Melatonin in Endometriosis: Mechanistic Understanding and Clinical Insight
Abstract
1. Introduction
2. The Biology of Melatonin
2.1. Synthesis
2.2. Metabolism
2.3. Receptors
2.4. Biological Effects
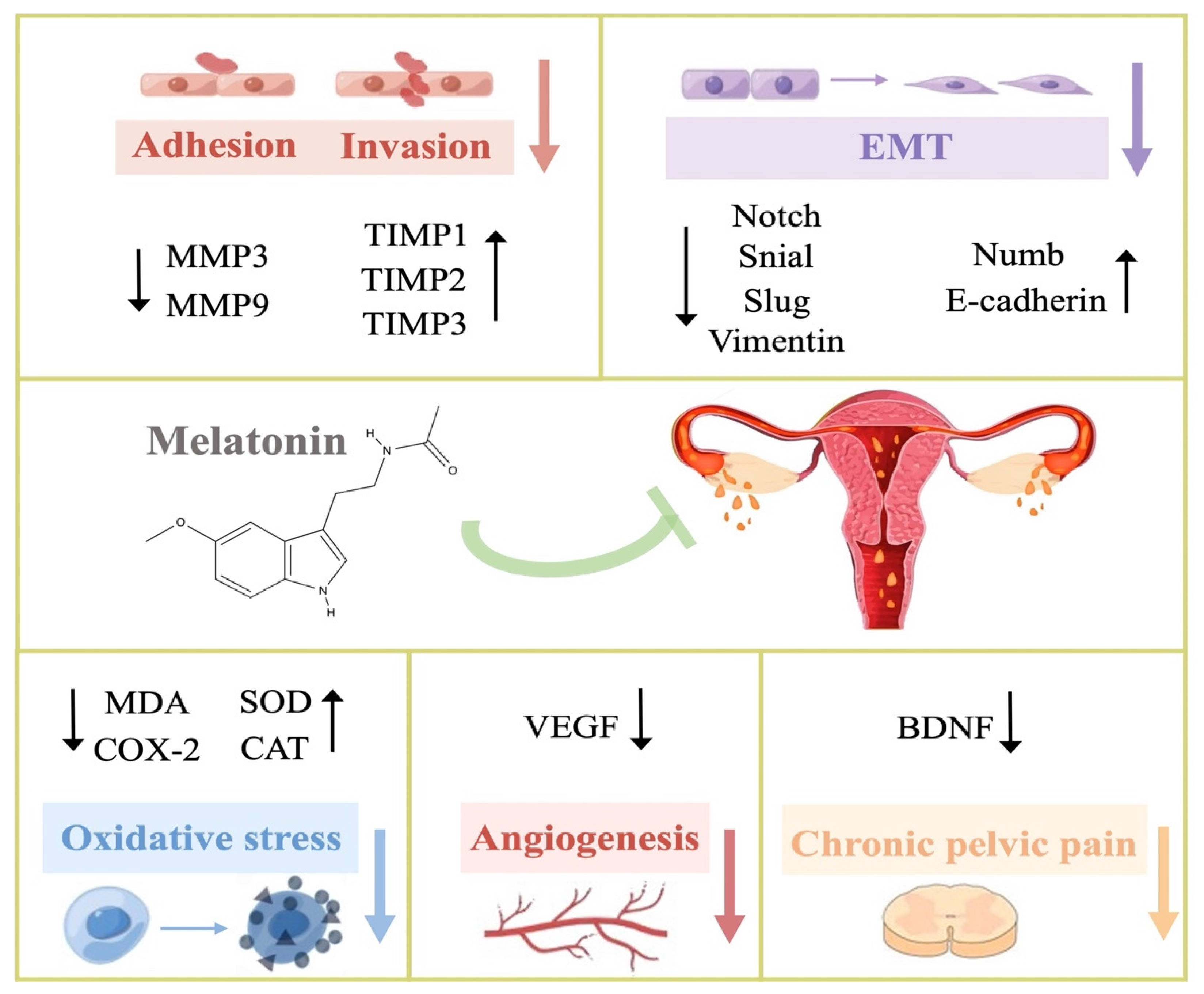
3. Potential Therapeutic Mechanisms of Melatonin in Endometriosis
| Studies | Study Type | Study Sample Specie | Models/Methods | Dose | Route | Duration | Results | Mechanism |
|---|---|---|---|---|---|---|---|---|
| Guney 2008 [58] | Animal in vivo | rats | Endometrium fragments sutured to the abdominal wall | 10 mg/kg daily | ip | 4 weeks | Decreased lesion sizes and weight | Increased SOD, CAT Decreased MDA, COX-2 |
| Paul 2008 [59] |
Animal in vivo | mice | Intraperitoneal transplantation | 48 mg/kg daily | ip | 10 or 20 days | / | Increased TIMP-1 Decreased MMP-9 |
| Paul 2010 [60] |
Animal in vivo | mice | Intraperitoneal transplantation | 48 mg/kg daily | ip | 10 or 20 days | / | Increased TIMP-3 Decreased MMP3 |
| Yildirim 2010 [61] |
Animal in vivo | rats | Endometrium fragments sutured to the abdominal wall | 10 mg/kg daily | ip | 2 weeks | Decreased lesion sizes Decreased histopathologic scores | Increased SOD, CAT |
| Koc 2010 [62] |
Animal in vivo | rats | Endometrium fragments sutured to the abdominal wall | 10 mg/kg daily | ip | 4 weeks | Decreased histopathologic score | Increased SOD, CAT Decreased MDA |
| Kocadal 2013 [63] |
Animal in vivo | rats | Intraperitoneal transplantation | 20 mg/kg daily | im and ip | 2 weeks | Decreased lesion sizes Decreased histopathologic score | / |
| Yilmaz 2015 [51] |
Animal in vivo | rats | Endometrium fragments sutured to the abdominal wall | 10 mg/kg daily | ip | 4 weeks | Decreased lesion weight Decreased histopathologic scores | Increased SOD, MDA, TIMP-2 Decreased VEGF, MMP-9 |
| Cetinkaya 2015 [50] |
Animal in vivo | rats | Endometrium fragments sutured to the abdominal wall | 10 or 20 mg/kg daily | im and ip | 2 weeks | Decreased lesion sizes in both treatment groups without significant difference | / |
| Shasha 2018 [64] | Cell in vitro | human | Endometriotic eutopic epithelial cell culture | 1 mM | culture medium | 72 h | / | Increased Numb, E-cadherin Decreased migration, invasion and Notch1, Vimentin, Slug, Snail |

3.1. Anti-Oxidation
3.2. Regulation of Steroid Hormone Production and Function
3.2.1. Estrogen
3.2.2. Progesterone
3.3. Anti-Proliferation and Pro-Apoptosis
3.4. Anti-Adhesion and Anti-Invasion
3.5. Anti-Epithelial–Mesenchymal Transition (Anti-EMT)
3.6. Anti-Angiogenesis
3.7. Immune Modulation
3.8. Neurotrophic Effect
4. Clinical Insights of Melatonin in Endometriosis and Other Reproductive Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavallo, A. Melatonin and human puberty: Current perspectives. J. Pineal Res. 1993, 15, 115–121. [Google Scholar] [CrossRef]
- Cagnacci, A. Melatonin in relation to physiology in adult humans. J. Pineal Res. 1996, 21, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B.; Daveau, A.; Maurice-Mandon, F.; Duarte, G.; Chemineau, P. Evidence That Melatonin Acts in the Premammillary Hypothalamic Area to Control Reproduction in the Ewe: Presence of Binding Sites and Stimulation of Luteinizing Hormone Secretion by in Situ Microimplant Delivery. Endocrinology 1998, 139, 1508–1516. [Google Scholar] [CrossRef]
- Gingerich, S.; Wang, X.; Lee, P.; Dhillon, S.; Chalmers, J.; Koletar, M.; Belsham, D. The generation of an array of clonal, immortalized cell models from the rat hypothalamus: Analysis of melatonin effects on kisspeptin and gonadotropin-inhibitory hormone neurons. Neuroscience 2009, 162, 1134–1140. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Li, L.; Ayre, E.; Pang, C.; Lee, P.; Xu, R.; Chow, P.; Yu, Z.; Shiu, S. Neuroendocrinology of melatonin in reproduction: Recent developments. J. Chem. Neuroanat. 1998, 14, 157–166. [Google Scholar] [CrossRef]
- Yellon, S.M.; Longo, L.D. Melatonin rhythms in fetal and maternal circulation during pregnancy in sheep. Am. J. Physiol. Metab. 1987, 252, E799–E802. [Google Scholar] [CrossRef] [PubMed]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2020, 2020, 8. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Bulun, S.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Horne, A.W.; Saunders, P. SnapShot: Endometriosis. Cell 2019, 179, 1677–1677.e1. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Greene, R.; Stratton, P.; Cleary, S.D.; Ballweg, M.L.; Sinaii, N. Diagnostic experience among 4334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 2009, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Evangelisti, G.; Barra, F. Current and emerging treatment options for endometriosis. Expert Opin. Pharmacother. 2018, 19, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.W.; Zhang, R.; Tan, Z.; Chung, J.P.W.; Zhang, T.; Wang, C.C. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: A review. Med. Res. Rev. 2021, 41, 2489–2564. [Google Scholar] [CrossRef]
- Schwertner, A.; Dos Santos, C.C.C.; Costa, G.D.; Deitos, A.; Souza, A.; Custodio-De-Souza, I.-C.; Torres, I.L.; Cunha-Filho, J.; Caumo, W. Efficacy of melatonin in the treatment of endometriosis: A phase II, randomized, double-blind, placebo-controlled trial. Pain 2013, 154, 874–881. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef]
- Kvetnoy, I.M. Extrapineal Melatonin: Location and Role within Diffuse Neuroendocrine System. Histochem. J. 1999, 31, 1–12. [Google Scholar] [CrossRef]
- Ekmekcioglu, C. Melatonin receptors in humans: Biological role and clinical relevance. Biomed. Pharmacother. 2006, 60, 97–108. [Google Scholar] [CrossRef]
- Yu, H.; Dickson, E.J.; Jung, S.-R.; Koh, D.-S.; Hille, B. High membrane permeability for melatonin. J. Gen. Physiol. 2015, 147, 63–76. [Google Scholar] [CrossRef]
- Ma, X.; Idle, J.R.; Krausz, K.W.; Gonzalez, F.J. Metabolism of melatonin by human cytochromes p450. Drug Metab. Dispos. 2005, 33, 489–494. [Google Scholar] [CrossRef]
- Harpsøe, N.G.; Andersen, L.P.H.; Gögenur, I.; Rosenberg, J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015, 71, 901–909. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, Classification, and Pharmacology of G Protein-Coupled Melatonin Receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- Mazzucchelli, C.; Pannacci, M.; Nonno, R.; Lucini, V.; Fraschini, F.; Stankov, B.M. The melatonin receptor in the human brain: Cloning experiments and distribution studies. Mol. Brain Res. 1996, 39, 117–126. [Google Scholar] [CrossRef]
- Weaver, D.R.; Reppert, S.M. The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. NeuroReport 1996, 8, 109–112. [Google Scholar] [CrossRef]
- Viswanathan, M.; Laitinen, J.T.; Saavedra, J.M. Expression of melatonin receptors in arteries involved in thermoregulation. Proc. Natl. Acad. Sci. USA 1990, 87, 6200–6203. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, P.C.; Brzozowska, I.; Pawlik, M.; Sliwowski, Z.; Cześnikiewicz-Guzik, M.; Kwiecień, S.; Brzozowski, T.; Bubenik, G.; Pawlik, W.W. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2007, 58, 381–405. [Google Scholar]
- Drew, J.; Williams, L.; Hannah, L.; Barrett, P. Abramovich Melatonin receptors in the human fetal kidney: 2-[125I]iodomelatonin binding sites correlated with expression of Mel1a and Mel1b receptor genes. J. Endocrinol. 1998, 156, 261–267. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.; Zmijewski, M.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Iwasaki, S.; Nakazawa, K.; Sakai, J.; Kometani, K.; Iwashita, M.; Yoshimura, Y.; Maruyama, T. Melatonin as a local regulator of human placental function. J. Pineal Res. 2005, 39, 261–265. [Google Scholar] [CrossRef]
- Schlabritz-Loutsevitch, N.; Hellner, N.; Middendorf, R.; Muller, D.; Olcese, J. The Human Myometrium as a Target for Melatonin. J. Clin. Endocrinol. Metab. 2003, 88, 908–913. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Itoh, M.T.; Kondo, H.; Okuma, Y.; Sato, S.; Kanishi, Y.; Hamada, N.; Kiguchi, K.; Ishizuka, B. Melatonin binding sites in estrogen receptor-positive cells derived from human endometrial cancer. J. Pineal Res. 2003, 35, 71–74. [Google Scholar] [CrossRef]
- Becker-André, M.; Wiesenberg, I.; Schaeren-Wiemers, N.; André, E.; Missbach, M.; Saurat, J.-H.; Carlberg, C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1997, 272, 28531–28534. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. J. Cereb. Blood Flow Metab. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Takayama, H.; Nakamura, Y.; Tamura, H.; Yamagata, Y.; Harada, A.; Nakata, M.; Sugino, N.; Kato, H. Pineal Gland(Melatonin) Affects the Parturition Time, but not Luteal Function and Fetal Growth, in Pregnant Rats. Endocr. J. 2003, 50, 37–43. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Salustiano, E.M.A.; Yen, P.W.; Soliman, A.; Vaillancourt, C. Melatonin in Pregnancy: Effects on Brain Development and CNS Programming Disorders. Curr. Pharm. Des. 2016, 22, 978–986. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Bahammam, A.S.; Ojike, N.I.; Akinseye, O.A.; Kendzerska, T.; Buttoo, K.; Dhandapany, P.S.; Brown, G.M.; Cardinali, D.P. Melatonin and Human Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2016, 22, 122–132. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Safa, M.; Kamrava, S.K.; Darabi, R.; Hayat, P.; Motevalian, M. Protective mechanisms of melatonin against hydrogen-peroxide-induced toxicity in human bone-marrow-derived mesenchymal stem cells. Can. J. Physiol. Pharmacol. 2017, 95, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Alves, M.; Martinez, M.; Camargo, I.C.C.; Pinheiro, P.F.F.; Domeniconi, R.F.; Júnior, L.A.L.; Martinez, F.E. Apoptosis is triggered by melatonin in an in vivo model of ovarian carcinoma. Endocr. Relat. Cancer 2015, 23, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.; Kireev, R.; Forman, K.; García, C.; Escames, G.; Ariznavarreta, C.; Vara, E.; Tresguerres, J.A. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8). Exp. Gerontol. 2010, 45, 950–956. [Google Scholar] [CrossRef] [PubMed]
- González-González, A.; González, A.; Alonso-González, C.; Menéndez-Menéndez, J.; Martínez-Campa, C.; Cos, S. Complementary actions of melatonin on angiogenic factors, the angiopoietin/Tie2 axis and VEGF, in co-cultures of human endothelial and breast cancer cells. Oncol. Rep. 2017, 39, 433–441. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores-Alvarado, L.J.; Manchester, L.C.; Tan, D.-X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2007, 44, 280–287. [Google Scholar] [CrossRef]
- Song, G.; Yoon, K.-A.; Chi, H.; Roh, J.; Kim, J.-H. Decreased concentration of serum melatonin in nighttime compared with daytime female medical technologists in South Korea. Chronobiol. Int. 2016, 33, 1305–1310. [Google Scholar] [CrossRef]
- Marino, J.L.; Holt, V.L.; Chen, C.; Davis, S. Shift Work, hCLOCK T3111C Polymorphism, and Endometriosis Risk. Epidemiology 2008, 19, 477–484. [Google Scholar] [CrossRef]
- Cetinkaya, N.; Attar, R.; Yildirim, G.; Ficicioglu, C.; Ozkan, F.; Yilmaz, B.; Yesildaglar, N. The effects of different doses of melatonin treatment on endometrial implants in an oophorectomized rat endometriosis model. Arch. Gynecol. Obstet. 2014, 291, 591–598. [Google Scholar] [CrossRef]
- Yilmaz, B.; Kilic, S.; Aksakal, O.; Ertas, I.E.; Tanrisever, G.G.; Aksoy, Y.; Lortlar, N.; Kelekci, S.; Gungor, T. Melatonin causes regression of endometriotic implants in rats by modulating angiogenesis, tissue levels of antioxidants and matrix metalloproteinases. Arch. Gynecol. Obstet. 2014, 292, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, G.; Attar, R.; Ozkan, F.; Kumbak, B.; Ficicioglu, C.; Yesildaglar, N. The effects of letrozole and melatonin on surgically induced endometriosis in a rat model: A preliminary study. Fertil. Steril. 2010, 93, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Mosher, A.A.; Tsoulis, M.W.; Lim, J.; Tan, C.; Agarwal, S.; Leyland, N.A.; Foster, W.G. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum. Reprod. 2019, 34, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Barchas, J.; Dacosta, F.; Spector, S. Acute Pharmacology of Melatonin. Nature 1967, 214, 919–920. [Google Scholar] [CrossRef]
- Cavalcante, A.G.D.M.; De Bruin, P.F.C.; De Bruin, V.M.S.; Nunes, D.M.; Pereira, E.D.B.; Cavalcante, M.M.; Andrade, G.M. Melatonin reduces lung oxidative stress in patients with chronic obstructive pulmonary disease: A randomized, double-blind, placebo-controlled study. J. Pineal Res. 2012, 53, 238–244. [Google Scholar] [CrossRef]
- Nickkholgh, A.; Schneider, H.; Sobirey, M.; Venetz, W.P.; Hinz, U.; Pelzl, L.H.; Gotthardt, D.N.; Cekauskas, A.; Manikas, M.; Mikalauskas, S.; et al. The use of high-dose melatonin in liver resection is safe: First clinical experience. J. Pineal Res. 2011, 50, 381–388. [Google Scholar] [CrossRef]
- Genario, R.; Morello, E.; Bueno, A.A.; Santos, H.O. The usefulness of melatonin in the field of obstetrics and gynecology. Pharmacol. Res. 2019, 147, 104337. [Google Scholar] [CrossRef]
- Güney, M.; Oral, B.; Karahan, N.; Mungan, T. Regression of endometrial explants in a rat model of endometriosis treated with melatonin. Fertil. Steril. 2008, 89, 934–942. [Google Scholar] [CrossRef]
- Paul, S.; Sharma, A.V.; Das Mahapatra, P.; Bhattacharya, P.; Reiter, R.J.; Swarnakar, S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal Res. 2008, 44, 439–449. [Google Scholar] [CrossRef]
- Paul, S.; Bhattacharya, P.; Das Mahapatra, P.; Swarnakar, S. Melatonin protects against endometriosis via regulation of matrix metalloproteinase-3 and an apoptotic pathway. J. Pineal Res. 2010, 49, 156–168. [Google Scholar] [CrossRef]
- Yıldırım, G.; Attar, R.; Fıçıcıoğlu, C.; Karateke, A.; Ozkan, F.; Kılıç, E.; Yılmaz, B.; Yeşildağlar, N. The combination of letrozole and melatonin causes regression in size not histopathological scores on endometriosis in an experimental rat model. J. Turk. Gynecol. Assoc. 2009, 10, 199–204. [Google Scholar]
- Koc, O.; Gunduz, B.; Topcuoglu, A.; Bugdayci, G.; Yilmaz, F.; Duran, B. Effects of pinealectomy and melatonin supplementation on endometrial explants in a rat model. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 153, 72–76. [Google Scholar] [CrossRef]
- Kocadal, N.C.; Attar, R.; Yıldırım, G.; Fıçıcıoğlu, C.; Ozkan, F.; Yılmaz, B.; Yesildaglar, N. Melatonin treatment results in regression of endometriotic lesions in an ooferectomized rat endometriosis model. J. Turk. Gynecol. Assoc. 2013, 14, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yan, L.; Liu, Z.; Mu, Y.-L.; Li, M.; Zhao, X.; Chen, Z.-J.; Zhang, H. Melatonin inhibits 17β-estradiol-induced migration, invasion and epithelial-mesenchymal transition in normal and endometriotic endometrial epithelial cells. Reprod. Biol. Endocrinol. 2018, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.-M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef]
- Yesildaglar, N.; Yıldırım, G.Y.; Attar, R.; Ozkan, F.; Akkaya, H.; Yilmaz, B. The effects of melatonin on endometriotic lesions induced by implanting human endometriotic cells in the first SCID-mouse endometriosis-model developed in Turkey. Clin. Exp. Obstet. Gynecol. 2016, 43, 25–30. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative Protection by Melatonin: Multiplicity of Mechanisms from Radical Detoxification to Radical Avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores-Alvarado, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2006, 42, 28–42. [Google Scholar] [CrossRef]
- Richter, H.G.; Hansell, J.A.; Raut, S.; Giussani, D.A. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. Pineal Res. 2009, 46, 357–364. [Google Scholar] [CrossRef]
- Mori, T.; Ito, F.; Koshiba, A.; Kataoka, H.; Takaoka, O.; Okimura, H.; Khan, K.N.; Kitawaki, J. Local estrogen formation and its regulation in endometriosis. Reprod. Med. Biol. 2019, 18, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; O’Malley, B.W. The dynamics of nuclear receptors and nuclear receptor coregulators in the pathogenesis of endometriosis. Hum. Reprod. Updat. 2014, 20, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-R.; Zhang, R.; Lian, Z.-X.; Deng, S.-L.; Yu, K. Estrogen-Receptor Expression and Function in Female Reproductive Disease. Cells 2019, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Gori, I.; Achtari, C.; Hornung, D.; Chardonnens, E.; Wunder, D.; Fiche, M.; Canny, G.O. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil. Steril. 2012, 98, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone Receptor Isoform A But Not B Is Expressed in Endometriosis. J. Clin. Endocrinol. Metab. 2000, 85, 2897–2902. [Google Scholar] [CrossRef]
- Flores, V.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef]
- Liang, B.; Wu, L.; Xu, H.; Cheung, C.W.; Fung, W.Y.; Wong, S.W.; Wang, C.C. Efficacy, safety and recurrence of new progestins and selective progesterone receptor modulator for the treatment of endometriosis: A comparison study in mice. Reprod. Biol. Endocrinol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Soares, J.M., Jr.; Gallo, C.C.; Furtado, A.; Cavaco, J.E.; Gonçalves, I.; Santos, C.R.A.; Quintela, T. The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology 2021, 112, 115–129. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Takiguchi, S.; Kashida, S.; Yamagata, Y.; Sugino, N.; Kato, H. Melatonin directly suppresses steroid production by preovulatory follicles in the cyclic hamster. J. Pineal Res. 1998, 25, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.; Seiva, F.R.; Fávaro, W.J.; Teixeira, G.R.; Amorim, J.P.; Mendes, L.O.; A Fioruci, B.; Pinheiro, P.F.F.; Fernandes, A.A.H.; Franci, J.A.; et al. Melatonin reduces LH, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod. Biol. Endocrinol. 2011, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Voordouw, B.C.; Euser, R.; Verdonk, R.E.; Alberda, B.T.; De Jong, F.H.; Drogendijk, A.C.; Fauser, B.C.; Cohen, M. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J. Clin. Endocrinol. Metab. 1992, 74, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Cos, S.; Sánchez-Barceló, E.J. Melatonin, experimental basis for a possible application in breast cancer prevention and treatment. Histol. Histopathol. 2000, 15, 637–647. [Google Scholar] [CrossRef] [PubMed]
- González, A. Melatonin: An Endogenous Antiestrogen with Oncostatic Properties. In Melatonin From Molecules to Therapy; Nova Science Publishers Inc.: New York, NY, USA, 2007; pp. 261–272. [Google Scholar]
- Hill, S.M.; Spriggs, L.L.; Simon, M.A.; Muraoka, H.; Blask, D.E. The growth inhibitory action of melatonin on human breast cancer cells is linked to the estrogen response system. Cancer Lett. 1992, 64, 249–256. [Google Scholar] [CrossRef]
- Bódis, J.; Koppán, M.; Kornya, L.; Tinneberg, H.; Török, A. Influence of Melatonin on Basal and Gonadotropin-Stimulated Progesterone and Estradiol Secretion of Cultured Human Granulosa Cells and in the Superfused Granulosa Cell System. Gynecol. Obstet. Investig. 2001, 52, 198–202. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Fang, L.; Li, Y.; Wang, S.; Li, Y.; Yan, Y.; Jia, Q.; Wu, Z.; Wang, Z.; Han, X.; et al. Melatonin stimulates aromatase expression and estradiol production in human granulosa-lutein cells: Relevance for high serum estradiol levels in patients with ovarian hyperstimulation syndrome. Exp. Mol. Med. 2020, 52, 1341–1350. [Google Scholar] [CrossRef]
- Chen, S.; Besman, M.J.; Sparkes, R.S.; Zollman, S.; Klisak, I.; Mohandas, T.; Hall, P.F.; Shively, J.E. Human Aromatase: cDNA Cloning, Southern Blot Analysis, and Assignment of the Gene to Chromosome 15. DNA 1988, 7, 27–38. [Google Scholar] [CrossRef]
- González, A.; Martínez-Campa, C.; Mediavilla, M.D.; Alonso-González, C.; Sánchez-Mateos, S.; Hill, S.M.; Sánchez-Barceló, E.J.; Cos, S. Effects of MT1 melatonin receptor overexpression on the aromatase-suppressive effect of melatonin in MCF-7 human breast cancer cells. Oncol. Rep. 2007, 17, 947–953. [Google Scholar] [CrossRef]
- Martínez-Campa, C.M.; González, A.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Alvarezgarcia, V.; Sanchez-Barcelo, E.J.; Cos, S. Melatonin inhibits aromatase promoter expression by regulating cyclooxygenases expression and activity in breast cancer cells. Br. J. Cancer 2009, 101, 1613–1619. [Google Scholar] [CrossRef]
- Alvarez-García, V.; González, A.; Martínez-Campa, C.; Alonso-González, C.; Cos, S. Melatonin modulates aromatase activity and expression in endothelial cells. Oncol. Rep. 2013, 29, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Martínez-Campa, C.M.; Mediavilla, M.D.; Alonso-Gonzalez, C.; Sanchez-Barcelo, E.J.; Cos, S. Inhibitory effects of pharmacological doses of melatonin on aromatase activity and expression in rat glioma cells. Br. J. Cancer 2007, 97, 755–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simpson, E.R.; Misso, M.; Hewitt, K.N.; Hill, R.; Boon, W.C.; Jones, M.; Kovacic, A.; Zhou, J.; Clyne, C.D. Estrogen—The Good, the Bad, and the Unexpected. Endocr. Rev. 2005, 26, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Reierstad, S.; Lu, M.; Lin, Z.; Ishikawa, H.; Bulun, S.E. Regulation of breast cancer-associated aromatase promoters. Cancer Lett. 2009, 273, 15–27. [Google Scholar] [CrossRef]
- Dardes, R.C.; Baracat, E.C.; Simões, M.J. Modulation of estrous cycle and LH, FSH and melatonin levels by pinealectomy and sham-pinealectomy in female rats. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2000, 24, 441–453. [Google Scholar] [CrossRef]
- Webley, G.E.; Luck, M.R. Melatonin directly stimulates the secretion of progesterone by human and bovine granulosa cells in vitro. Reproduction 1986, 78, 711–717. [Google Scholar] [CrossRef]
- Fang, L.; Li, Y.; Wang, S.; Yu, Y.; Li, Y.; Guo, Y.; Yan, Y.; Sun, Y.-P. Melatonin induces progesterone production in human granulosa-lutein cells through upregulation of StAR expression. Aging 2019, 11, 9013–9024. [Google Scholar] [CrossRef]
- Schaeffer, H.-J.; Sirotkin, A.V. Melatonin and serotonin regulate the release of insulin-like growth factor-I, oxytocin and progesterone by cultured human granulosa cells. Exp. Clin. Endocrinol. Diabetes 1997, 105, 109–112. [Google Scholar] [CrossRef]
- Woo, M.M.M.; Tai, C.-J.; Kang, S.K.; Nathwani, P.S.; Pang, S.F.; Leung, P. Direct Action of Melatonin in Human Granulosa-Luteal Cells. J. Clin. Endocrinol. Metab. 2001, 86, 4789–4797. [Google Scholar] [CrossRef]
- Taketani, T.; Tamura, H.; Takasaki, A.; Lee, L.; Kizuka, F.; Tamura, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Shimamura, K.; et al. Protective role of melatonin in progesterone production by human luteal cells. J. Pineal Res. 2011, 51, 207–213. [Google Scholar] [CrossRef]
- Wang, X.; Meng, K.; He, Y.; Wang, H.; Zhang, Y.; Quan, F. Melatonin Stimulates STAR Expression and Progesterone Production via Activation of the PI3K/AKT Pathway in Bovine Theca Cells. Int. J. Biol. Sci. 2019, 15, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Guan, S.; Tao, J.; Zhu, K.; Lv, D.; Wang, J.; Li, G.; Gao, Y.; Wu, H.; Liu, J.; et al. Melatonin promotes male reproductive performance and increases testosterone synthesis in mammalian Leydig cells. Biol. Reprod. 2021, 104, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Roshangar, L.; Soleimani, J.; Karimian, N. Evaluating the Effect of Melatonin on HAS2, PGR, Cumulus Expansion, and Fertility Potential in Mouse. Cell J. 2018, 20, 108–112. [Google Scholar] [CrossRef]
- Ram, P.; Yuan, L.; Dai, J.; Kiefer, T.; Klotz, D.M.; Spriggs, L.L.; Hill, S.M. Differential responsiveness of MCF-7 human breast cancer cell line stocks to the pineal hormone, melatonin. J. Pineal Res. 2000, 28, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.D.A.; Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. Int. J. Mol. Sci. 2019, 21, 300. [Google Scholar] [CrossRef]
- Sainz, R.M.; Mayo, J.C.; Rodriguez, C.; Tan, D.X.; Lopez-Burillo, S.; Reiter, R.J. Melatonin and cell death: Differential actions on apoptosis in normal and cancer cells. Cell Mol. Life Sci. 2003, 60, 1407–1426. [Google Scholar] [CrossRef]
- Vermeulen, K.; Berneman, Z.N.; Van Bockstaele, D.R. Cell cycle and apoptosis. Cell Prolif. 2003, 36, 165–175. [Google Scholar] [CrossRef]
- Lu, J.-J.; Fu, L.; Tang, Z.; Zhang, C.; Qin, L.; Wang, J.; Yu, Z.; Shi, D.; Xiao, X.; Xie, F.; et al. Melatonin inhibits AP-2β/hTERT, NF-κB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget 2015, 7, 2985–3001. [Google Scholar] [CrossRef]
- Ordoñez, R.; Carbajo-Pescador, S.; Prieto-Domínguez, N.; Palomo, A.G.; González-Gallego, J.; Mauriz, J.L. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J. Pineal Res. 2013, 56, 20–30. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, X.; Zhang, Y.; Shi, D.; Chen, W.; Fu, L.; Liu, L.; Xie, F.; Kang, T.; Huang, W.; et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J. Pineal Res. 2012, 53, 77–90. [Google Scholar] [CrossRef]
- Proietti, S.; Cucina, A.; D’Anselmi, F.; DiNicola, S.; Pasqualato, A.; Lisi, E.; Bizzarri, M. Melatonin and vitamin D3 synergistically down-regulate Akt and MDM2 leading to TGFβ-1-dependent growth inhibition of breast cancer cells. J. Pineal Res. 2010, 50, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, J.G.; Yeste-Velasco, M.; Esparza, J.L.; Verdaguer, E.; Palls, M.; Camins, A.; Folch, J.; Pallàs, M.; Esparza, J.L. The antiproliferative activity of melatonin in B65 rat dopaminergic neuroblastoma cells is related to the downregulation of cell cycle-related genes. J. Pineal Res. 2008, 45, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Cini, G.; Neri, B.; Pacini, A.; Cesati, V.; Sassoli, C.; Quattrone, S.; D’Apolito, M.; Fazio, A.; Scapagnini, G.; Provenzani, A.; et al. Antiproliferative activity of melatonin by transcriptional inhibition of cyclin D1 expression: A molecular basis for melatonin-induced oncostatic effects. J. Pineal Res. 2005, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, Y.; Reiter, R.J. Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone 2013, 55, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Najafi, M.; Farhood, B.; Ahmadi, A.; Potes, Y.; Shabeeb, D.; Musa, A.E. Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 2019, 228, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Klemmt, P.A.; Carver, J.G.; Koninckx, P.; McVeigh, E.J.; Mardon, H.J. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: Towards a mechanistic model for endometriosis progression. Hum. Reprod. 2007, 22, 3139–3147. [Google Scholar] [CrossRef]
- Rai, V.; Hopkisson, J.; Kennedy, S.; Bergqvist, A.; Barlow, D.H.; Mardon, H.J. Integrins alpha 3 and alpha 6 are differentially expressed in endometrium and endometriosis. J. Pathol. 1996, 180, 181–187. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, J.H.; Lee, S.H. Melatonin suppresses ischemia-induced fibrosis by regulating miR-149. Biochem. Biophys. Res. Commun. 2020, 525, 354–359. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Chung, H.-W.; Wen, Y.; Chun, S.-H.; Nezhat, C.; Woo, B.-H.; Polan, M.L. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: A rationale for endometriotic invasiveness. Fertil. Steril. 2001, 75, 152–159. [Google Scholar] [CrossRef]
- Shaco-Levy, R.; Sharabi, S.; Benharroch, D.; Piura, B.; Sion-Vardy, N. Matrix metalloproteinases 2 and 9, E-cadherin, and β-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 139, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, C.; Bonifacio, M.; Tommaselli, G.A.; Bifulco, G.; Guerra, G.; Nappi, C. Metalloproteinases, vascular endothelial growth factor, and angiopoietin 1 and 2 in eutopic and ectopic endometrium. Fertil. Steril. 2009, 91, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Collette, T.; Maheux, R.; Mailloux, J.; Akoum, A. Increased expression of matrix metalloproteinase-9 in the eutopic endometrial tissue of women with endometriosis. Hum. Reprod. 2006, 21, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Lee, J.Y.; Moon, H.-S.; Hur, S.E.; Park, M.H.; Wen, Y.; Polan, M.L. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil. Steril. 2002, 78, 787–795. [Google Scholar] [CrossRef]
- Collette, T.; Bellehumeur, C.; Kats, R.; Maheux, R.; Mailloux, J.; Villeneuve, M.; Akoum, A. Evidence for an increased release of proteolytic activity by the eutopic endometrial tissue in women with endometriosis and for involvement of matrix metalloproteinase-9. Hum. Reprod. 2004, 19, 1257–1264. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Botchorishvili, R.; Pouly, J.L.; Canis, M. Targeting the Wnt/β-catenin pathway in endometriosis: A potentially effective approach for treatment and prevention. Mol. Cell. Ther. 2014, 2, 36. [Google Scholar] [CrossRef]
- Cox, K.E.; Piva, M.; Sharpe-Timms, K.L. Differential regulation of matrix metalloproteinase-3 gene expression in endometriotic lesions compared with endometrium. Biol. Reprod. 2001, 65, 1297–1303. [Google Scholar] [CrossRef]
- Ganguly, K.; Maity, P.; Reiter, R.J.; Swarnakar, S. Effect of melatonin on secreted and induced matrix metalloproteinase-9 and -2 activity during prevention of indomethacin-induced gastric ulcer. J. Pineal Res. 2005, 39, 307–315. [Google Scholar] [CrossRef]
- Liu, D.; Shi, K.; Fu, M.; Chen, F. Melatonin indirectly decreases gastric cancer cell proliferation and invasion via effects on cancer-associated fibroblasts. Life Sci. 2021, 277, 119497. [Google Scholar] [CrossRef]
- Mao, L.; Yuan, L.; Slakey, L.M.; Jones, F.E.; E Burow, M.; Hill, S.M. Inhibition of breast cancer cell invasion by melatonin is mediated through regulation of the p38 mitogen-activated protein kinase signaling pathway. Breast Cancer Res. 2010, 12, R107. [Google Scholar] [CrossRef]
- Djordjevic, B.; Cvetkovic, T.; Stoimenov, T.J.; Despotovic, M.; Zivanovic, S.; Basic, J.; Veljkovic, A.; Velickov, A.; Kocic, G.; Pavlovic, D.; et al. Oral supplementation with melatonin reduces oxidative damage and concentrations of inducible nitric oxide synthase, VEGF and matrix metalloproteinase 9 in the retina of rats with streptozotocin/nicotinamide induced pre-diabetes. Eur. J. Pharmacol. 2018, 833, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.A.; Movassaghpour, A.A.; Ghanbari, H.; Kheirandish, M.; Maroufi, N.F.; Rahbarghazi, R.; Nouri, M.; Samadi, N. The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells. Sci. Rep. 2017, 7, 17062. [Google Scholar] [CrossRef]
- Jardim-Perassi, B.V.; Lourenço, M.R.; Doho, G.M.; Grígolo, I.H.; Gelaleti, G.B.; Ferreira, L.C.; Borin, T.F.; Moschetta, M.G.; Zuccari, D.A.P.D.C. Melatonin Regulates Angiogenic Factors under Hypoxia in Breast Cancer Cell Lines. Anti-Cancer Agents Med. Chem. 2016, 16, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hu, A.; Luo, Y.; Sun, W.; Hu, X.; Tang, S. Interleukin-4 and melatonin ameliorate high glucose and interleukin-1β stimulated inflammatory reaction in human retinal endothelial cells and retinal pigment epithelial cells. Mol. Vis. 2014, 20, 921–928. [Google Scholar] [PubMed]
- Černyšiov, V.; Mauricas, M.; Girkontaite, I. Melatonin inhibits granulocyte adhesion to ICAM via MT3/QR2 and MT2 receptors. Int. Immunol. 2015, 27, 599–608. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. Available online: http://www.clintransmed.com/%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed13&NEWS=N&AN=2015793182 (accessed on 26 September 2022). [CrossRef]
- Min, C.; Eddy, S.F.; Sherr, D.H.; Sonenshein, G.E. NF-κB and epithelial to mesenchymal transition of cancer. J. Cell. Biochem. 2008, 104, 733–744. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Qian, X.; Wu, Q.; Xia, J.; Miele, L.H.; Sarkar, F.H.; Wang, Z. Regulation of EMT by Notch signaling pathway in tumor progression. Curr. Cancer Drug Targets 2013, 13, 957–962. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Maggiolini, M.; Musti, A.M. Crosstalk between Notch, HIF-1α and GPER in Breast Cancer EMT. Int. J. Mol. Sci. 2018, 19, 2011. [Google Scholar] [CrossRef]
- Wu, S.-M.; Lin, W.-Y.; Shen, C.-C.; Pan, H.-C.; Bin, W.K.; Chen, Y.-C.; Jan, Y.-J.; Lai, D.-W.; Tang, S.-C.; Tien, H.-R.; et al. Melatonin set out to ER stress signaling thwarts epithelial mesenchymal transition and peritoneal dissemination via calpain-mediated C/EBPβ and NFκ B cleavage. J. Pineal Res. 2015, 60, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, B.; Xie, J.; Hou, D.; Zhang, H.; Huang, H. Melatonin inhibits epithelial-to-mesenchymal transition in gastric cancer cells via attenuation of IL-1β/NF-κB/MMP2/MMP9 signaling. Int. J. Mol. Med. 2018, 42, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Ndo, N.G.; Colombo, J.; Lopes, J.R.; Gelaleti, G.B.; Moschetta, M.G.; Sonehara, N.M.; Hellmén, E.; Cde, F.Z.; Oliani, S.M.; Zuccari, D.A. Effect of Melatonin in Epithelial Mesenchymal Transition Markers and Invasive Properties of Breast Cancer Stem Cells of Canine and Human Cell Lines. PLoS ONE 2016, 11, e0150407. [Google Scholar] [CrossRef]
- Mao, L.; Dauchy, R.T.; Blask, D.E.; Slakey, L.M.; Xiang, S.; Yuan, L.; Dauchy, E.M.; Shan, B.; Brainard, G.C.; Hanifin, J.P.; et al. Circadian Gating of Epithelial-to-Mesenchymal Transition in Breast Cancer Cells Via Melatonin-Regulation of GSK3β. Mol. Endocrinol. 2012, 26, 1808–1820. [Google Scholar] [CrossRef]
- Mao, L.; Summers, W.; Xiang, S.; Yuan, L.; Dauchy, R.T.; Reynolds, A.; Wren-Dail, M.A.; Pointer, D.; Frasch, T.; Blask, D.E.; et al. Melatonin Represses Metastasis in Her2-Postive Human Breast Cancer Cells by Suppressing RSK2 Expression. Mol. Cancer Res. 2016, 14, 1159–1169. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, T.; Liu, X.; Li, Z.; Zhou, D.; Xu, W. Melatonin suppresses epithelial-to-mesenchymal transition in the MG-63 cell line. Mol. Med. Rep. 2019, 21, 1356–1364. [Google Scholar] [CrossRef]
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J. Cell. Physiol. 2019, 234, 19384–19392. [Google Scholar] [CrossRef]
- McLaren, J.; Prentice, A.; Charnock-Jones, D.S.; Smith, S.K. Vascular Endothelial Growth Factor (VEGF) Concentrations Are Elevated in Peritoneal Fluid of Women With Endometriosis. Obstet. Gynecol. Surv. 1996, 51, 488–490. [Google Scholar] [CrossRef]
- McLaren, J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum. Reprod. Updat. 2000, 6, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Liu, S.; Xin, X.; Hua, T.; Shi, R.; Chi, S.; Jin, Z.; Wang, H. Efficacy of Anti-VEGF/VEGFR Agents on Animal Models of Endometriosis: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0166658. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, T.; Man, G.C.W.; May, K.E.; Becker, C.M.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wong, A.W.Y.; et al. Vascular endothelial growth factor C is increased in endometrium and promotes endothelial functions, vascular permeability and angiogenesis and growth of endometriosis. Angiogenesis 2013, 16, 541–551. [Google Scholar] [CrossRef]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.; Birsner, A.E.; D’Amato, R.J.; Man, C.W.; Wang, R. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011, 96, 1021–1028.e1. [Google Scholar] [CrossRef]
- Jardim-Perassi, B.V.; Arbab, A.S.; Ferreira, L.C.; Borin, T.F.; Varma, N.R.S.; Iskander, A.S.M.; Shankar, A.; Ali, M.M.; Zuccari, D.A.P.D.C. Effect of Melatonin on Tumor Growth and Angiogenesis in Xenograft Model of Breast Cancer. PLoS ONE 2014, 9, e85311. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Troncoso, A.M.; García-Parrilla, M.C. Inhibition of VEGF-Induced VEGFR-2 Activation and HUVEC Migration by Melatonin and Other Bioactive Indolic Compounds. Nutrients 2017, 9, 249. [Google Scholar] [CrossRef]
- Lissoni, P.; Rovelli, F.; Malugani, F.; Bucovec, R.; Conti, A.; Maestroni, G.J. Anti-angiogenic activity of melatonin in advanced cancer patients. Neuro Endocrinol. Lett. 2001, 22, 45–48. [Google Scholar]
- Hur, S.E.; Lee, J.Y.; Moon, H.-S.; Chung, H.W. Angiopoietin-1, angiopoietin-2 and Tie-2 expression in eutopic endometrium in advanced endometriosis. Mol. Hum. Reprod. 2006, 12, 421–426. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Lebovic, D.I.; Taylor, R.N. Eutopic Endometrium in Women with Endometriosis: Ground Zero for the Study of Implantation Defects. Semin. Reprod. Med. 2013, 31, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Agic, A.; Xu, H.; Finas, D.; Banz, C.; Diedrich, K.; Hornung, D. Is Endometriosis Associated with Systemic Subclinical Inflammation? Gynecol. Obstet. Investig. 2006, 62, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Updat. 2019, 25, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Mihalyi, A.; Gevaert, O.; Kyama, C.M.; Simsa, P.; Pochet, N.; De Smet, F.; De Moor, B.; Meuleman, C.; Billen, J.; Blanckaert, N.; et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum. Reprod. 2009, 25, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Vodolazkaia, A.; El-Aalamat, Y.; Popovic, D.; Mihalyi, A.; Bossuyt, X.; Kyama, C.M.; Fassbender, A.; Bokor, A.; Schols, D.; Huskens, D.; et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum. Reprod. 2012, 27, 2698–2711. Available online: http://linker2.worldcat.org/?rft.institution_id=129954&spage=2698&pkgName=UKPMC&issn=0268-1161&linkclass=to_article&jKey=Hum%2BReprod&issue=9&provider=NLM&date=2012-09&aulast=Vodolazkaia+A.%3B+Mihalyi+A.%3B+Kyama+C.M.%3B+Fassbender+A.%3B+Bokor+A.%3B+Meule (accessed on 26 September 2022). [CrossRef]
- Currier, N.L.; Sun, L.Z.-Y.; Miller, S.C. Exogenous melatonin: Quantitative enhancement in vivo of cells mediating non-specific immunity. J. Neuroimmunol. 2000, 104, 101–108. [Google Scholar] [CrossRef]
- Miller, S.C.; Pandi, P.S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J.M. The role of melatonin in immuno-enhancement: Potential application in cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef]
- Martins, E., Jr.; Fernandes, L.; Bartol, I.; Cipolla-Neto, J.; Rosa, L.C. The effect of melatonin chronic treatment upon macrophage and lymphocyte metabolism and function in Walker-256 tumour-bearing rats. J. Neuroimmunol. 1998, 82, 81–89. [Google Scholar] [CrossRef]
- Cho, J.H.; Bhutani, S.; Kim, C.H.; Irwin, M.R. Anti-inflammatory effects of melatonin: A systematic review and meta-analysis of clinical trials. Brain Behav. Immun. 2021, 93, 245–253. [Google Scholar] [CrossRef]
- Stratton, P.; Berkley, K.J. Chronic pelvic pain and endometriosis: Translational evidence of the relationship and implications. Hum. Reprod. Update 2010, 17, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Vincent, K.; Brawn, J.; Zondervan, K.T.; Becker, C.M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Update 2014, 20, 717–736. [Google Scholar] [CrossRef] [PubMed]
- Nockher, W.A.; Renz, H. Neurotrophins in allergic diseases: From neuronal growth factors to intercellular signaling molecules. J. Allergy Clin. Immunol. 2006, 117, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Meeus, M.; Versijpt, J.; Moens, M.; Bos, I.; Knaepen, K.; Meeusen, R. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: A new therapeutic target? Expert Opin. Ther. Targets 2014, 19, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.J.; Faulkner, S.; Fleiss, B.; Bainbridge, A.; Andorka, C.; Price, D.; Powell, E.; Lecky-Thompson, L.; Thei, L.; Chandrasekaran, M.; et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2012, 136, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Babaee, A.; Eftekhar-Vaghefi, S.H.; Asadi-Shekaari, M.; Shahrokhi, N.; Soltani, S.D.; Malekpour-Afshar, R.; Basiri, M. Melatonin treatment reduces astrogliosis and apoptosis in rats with traumatic brain injury. Iran. J. Basic Med. Sci. 2015, 18, 867–872. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Maestroni, G.J.M.; Esquifino, A.I.; Hardeland, R.; Cardinali, D.P. Role of melatonin in neurodegenerative diseases. Neurotox. Res. 2005, 7, 293–318. [Google Scholar] [CrossRef]
- Morris, R.W.; Lutsch, E.F. Daily Susceptibility Rhythm to Morphine Analgesia. J. Pharm. Sci. 1969, 58, 374–376. [Google Scholar] [CrossRef]
- Yu, C.-X.; Zhu, C.-B.; Xu, S.-F.; Cao, X.-D.; Wu, G.-C. The analgesic effects of peripheral and central administration of melatonin in rats. Eur. J. Pharmacol. 2000, 403, 49–53. [Google Scholar] [CrossRef]
- El-Shenawy, S.M.; Abdel-Salam, O.M.; Baiuomy, A.R.; El-Batran, S.; Arbid, M.S. Studies on the anti-inflammatory and anti-nociceptive effects of melatonin in the rat. Pharmacol. Res. 2002, 46, 235–243. [Google Scholar] [CrossRef]
- Lopez-Canul, M.; Palazzo, E.; Dominguez-Lopez, S.; Luongo, L.; Lacoste, B.; Comai, S.; Angeloni, D.; Fraschini, F.; Boccella, S.; Spadoni, G.; et al. Selective melatonin MT2 receptor ligands relieve neuropathic pain through modulation of brainstem descending antinociceptive pathways. Pain 2015, 156, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Masruha, M.R.; Vieira, D.S.D.S.; Minett, T.S.C.; Cipolla-Neto, J.; Zukerman, E.; Vilanova, L.C.P.; Peres, M.F.P. Low urinary 6-sulphatoxymelatonin concentrations in acute migraine. J. Headache Pain 2008, 9, 221–224. [Google Scholar] [CrossRef]
- Leone, M.; D’Amico, D.; Moschiano, F.; Fraschini, F.; Bussone, G. Melatonin Versus Placebo in the Prophylaxis of Cluster Headache: A double-blind pilot study with parallel groups. Cephalalgia 1996, 16, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Miano, S.; Parisi, P.; Pelliccia, A.; Luchetti, A.; Paolino, M.C.; Villa, M.P. Melatonin to prevent migraine or tension-type headache in children. Neurol. Sci. 2008, 29, 285–287. [Google Scholar] [CrossRef]
- Kurganova, Y.M.; Danilov, A. A role of melatonin in the treatment of low back pain. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2015, 115, 30–35. [Google Scholar] [CrossRef]
- Hussain, S.A.-R.; Alkhalifa, I.; Jasim, N.A.; Gorial, F.I. Adjuvant use of melatonin for treatment of fibromyalgia. J. Pineal Res. 2010, 50, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2018, 15, 105–125. [Google Scholar] [CrossRef]
- Wu, H.; Liu, J.; Yin, Y.; Zhang, D.; Xia, P.; Zhu, G. Therapeutic Opportunities in Colorectal Cancer: Focus on Melatonin Antioncogenic Action. BioMed Res. Int. 2019, 2019, 9740568. [Google Scholar] [CrossRef]
- Alston, M.; Cain, S.W.; Rajaratnam, S.M.W. Advances of Melatonin-Based Therapies in the Treatment of Disturbed Sleep and Mood. Sleep-Wake Neurobiol. Pharmacol. 2018, 253, 305–319. [Google Scholar] [CrossRef]
- Eryilmaz, O.G.; Devran, A.; Sarikaya, E.; Aksakal, F.N.; Mollamahmutoğlu, L.; Cicek, N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J. Assist. Reprod. Genet. 2011, 28, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Batıoğlu, A.S.; Şahin, U.; Gürlek, B.; Öztürk, N.; Unsal, E. The efficacy of melatonin administration on oocyte quality. Gynecol. Endocrinol. 2011, 28, 91–93. [Google Scholar] [CrossRef]
- Fernando, S.; Wallace, E.M.; Vollenhoven, B.; Lolatgis, N.; Hope, N.; Wong, M.; Lawrence, M.; Lawrence, A.; Russell, C.; Leong, K.; et al. Melatonin in Assisted Reproductive Technology: A Pilot Double-Blind Randomized Placebo-Controlled Clinical Trial. Front. Endocrinol. 2018, 9, 545. [Google Scholar] [CrossRef]
- Takasaki, A.; Nakamura, Y.; Tamura, H.; Shimamura, K.; Morioka, H. Melatonin as a new drug for improving oocyte quality. Reprod. Med. Biol. 2003, 2, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Espino, J.; Macedo, M.; Lozano, G.; Ortiz, A.; Rodríguez, C.; Rodríguez, A.B.; Bejarano, I. Impact of Melatonin Supplementation in Women with Unexplained Infertility Undergoing Fertility Treatment. Antioxidants 2019, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Fernando, S.; Osianlis, T.; Vollenhoven, B.; Wallace, E.; Rombauts, L. A pilot double-blind randomised placebo-controlled dose-response trial assessing the effects of melatonin on infertility treatment (MIART): Study protocol. BMJ Open 2014, 4, e005986. [Google Scholar] [CrossRef]
- Tagliaferri, V.; Romualdi, D.; Scarinci, E.; De Cicco, S.; Di Florio, C.; Immediata, V.; Tropea, A.; Santarsiero, C.M.; Lanzone, A.; Apa, R. Melatonin Treatment May Be Able to Restore Menstrual Cyclicity in Women With PCOS: A Pilot Study. Reprod. Sci. 2017, 25, 269–275. [Google Scholar] [CrossRef]
- Mokhtari, F.; Asbagh, F.A.; Azmoodeh, O.; Bakhtiyari, M.; Almasi-Hashiani, A. Effects of Melatonin Administration on Chemical Pregnancy Rates of Polycystic Ovary Syndrome Patients Undergoing Intrauterine Insemination: A Randomized Clinical Trial. Int. J. Fertil. Steril. 2019, 13, 225–229. [Google Scholar] [CrossRef]
- Miller, S.L.; Yawno, T.; Alers, N.O.; Castillo-Melendez, M.; Supramaniam, V.G.; Vanzyl, N.; Sabaretnam, T.; Loose, J.M.; Drummond, G.R.; Walker, D.W.; et al. Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J. Pineal Res. 2014, 56, 283–294. [Google Scholar] [CrossRef]
- Hobson, S.; Gurusinghe, S.; Lim, R.; Alers, N.O.; Miller, S.L.; Kingdom, J.C.; Wallace, E.M. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J. Pineal Res. 2018, 65, e12508. [Google Scholar] [CrossRef]
- Parandavar, N.; Abdali, K.; Keshtgar, S.; Emamghoreishi, M.; Amooee, S. The Effect of Melatonin on Climacteric Symptoms in Menopausal Women; A Double-Blind, Randomized Controlled, Clinical Trial. Iran. J. Public Health 2014, 43, 1405–1416. [Google Scholar] [PubMed]
- Rizzo, P.; Raffone, E.; Benedetto, V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and pregnancy outcome in IVF cycles. A prospective, clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 555–561. [Google Scholar] [PubMed]
- Nishihara, T.; Hashimoto, S.; Ito, K.; Nakaoka, Y.; Matsumoto, K.; Hosoi, Y.; Morimoto, Y. Oral melatonin supplementation improves oocyte and embryo quality in women undergoing in vitro fertilization-embryo transfer. Gynecol. Endocrinol. 2014, 30, 359–362. [Google Scholar] [CrossRef]
- Fernando, S.; Biggs, S.N.; Horne, R.S.C.; Vollenhoven, B.; Lolatgis, N.; Hope, N.; Wong, M.; Lawrence, M.; Lawrence, A.; Russell, C.; et al. The impact of melatonin on the sleep patterns of women undergoing IVF: A double blind RCT. Hum. Reprod. Open 2018, 2017, hox027. [Google Scholar] [CrossRef]
- Wdowiak, A.; Filip, M. The effect of myo-inositol, vitamin D3 and melatonin on the oocyte quality and pregnancy in in vitro fertilization: A randomized prospective controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, B.N.; Sadeghi, S.; Alipour, S.; Parsanezhad, M.E.; Alamdarloo, S.M. Effect of melatonin on the outcome of assisted repro-ductive technique cycles in women with diminished ovarian reserve: A double-blinded randomized clinical trial. Iran J. Med. Sci. 2017, 42, 73–78. [Google Scholar]
- Parandavar, N.; Hojat, M.; Abdali, K.; Keshtgar, S.; Emamghoreishi, M.; Yeganeh, B.S. The effect of melatonin on the lipid levels in menopausal women: A double-blind, controlled, clinical trial. J. Educ. Health Promot. 2018, 7, 144. [Google Scholar] [CrossRef]
- Chojnacki, C.; Kaczka, A.; Gasiorowska, A.; Fichna, J.; Chojnacki, J.; Brzozowski, T. The effect of long-term melatonin supplementation on psychosomatic disorders in postmenopausal women. J. Physiol. Pharmacol. 2018, 69, 297–304. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2015, 36, 169–175. [Google Scholar] [CrossRef]
| Studies | Disorder | Sample Size (Melatonin/ Control) | Age (Means ± SD/ SEM or Range) | Daily Dose | Duration or Period | Main Outcomes | Adverse Events | Trial Registration Number |
|---|---|---|---|---|---|---|---|---|
| Schwertner 2013 [18] | Endometriosis | 20/20 | 18–45 | 10 mg | 8 weeks | Reduced pain scores and BDNF levels Increased sleep quality | / | / |
| Takasaki 2003 [195] | Infertility | 27/NA | 34 ± 4.4 | 1 or 3 mg | From 2nd to 5th day of the menstrual cycle to hCG day | Decreased number of degenerate oocytes (only in 3 mg group) | / | / |
| Tamura 2008 [47] | Infertility | 56/59 | 34.8 ± 4.8 | 3 mg | From 2nd to 5th day of the menstrual cycle to OPU day | Increased oocytes quality and fertility rate | / | / |
| Rizzo 2010 [203] | Infertility | 32/33 | 35–42 | 3 mg | From GnRH administration day to pregnancy test result was confirmed | Increased tendency on number of mature oocytes, embryo quality, clinical pregnancy rate, and implantation rate | / | / |
| Batioglu 2012 [193] | Infertility | 40/45 | 20–40 | 3 mg | During the IVF-ET procedure | Increased number and percentage of mature oocytes Increased number of embryos of top quality Increased tendency on clinical pregnancy rate | / | / |
| Nishihara 2014 [204] | Infertility | 97/NA | ≤42 | 3 mg | At least 2 weeks | Increased fertilization and embryo quality | / | / |
| Fernando 2018 [205] | Infertility | 32/29 29/29 26/29 | 18–45 | 2, 4, or 8 mg | From 2nd to 5th day of the menstrual cycle to OPU day | No significant differences in daytime Karolinska sleepiness score between groups No differences in objective measures of sleep quantity or quality Improved the subjective sleep quality scores except 8 mg group | / | ACTRN12613001317785 |
| Fernando 2018 [194] | Infertility | 41/41 39/41 40/41 | 18–45 | 2, 4, or 8 mg | From 2nd to 5th day of the menstrual cycle to OPU day | There were no differences between all the groups in total oocyte number, number of MII oocytes, number of fertilized oocytes, the number or quality of embryos, clinical pregnancy rate, or live birth rate | Headache, fatigue (no significant difference) | ACTRN12613001317785 |
| Espino 2019 [196] | Infertility | 20/20 | 3 or 6 mg | From 2nd to 5th day of the menstrual cycle to OPU day | Ameliorated intrafollicular oxidative balance and oocyte quality Increased the rate of pregnancies/live births No significant difference between 3 mg group and 6 mg group | / | / | |
| Wdowiak 2020 [206] | Infertility | 50/50 | 20–35 | 1 mg | 6 months | Increased blastocyst and oocyte quality Increased rate of clinical pregnancy | / | / |
| Jahromi 2017 [207] | Diminished ovarian reserve | 40/40 | 22–42 | 3 mg | From 2nd to 5th day of the menstrual cycle to OPU day | Increased serum estradiol level on the triggering day Increased number of MII oocytes, top quality embryos with G1 and G2 | / | IRCT2014041417264N1 |
| Tagliaferri 2017 [198] | PCOS | 40/NA | 23.25 ± 4.07 | 2 mg | 6 months |
Improved the menstrual irregularities Decreased biochemical hyperandrogenism | / | / |
| Mokhtari 2019 [199] | PCOS | 98/100 | 28.9 ± 5.5 | 3 mg | From 2nd to 5th day of the menstrual cycle to hCG day | Increased chemical pregnancies and endometrial thickness | / | / |
| Parandavar 2018 [208] | Menopausal women | 98/142 | 53.22 ± 4.21 | 3 mg | 3 months | Increased amount of triglyceride | / | / |
| Chojnacki 2018 [209] | Postmenopausal women | 30/30 | 51–64 | 8 mg | 12 months | Decreased Kupperman Index and body mass index | No adverse influence on patients’ psychosomatic activity | / |
| Miller 2014 [200] | Fetal growth restriction | 6/6 | / | 8 mg | For the duration of pregnancy | Decreased placental oxidative stress | No adverse maternal or fetal effects | NCT01695070 |
| Parandavar 2014 [202] | Climacteric symptoms | 99/101 | 40–60 | 3 mg | 3 months | Decreased climacteric symptoms score | Sleepiness, nausea, vomiting, and headache (no significant difference) | / |
| Hobson 2018 [201] | Preeclampsia | 20/48 | Control: 30.6 ± 0.8 Melatonin: 32.7 ± 1.1 | 30 mg | From recruitment until delivery |
No adverse events and adverse drug reactions Prolonged gestation Reduced the pharmacological need for antihypertensives | No adverse events | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Hung, S.-W.; Zhang, R.; Man, G.C.-W.; Zhang, T.; Chung, J.P.-W.; Fang, L.; Wang, C.-C. Melatonin in Endometriosis: Mechanistic Understanding and Clinical Insight. Nutrients 2022, 14, 4087. https://doi.org/10.3390/nu14194087
Li Y, Hung S-W, Zhang R, Man GC-W, Zhang T, Chung JP-W, Fang L, Wang C-C. Melatonin in Endometriosis: Mechanistic Understanding and Clinical Insight. Nutrients. 2022; 14(19):4087. https://doi.org/10.3390/nu14194087
Chicago/Turabian StyleLi, Yiran, Sze-Wan Hung, Ruizhe Zhang, Gene Chi-Wai Man, Tao Zhang, Jacqueline Pui-Wah Chung, Lanlan Fang, and Chi-Chiu Wang. 2022. "Melatonin in Endometriosis: Mechanistic Understanding and Clinical Insight" Nutrients 14, no. 19: 4087. https://doi.org/10.3390/nu14194087
APA StyleLi, Y., Hung, S.-W., Zhang, R., Man, G. C.-W., Zhang, T., Chung, J. P.-W., Fang, L., & Wang, C.-C. (2022). Melatonin in Endometriosis: Mechanistic Understanding and Clinical Insight. Nutrients, 14(19), 4087. https://doi.org/10.3390/nu14194087








