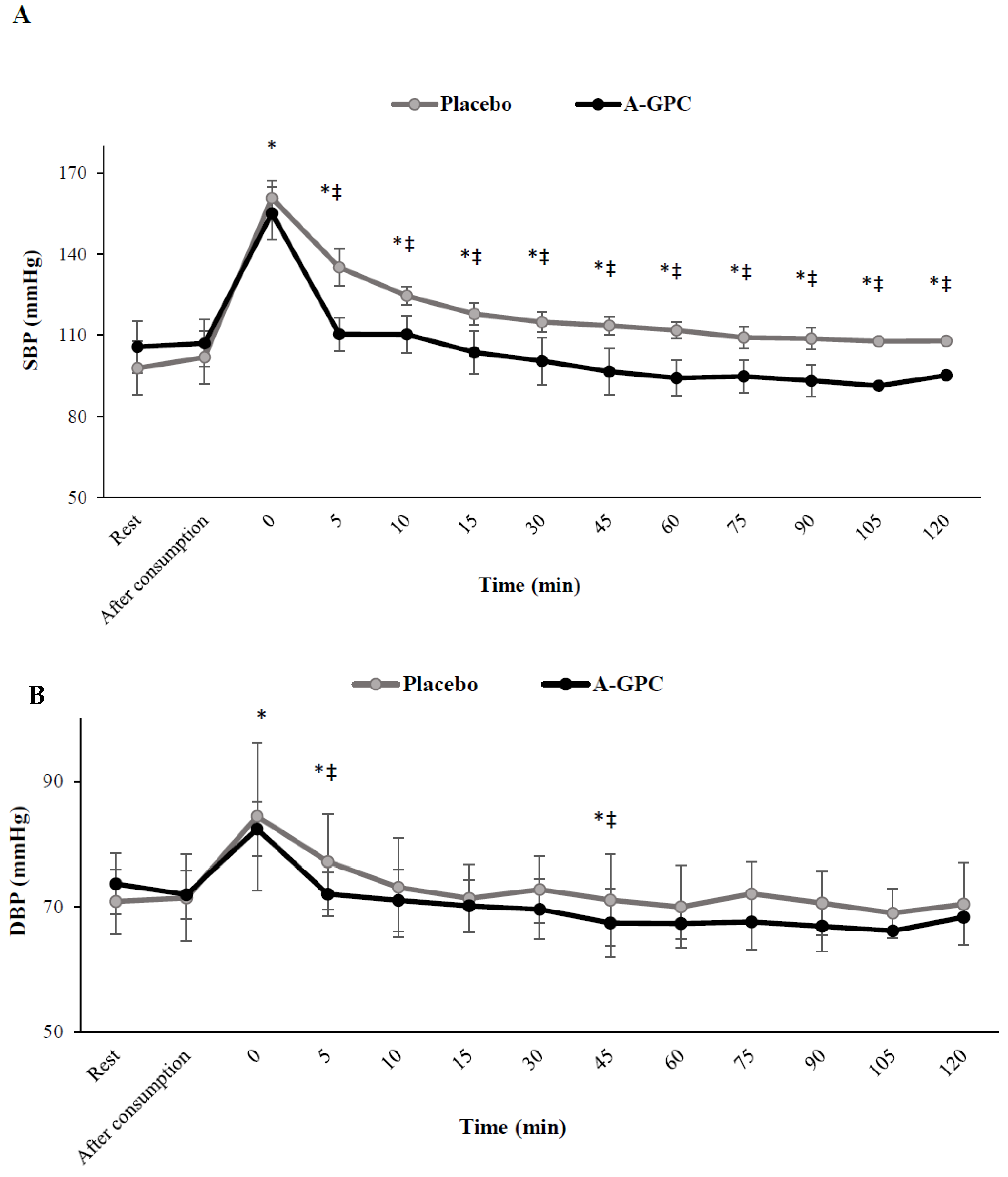
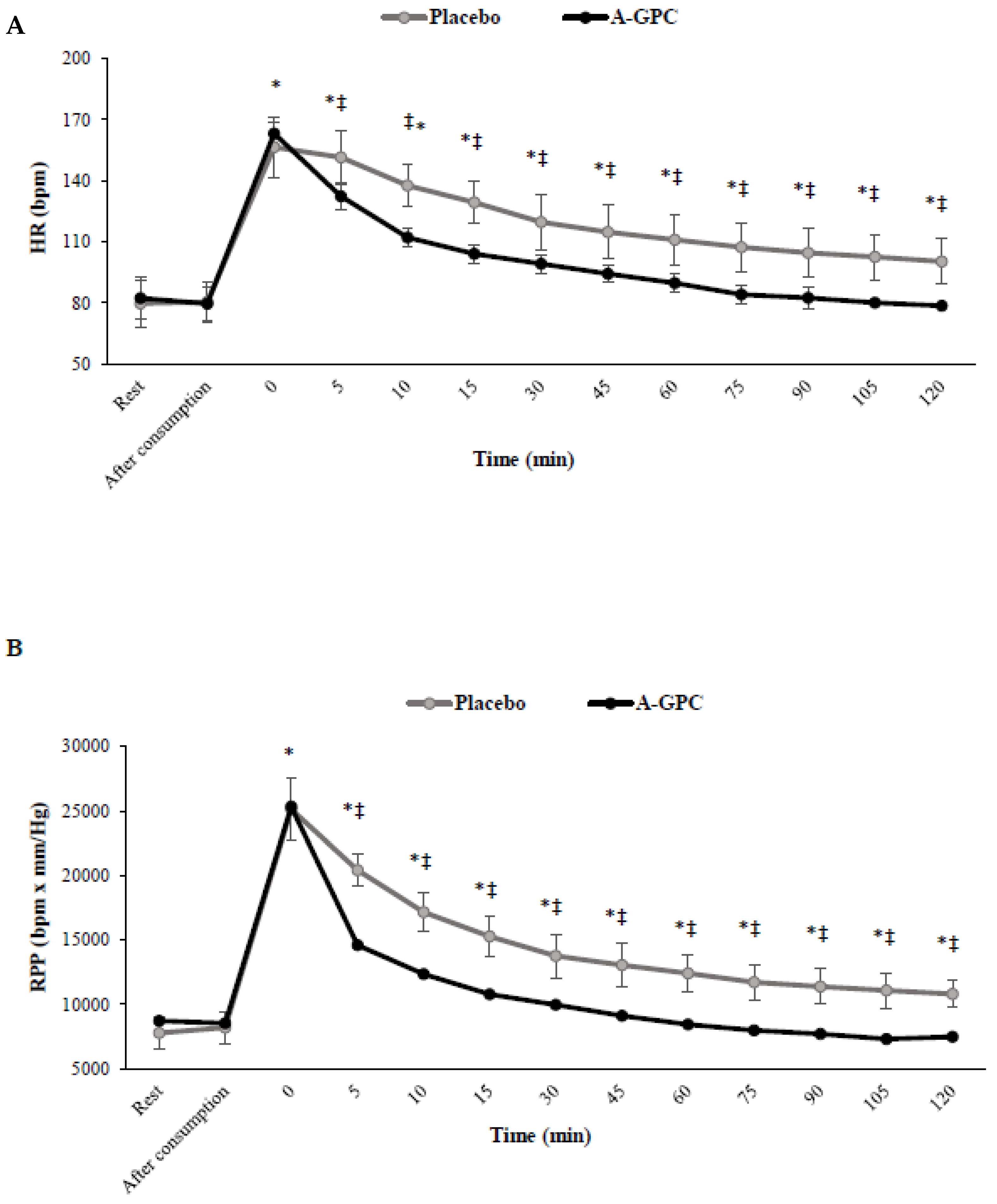
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Rankin, P.; Morton, D.P.; Diehl, H.; Gobble, J.; Morey, P.; Chang, E. Effectiveness of a volunteer-delivered lifestyle modification pro-gram for reducing cardiovascular disease risk factors. Am. J. Cardiol. 2012, 109, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J., III; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.L.; Yadav, P.K.; Yadav, L.K.; Agrawal, K.; Sah, S.K.; Islam, N. Association between obesity and heart rate variability indices: An intuition toward cardiac autonomic alteration—A risk of CVD. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Mittleman, M.A.; Chae, C.U.; Lee, I.-M.; Hennekens, C.H.; Manson, J.E. Triggering of Sudden Death from Cardiac Causes by Vigorous Exertion. N. Engl. J. Med. 2000, 343, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Ikarugi, H.; Shibata, M.; Shibata, S.; Ishii, H.; Taka, T.; Yamamoto, J. High Intensity Exercise Enhances Platelet Reactivity to Shear Stress and Coagulation during and after Exercise. Pathophysiol. Haemost. Thromb. 2003, 33, 127–133. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine; Thompson, P.D.; Franklin, B.A.; Balady, G.J.; Blair, S.N.; Corrado, D.; EstesIII, N.A.M.; Fulton, J.E.; Gordon, N.F.; Haskell, W.L.; et al. Exercise and acute cardiovascular events: Placing the risks into perspective: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007, 115, 2358–2368. [Google Scholar] [PubMed]
- Stuckey, M.I.; Tordi, N.; Mourot, L.; Gurr, L.J.; Rakobowchuk, M.; Millar, P.J.; Toth, R.; MacDonald, M.J.; Kamath, M.V. Autonomic recovery following sprint interval exercise. Scand. J. Med. Sci. Sports 2011, 22, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Vollaard, N.; Metcalfe, R. Research into the Health Benefits of Sprint Interval Training Should Focus on Protocols with Fewer and Shorter Sprints. Sports Med 2017, 12, 2443–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollaard, N.; Metcalfe, R.; Williams, S. Effect of Number of Sprints in an SIT Session on Change in V’O2max. Med. Sci. Sports Exerc. 2017, 49, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Koral, J.; Oranchuk, D.J.; Herrera, R.; Millet, G.Y. Six Sessions of Sprint Interval Training Improves Running Performance in Trained Athletes. J. Strength Cond. Res. 2018, 32, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Tong, T.K.; Kong, Z.; Tao, E.D.; Ying, X.; Nie, J. Cardiac autonomic disturbance following sprint-interval exercise in untrained young males: Does exercise volume matter? J. Exerc. Sci. Fit. 2022, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Fet, N.; Garab, D.; Szabó, A.; Kaszaki, J.; Srinivasan, P.K.; Tolba, R.H.; Boros, M. L-alpha-glycerylphosphorylcholine reduces the mi-crocirculatory dysfunction and nicotinamide adenine dinucleotide phosphate-oxidase type 4 induction after partial hepatic ischemia in rats. J. Surg. Res. 2014, 189, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Okubo, T.; Sato, K.; Fujita, S.; Goto, K.; Hamaoka, T.; Iemitsu, M. Glycerophosphocholine enhances growth hormone secretion and fat oxidation in young adults. Nutrition 2012, 28, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Soileau, J.; Judge, L.W.; Bellar, D. Evaluation of the effects of two doses of alpha glycerylphosphorylcholine on physical and psychomotor performance. J. Int. Soc. Sports Nutr. 2017, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Tuboly, E.; Gáspár, R.; Ibor, M.O.; Gömöri, K.; Kiss, B.; Strifler, G.; Hartmann, P.; Ferdinandy, P.; Bartekova, M.; Boros, M.; et al. L-Alpha-glycerylphosphorylcholine can be cytoprotective or cytotoxic in neonatal rat cardiac myocytes: A double-edged sword phenomenon. Mol. Cell. Biochem. 2019, 460, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Brownawell, A.M.; Carmines, E.L.; Montesano, F. Safety assessment of AGPC as a food ingredient. Food Chem. Toxicol. 2011, 49, 1303–1315. [Google Scholar] [CrossRef]
- Lopez, C.; Govoni, S.; Battaini, F.; Bergamaschi, S.; Longoni, A.; Giaroni, C.; Trabucchi, M. Effect of a new cognition enhancer, alpha-glycerylphosphorylcholine, on scopolamine-induced amnesia and brain acetylcholine. Pharmacol. Biochem. Behav. 1991, 39, 835–840. [Google Scholar] [CrossRef]
- Sartori, C.; Lepori, M.; Scherrer, U. Interaction between nitric oxide and the cholinergic and sympathetic nervous system in car-diovascular control in humans. Pharm. Ther. 2005, 106, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lu, Y.; Bi, X.; Xu, M.; Yu, X.; Xue, R.; He, X.; Zang, W. Choline ameliorates cardiovascular damage by improving vagal activity and in-hibiting the inflammatory response in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Meyer, K.A.; Shea, J.W. Dietary Choline and Betaine and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2017, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.J.; Zhang, S.; Bhadelia, R.A.; Johnson, E.J.; Lichtenstein, A.H.; Rogers, G.T.; Rosenberg, I.H.; Smith, C.E.; Zeisel, S.H.; Scott, T.M.; et al. Choline and its metabolites are differently as-sociated with cardiometabolic risk factors, history of cardiovascular disease, and MRI-documented cerebrovascular disease in older adults. Am. J. Clin. Nutr. 2017, 105, 1283–1290. [Google Scholar] [PubMed]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can. J. Sport Sci. 1992, 17, 338–345. [Google Scholar] [PubMed]
- Malik, M. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Ann. Noninvasive Elec-Trocardiol. 1996, 1, 151–181. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV–heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; Asmar, R.; Beilin, L.; Imai, Y.; Mancia, G.; Mengden, T.; Myers, M.; Padfield, P.; Palatini, P.; Parati, G.; et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J. Hypertens. 2005, 23, 697–701. [Google Scholar] [CrossRef]
- Abbiati, G.; Fossati, T.; Lachmann, G.; Bergamaschi, M.; Castiglioni, C. Absorption, tissue distribution and excretion of radiolabelled compounds in rats after administration of [14C]-l-α-glycerylphosphorylcholine. Eur. J. Drug Metab. Pharmacokinet. 1993, 18, 173–180. [Google Scholar] [CrossRef]
- Strifler, G.; Tuboly, E.; Görbe, A.; Boros, M.; Pécz, D.; Hartmann, P. Targeting mitochondrial dysfunction with L-alpha glycer-ylphosphorylcholine. PLoS ONE 2016, 11, e0166682. [Google Scholar] [CrossRef]
- Zimmer, A.; Teixeira, R.; Bonetto, J.; Bahr, A.; Türck, P.; de Castro, A.L.; Campos-Carraro, C.; Visioli, F.; Fernandes-Piedras, T.R.; Casali, K.R.; et al. Role of inflammation, oxidative stress, and autonomic nervous system activation during the development of right and left cardiac remodeling in experimental pulmonary arterial hyper-tension. Mol. Cell. Biochem. 2020, 464, 93–109. [Google Scholar] [CrossRef]
- Koopman, F.A.; Vosters, J.L.; Roescher, N.; Broekstra, N.; Tak, P.P.; Vervoordeldonk, M.J. Cholinergic anti-inflammatory pathway in the non-obese diabetic mouse model. Oral Dis. 2015, 21, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Zouhal, H.; Prioux, J.; Gratas-Delamarche, A.; Bentue-Ferrer, D.; Delamarche, P. Effect of the intensity of training on cat-echolamine responses to supramaximal exercise in endurance-trained men. Eur. J. Appl. Physiol. 2004, 91, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Jacob, C.; Rannou, F.; Gratas-Delamarche, A. Effect of training status on the sympathoadrenal activity during a su-pramaximal exercise in human. J. Sports Med. Phys. Fit. 2001, 41, 330. [Google Scholar]
- Aoi, W.; Naito, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Ichikawa, H.; Yoshida, N.; Yoshikawa, T. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic. Biol. Med. 2004, 37, 480–487. [Google Scholar] [CrossRef]
- Pyne, D. Exercise-induced muscle damage and inflammation: A review. Aust. J. Sci. Med. Sport 1994, 26, 49–58. [Google Scholar]
- Bellar, D.; LeBlanc, N.R.; Campbell, B. The effect of 6 days of alpha glycerylphosphorylcholine on isometric strength. J. Int. Soc. Sports Nutr. 2015, 12, 42. [Google Scholar] [CrossRef]
- Jagim, A.R.; Jones, M.T.; Wright, G.A.; St Antoine, C.; Kovacs, A.; Oliver, J.M. The acute effects of multi-ingredient pre-workout in-gestion on strength performance, lower body power, and anaerobic capacity. J. Int. Soc. Sports Nutr. 2016, 13, 1–10. [Google Scholar] [CrossRef]
- Traini, E.; Bramanti, V.; Amenta, F. Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline-containing phos-pholipid with a still interesting profile as cognition enhancing agent. Curr. Alzheimer Res. 2013, 10, 1070–1079. [Google Scholar] [CrossRef]

| Variable | Means ± SD |
|---|---|
| Age (year) | 31.0 ± 4.6 |
| Height (cm) | 160 ± 7.2 |
| Weight (kg) | 75.3 ± 4.0 |
| BMI (Kg/m2) | 29.4 ± 2.1 |
| BF (%) | 37.7 ± 4.8 |
| SDNN (m/s) | 66.7 ± 8.8 |
| pNN50 (%) | 30.5 ± 6.3 |
| LF (m/s) | 1168 ± 113 |
| HF (m/s) | 1091 ± 158 |
| LF/HF (ratio) | 0 ± 0.1 |
| SBP (mmHg) | 109 ± 5.8 |
| DBP(mmHg) | 73.2 ± 6.8 |
| HR (bpm) | 76.7 ± 9.2 |
| RPP (bpm×mmHg) | 8360 ± 921 |
| Characteristics | Placebo 1st Bout | Supplement 1st Bout | Placebo 2nd Bout | Supplement 2nd Bout |
|---|---|---|---|---|
| Peak power (W) | 407 ± 63.1 | 465 ± 88.1 ‡ | 362 ± 54.5 | 416 ± 77.4 ‡ |
| Mean power (W) | 304 ± 40.4 | 338 ± 59.6 ‡ | 257 ± 45.6 | 292 ± 69.9 ‡ |
| Fatigue index (%) | 55.8 ± 15.8 | 51.8 ± 6.0 | 60.4 ± 10.2 | 57.7 ± 9.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barzanjeh, S.P.; Pescatello, L.S.; Figueroa, A.; Ahmadizad, S. The Effects of Alpha-Glycerylphosphorylcholine on Heart Rate Variability and Hemodynamic Variables Following Sprint Interval Exercise in Overweight and Obese Women. Nutrients 2022, 14, 3970. https://doi.org/10.3390/nu14193970
Barzanjeh SP, Pescatello LS, Figueroa A, Ahmadizad S. The Effects of Alpha-Glycerylphosphorylcholine on Heart Rate Variability and Hemodynamic Variables Following Sprint Interval Exercise in Overweight and Obese Women. Nutrients. 2022; 14(19):3970. https://doi.org/10.3390/nu14193970
Chicago/Turabian StyleBarzanjeh, Seyedeh Parya, Linda S. Pescatello, Arturo Figueroa, and Sajad Ahmadizad. 2022. "The Effects of Alpha-Glycerylphosphorylcholine on Heart Rate Variability and Hemodynamic Variables Following Sprint Interval Exercise in Overweight and Obese Women" Nutrients 14, no. 19: 3970. https://doi.org/10.3390/nu14193970
APA StyleBarzanjeh, S. P., Pescatello, L. S., Figueroa, A., & Ahmadizad, S. (2022). The Effects of Alpha-Glycerylphosphorylcholine on Heart Rate Variability and Hemodynamic Variables Following Sprint Interval Exercise in Overweight and Obese Women. Nutrients, 14(19), 3970. https://doi.org/10.3390/nu14193970







