Negative Effects of Chronic High Intake of Fructose on Lung Diseases
Abstract
1. Introduction
2. The Effect of the Amount of Fructose Intake from SSBs on Non-Communicable Diseases
3. Pulmonary Disease in LATAM
4. The Effect of Fructose Intake from SSBs on Lung Diseases
5. Uric Acid on Lung Function and Chronic High Intake of Fructose
6. The Renin–Angiotensin System on Lung Function and Chronic High Intake of Fructose
7. Receptor for Advanced Glycation End-Products in Lung Function and Chronic High Intake of Fructose
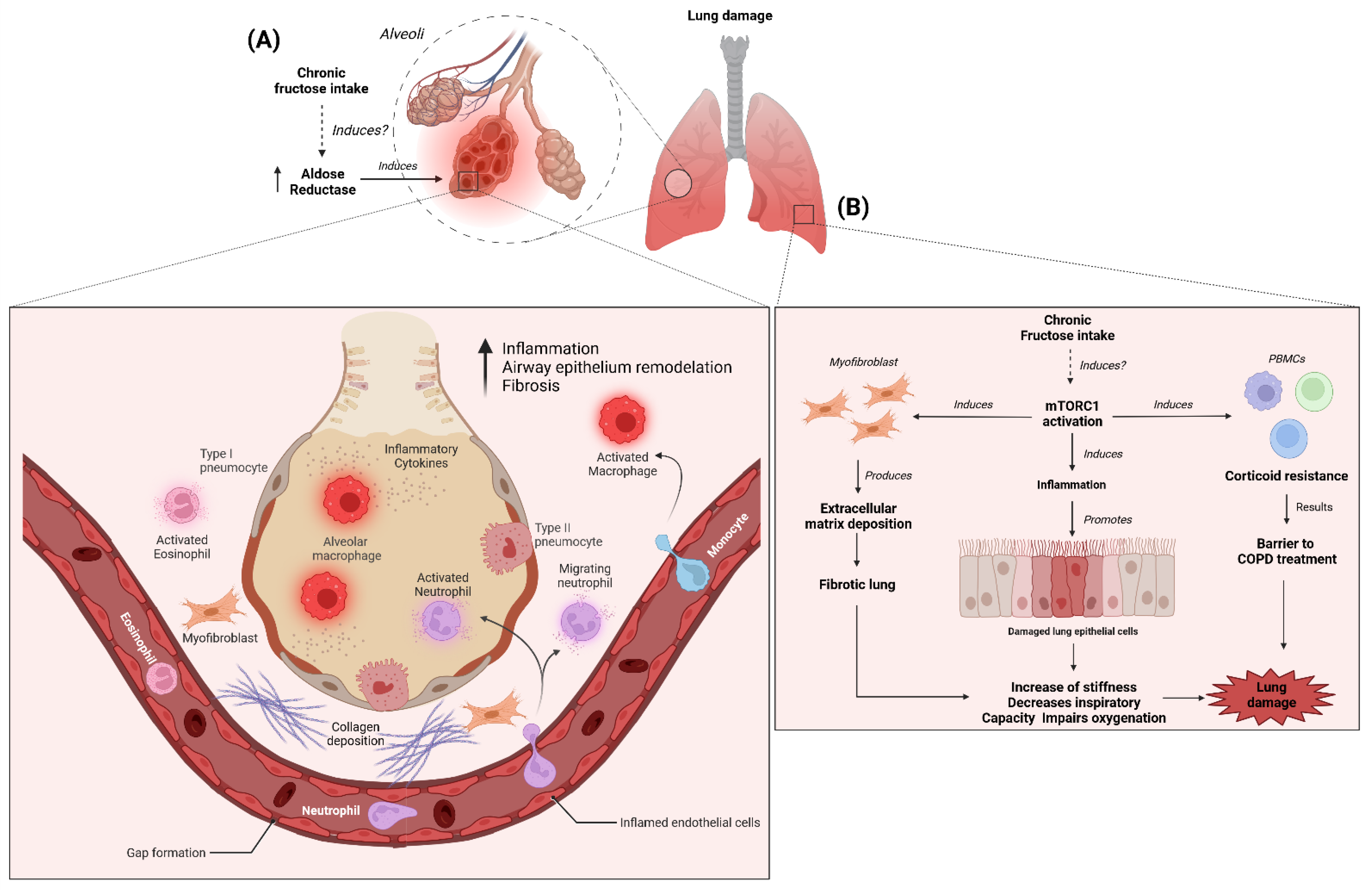
8. Aldose Reductase Activity in Lung Function and Chronic High Intake of Fructose
9. Activation of mTOR Signaling in Lung Function and Chronic High Intake of Fructose
10. Chronic High Intake of Fructose and Its Potential Involvement with COVID-19 Severity
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| AGEs | Advanced glycation end-products |
| AGT | Angiotensinogen |
| ALI | Acute lung injury |
| AMPK | AMP-activated protein kinase |
| Ang | Angiotensin |
| AR | Aldose reductase |
| ARB | Ang II receptor blockers |
| ARDS | Acute respiratory distress syndrome |
| AT1R | Ang-1 receptor |
| AT2R | Ang-2 receptor |
| BALF | Bronchoalveolar lavage fluid |
| BMI | Body mass index |
| CI | Confidence intervals |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| CVD | Cardiovascular disease |
| ET-1 | Endothelin-1 |
| Fru-AGEs | Fructose-derived AGEs |
| GSK3β | Glycogen synthase kinase 3β |
| HDL | High-density lipoprotein |
| HFCS | High-fructose corn syrup |
| HMGB1 | High-mobility group box 1 |
| HR | Hazard regression |
| HUVEC | Human umbilical vein endothelial cells |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| KC | Keratinocyte-derived chemokine |
| KHK | Ketohexokinase |
| KO | Knock-out |
| LATAM | Latin America |
| LDL | Low density lipoprotein |
| LPS | Lipopolysaccharide |
| MasR | Mas receptor |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MeS | Metabolic syndrome |
| MGO | Methylglyoxal |
| MIP-2 | Macrophage inflammatory protein 2 |
| mRAGE | Membrane-bound RAGE |
| mTOR | Mammalian target of rapamycin |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NCDs | Non-communicable diseases |
| NF-κB | Factor nuclear factor kappa B |
| NLRP3 | Nucleotide oligomerization domain (NOD)-like receptor protein 3 |
| NO | Nitric oxide |
| OR | Odds ratio |
| ox-LDL | Oxidized LDL |
| PAH | Pulmonary artery hypertension |
| PI3K | Phosphatidylinositol 3-kinase |
| PRR | Prorenin receptor |
| RAGE | Receptor for advanced glycation end-products |
| RAS | Renin-angiotensin system |
| ROS | Reactive oxygen species |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| sRAGE | Soluble RAGE |
| SSBs | Sugar-sweetened beverages |
| T2DM | Type 2 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TGFβ1 | Transforming growth factor-β1 |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| UA | Uric acid |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
| VSMC | Vascular smooth muscle cells |
| α-SMA | α-smooth muscle actin |
References
- Marriott, B.P.; Cole, N.; Lee, E. National Estimates of Dietary Fructose Intake Increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Ettridge, K.; Wakefield, M.; Pettigrew, S.; Coveney, J.; Roder, D.; Durkin, S.; Wittert, G.; Martin, J.; Dono, J. Consumption of sugar-sweetened beverages, juice, artificially-sweetened soda and bottled water: An Australian population study. Nutrients 2020, 12, 817. [Google Scholar] [CrossRef] [PubMed]
- Vanderlee, L.; White, C.M.; Kirkpatrick, S.I.; Rynard, V.L.; Jáuregui, A.; Adams, J.; Sacks, G.; Hammond, D. Nonalcoholic and Alcoholic Beverage Intakes by Adults across 5 Upper-Middle- and High-Income Countries. J. Nutr. 2021, 151, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Lim, S.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D.; Musaiger, A.O. Correction: Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: A systematic assessment of beverage intake in 187 countries. PLoS ONE 2019, 14, e0214344. [Google Scholar] [CrossRef]
- Stern, D.; Piernas, C.; Barquera, S.; Rivera, J.A.; Popkin, B.M. Caloric beverages were major sources of energy among children and adults in Mexico, 1999-2012. J. Nutr. 2014, 144, 949–956. [Google Scholar] [CrossRef]
- Matos, R.A.; Adams, M.; Sabaté, J. Review: The Consumption of Ultra-Processed Foods and Non-communicable Diseases in Latin America. Front. Nutr. 2021, 8, 110. [Google Scholar] [CrossRef]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Lim, S.; Ezzati, M.; Mozaffarian, D. Estimated global, regional, and national disease burdens related to sugar-sweetened beverage consumption in 2010. Circulation 2015, 132, 639–666. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Medici, V.; Bremer, A.A.; Lee, V.; Lam, H.D.; Nunez, M.V.; Chen, G.X.; Keim, N.L.; Havel, P.J. A dose-response study of consuming high-fructose corn syrup–sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am. J. Clin. Nutr. 2015, 101, 1144–1154. [Google Scholar] [CrossRef]
- Taskinen, M.-R.; Söderlund, S.; Bogl, L.H.; Hakkarainen, A.; Matikainen, N.; Pietiläinen, K.H.; Räsänen, S.; Lundbom, N.; Björnson, E.; Eliasson, B.; et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J. Intern. Med. 2017, 282, 187–201. [Google Scholar] [CrossRef]
- Geidl-Flueck, B.; Hochuli, M.; Németh, Á.; Eberl, A.; Derron, N.; Köfeler, H.C.; Tappy, L.; Berneis, K.; Spinas, G.A.; Gerber, P.A. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J. Hepatol. 2021, 75, 46–54. [Google Scholar] [CrossRef]
- Schulze, M.B.; Manson, J.A.E.; Ludwig, D.S.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Sugar-Sweetened Beverages, Weight Gain, and Incidence of Type 2 Diabetes in Young and Middle-Aged Women. JAMA 2004, 292, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.R.; Boggs, D.A.; Krishnan, S.; Hu, F.B.; Singer, M.; Rosenberg, L. Sugar-Sweetened Beverages and Incidence of Type 2 Diabetes Mellitus in African American Women. Arch. Intern. Med. 2008, 168, 1487. [Google Scholar] [CrossRef]
- Weber, K.S.; Simon, M.C.; Strassburger, K.; Markgraf, D.F.; Buyken, A.E.; Szendroedi, J.; Müssig, K.; Roden, M.; Al-Hasani, H.; Belgardt, B.; et al. Habitual Fructose Intake Relates to Insulin Sensitivity and Fatty Liver Index in Recent-Onset Type 2 Diabetes Patients and Individuals without Diabetes. Nutrients 2018, 10, 774. [Google Scholar] [CrossRef]
- Aeberli, I.; Gerber, P.A.; Hochuli, M.; Kohler, S.; Haile, S.R.; Gouni-Berthold, I.; Berthold, H.K.; Spinas, G.A.; Berneis, K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 94, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Couch, S.C.; Crandell, J.L.; Shah, A.S.; Dolan, L.M.; Merchant, A.T.; Liese, A.D.; Lawrence, J.M.; Pihoker, C.; Mayer-Davis, E.J. Fructose intake and cardiovascular risk factors in youth with type 1 diabetes: SEARCH for diabetes in youth study. Diabetes Res. Clin. Pract. 2013, 100, 265–271. [Google Scholar] [CrossRef]
- Semnani-Azad, Z.; Khan, T.A.; Blanco Mejia, S.; De Souza, R.J.; Leiter, L.A.; Kendall, C.W.C.; Hanley, A.J.; Sievenpiper, J.L. Association of Major Food Sources of Fructose-Containing Sugars With Incident Metabolic Syndrome: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e209993. [Google Scholar] [CrossRef] [PubMed]
- Choo, V.L.; Viguiliouk, E.; Blanco Mejia, S.; Cozma, A.I.; Khan, T.A.; Ha, V.; Wolever, T.M.S.; Leiter, L.A.; Vuksan, V.; Kendall, C.W.C.; et al. Food sources of fructose-containing sugars and glycaemic control: Systematic review and meta-analysis of controlled intervention studies. BMJ 2018, 363, 4644. [Google Scholar] [CrossRef]
- Bahrami, M.; Ataie-Jafari, A.; Hosseini, S.; Foruzanfar, M.H.; Rahmani, M.; Pajouhi, M. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. Int. J. Food Sci. Nutr. 2009, 60, 618–626. [Google Scholar] [CrossRef]
- Park, S.; Akinbami, L.J.; McGuire, L.C.; Blanck, H.M. Association of sugar-sweetened beverage intake frequency and asthma among U.S. adults, 2013. Prev. Med. 2016, 91, 58–61. [Google Scholar] [CrossRef]
- Wright, L.S.; Rifas-Shiman, S.L.; Oken, E.; Litonjua, A.A.; Gold, D.R. Prenatal and early life fructose, fructose-containing beverages, and midchildhood asthma. Ann. Am. Thorac. Soc. 2018, 15, 217–224. [Google Scholar] [CrossRef]
- Suehiro, C.L.; Toledo-Arruda, A.C.D.; Vieira, R.D.P.; Almeida, F.M.D.; Olivo, C.R.; Martins, M.D.A.; Lin, C.J. A possible association between fructose consumption and pulmonary emphysema. Sci. Rep. 2019, 9, 9344. [Google Scholar] [CrossRef]
- The Lancet GBD 2017: A fragile world. Lancet 2018, 392, 1683. [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for chronic obstructive pulmonary disease. Available online: https://journals.lww.com/co-pulmonarymedicine/Abstract/2002/03000/Global_Initiative_for_Chronic_Obstructive_Lung.1.aspx (accessed on 26 April 2022).
- Perez-Padilla, R.; Menezes, A.M.B. Chronic Obstructive Pulmonary Disease in Latin America. Ann. Glob. Health 2019, 85, 7. [Google Scholar] [CrossRef] [PubMed]
- PAHO/WHO. Chronic Respiratory Disease Burden. Available online: https://www.paho.org/en/noncommunicable-diseases-and-mental-health/noncommunicable-diseases-and-mental-health-data-20 (accessed on 26 April 2022).
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Forno, E.; Gogna, M.; Cepeda, A.; Yañez, A.; Solé, D.; Cooper, P.; Avila, L.; Soto-Quiros, M.; Castro-Rodriguez, J.A.; Celedón, J.C. Asthma in Latin America. Thorax 2015, 70, 898. [Google Scholar] [CrossRef]
- DeChristopher, L.R.; Tucker, K.L. Excess free fructose, high-fructose corn syrup and adult asthma: The Framingham Offspring Cohort. Br. J. Nutr. 2018, 119, 1157–1167. [Google Scholar] [CrossRef]
- DeChristopher, L.R.; Uribarri, J.; Tucker, K.L. Intakes of apple juice, fruit drinks and soda are associated with prevalent asthma in US children aged 2-9 years. Public Health Nutr. 2016, 19, 123–130. [Google Scholar] [CrossRef]
- Shi, Z.; Dal Grande, E.; Taylor, A.W.; Gill, T.K.; Adams, R.; Wittert, G.A. Association between soft drink consumption and asthma and chronic obstructive pulmonary disease among adults in Australia. Respirology 2012, 17, 363–369. [Google Scholar] [CrossRef]
- Dechristopher, L.R.; Uribarri, J.; Tucker, K.L. Intake of high fructose corn syrup sweetened soft drinks is associated with prevalent chronic bronchitis in U.S. Adults, ages 20-55 y. Nutr. J. 2015, 14, 107. [Google Scholar] [CrossRef]
- Kaluza, J.; Harris, H.R.; Linden, A.; Wolk, A. Long-term consumption of fruits and vegetables and risk of chronic obstructive pulmonary disease: A prospective cohort study of women. Int. J. Epidemiol. 2018, 47, 1897–1909. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added Sugar Intake and Cardiovascular Diseases Mortality Among US Adults. JAMA Intern. Med. 2014, 174, 516. [Google Scholar] [CrossRef] [PubMed]
- Vedova, M.C.D.; Soler Garcia, F.M.; Muñoz, M.D.; Fornes, M.W.; Gomez Mejiba, S.E.; Gómez, N.N.; Ramirez, D.C. Diet-Induced Pulmonary Inflammation and Incipient Fibrosis in Mice: A Possible Role of Neutrophilic Inflammation. Inflammation 2019, 42, 1886–1900. [Google Scholar] [CrossRef]
- Suehiro, C.; Toledo-Arruda, A.; Almeida, F.; Olivo, C.; Oliveira Jr, M.; Sousa, A.; Vieira, R.; Martins, M.; Lin, C. High fructose intake increases alveolar enlargement and muscle inflammation in mice exposed to cigarette smoke. Eur. Respir. J. 2016, 48, PA4010. [Google Scholar] [CrossRef]
- Becker, B.F.; Reinholz, N.; Leipert, B.; Ruschke, P.; Permanetter, B.; Gerlach, E. Role of Uric Acid as an Endogenous Radical Scavenger and Antioxidant. Chest 1991, 100, 176S–181S. [Google Scholar] [CrossRef] [PubMed]
- Nicks, M.E.; O’Brien, M.M.; Bowler, R.P. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD J. Chronic Obstr. Pulm. Dis. 2011, 8, 264–269. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Tapia, E.; Soto, V.; Sautin, Y.; Sánchez-Lozada, L.G. Uric Acid and Fructose: Potential Biological Mechanisms. Semin. Nephrol. 2011, 31, 426–432. [Google Scholar] [CrossRef]
- Akhavan, T.; Anderson, G.H. Effects of glucose-to-fructose ratios in solutions on subjective satiety, food intake, and satiety hormones in young men. Am. J. Clin. Nutr. 2007, 86, 1354–1363. [Google Scholar] [CrossRef]
- Cox, C.L.; Stanhope, K.L.; Schwarz, J.M.; Graham, J.L.; Hatcher, B.; Griffen, S.C.; Bremer, A.A.; Berglund, L.; McGahan, J.P.; Keim, N.L.; et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr. Metab. 2012, 9, 68. [Google Scholar] [CrossRef]
- Carran, E.L.; White, S.J.; Reynolds, A.N.; Haszard, J.J.; Venn, B.J. Acute effect of fructose intake from sugar-sweetened beverages on plasma uric acid: A randomised controlled trial. Eur. J. Clin. Nutr. 2016, 70, 1034–1038. [Google Scholar] [CrossRef]
- Olofsson, C.; Anderstam, B.; Bragfors-Helin, A.-C.; Eriksson, M.; Qureshi, A.R.; Lindholm, B.; Hilding, A.; Wiczkowski, W.; Orsini, N.; Stenvinkel, P.; et al. Effects of acute fructose loading on levels of serum uric acid-a pilot study. Eur. J. Clin. Investig. 2019, 49, e13040. [Google Scholar] [CrossRef]
- Romi, M.M.; Arfian, N.; Tranggono, U.; Setyaningsih, W.A.W.; Sari, D.C.R. Uric acid causes kidney injury through inducing fibroblast expansion, Endothelin-1 expression, and inflammation. BMC Nephrol. 2017, 18, 326. [Google Scholar] [CrossRef] [PubMed]
- Spiropoulos, K.; Trakada, G.; Nikolaou, E.; Prodromakis, E.; Efremidis, G.; Pouli, A.; Koniavitou, A. Endothelin-1 levels in the pathophysiology of chronic obstructive pulmonary disease and bronchial asthma. Respir. Med. 2003, 97, 983–989. [Google Scholar] [CrossRef]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.G.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric acid and inflammatory markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Cherubini, A.; Miller, E.; Maggio, M.; Najjar, S.S.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Usefulness of Uric Acid to Predict Changes in C-Reactive Protein and Interleukin-6 in 3-Year Period in Italians Aged 21 to 98 Years. Am. J. Cardiol. 2007, 100, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, M.T.; Aslami, H.; Vlaar, A.P.J.; Juffermans, N.P.; Tuip-de Boer, A.M.; Hegeman, M.A.; Jongsma, G.; Roelofs, J.J.T.H.; van der Poll, T.; Schultz, M.J.; et al. Pre-Treatment with Allopurinol or Uricase Attenuates Barrier Dysfunction but Not Inflammation during Murine Ventilator-Induced Lung Injury. PLoS ONE 2012, 7, e50559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Ma, L.-L.; Wang, L.-X. Relationship between serum uric acid levels and ventricular function in patients with idiopathic pulmonary hypertension. Exp. Clin. Cardiol. 2013, 18, e37–e39. [Google Scholar]
- Li, L.; Wan, C.; Wen, F. An unexpected role for serum uric acid as a biomarker for severity of asthma exacerbation. Asian Pac. J. Allergy Immunol. 2014, 32, 93–99. [Google Scholar] [CrossRef]
- Bartziokas, K.; Papaioannou, A.I.; Loukides, S.; Papadopoulos, A.; Haniotou, A.; Papiris, S.; Kostikas, K. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur. Respir. J. 2014, 43, 43–53. [Google Scholar] [CrossRef]
- Sharma, A. A Prospective Study on Serum Uric Acid levels in Chronic Obstructive Lung Disease Cases. Int. Arch. Clin. Res. Diagn. Lab. Med. 2019, 1, 10–12. [Google Scholar]
- Rumora Id, L.; Hlapčić, I.; Popović-Grle, S.; Rako, I.; Rogić, D.; Čepelak, I. Uric acid and uric acid to creatinine ratio in the assessment of chronic obstructive pulmonary disease: Potential biomarkers in multicomponent models comprising IL-1beta. PLoS ONE 2020, 15, e0234363. [Google Scholar] [CrossRef]
- Fujikawa, H.; Sakamoto, Y.; Masuda, N.; Oniki, K.; Kamei, S.; Nohara, H.; Nakashima, R.; Maruta, K.; Kawakami, T.; Eto, Y.; et al. Higher Blood Uric Acid in Female Humans and Mice as a Protective Factor against Pathophysiological Decline of Lung Function. Antioxidants 2020, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Waring, W.S.; McKnight, J.A.; Webb, D.J.; Maxwell, S.R.J. Uric Acid Restores Endothelial Function in Patients With Type 1 Diabetes and Regular Smokers. Diabetes 2006, 55, 3127–3132. [Google Scholar] [CrossRef] [PubMed]
- Gasse, P.; Riteau, N.; Charron, S.; Girre, S.; Fick, L.; Pétrilli, V.; Tschopp, J.; Lagente, V.; Quesniaux, V.F.J.; Ryffel, B.; et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 2009, 179, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ishikawa, M.; Abe, K.; Ishikawa, T.; Imakiire, S.; Masaki, K.; Hosokawa, K.; Fukuuchi, T.; Kaneko, K.; Ohtsubo, T.; et al. Increased lung uric acid deteriorates pulmonary arterial hypertension. J. Am. Heart Assoc. 2021, 10, e022712. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-G.; Tae, Y.-M.; Kim, Y.-S.; Gyu Jeon, S.; Oh, S.-Y.; Song Gho, Y.; Zhu, Z.; Kim, Y.-K. Conversion of Th17-type into Th2-type inflammation by acetyl salicylic acid via the adenosine and uric acid pathway in the lung. Allergy 2010, 65, 1093–1103. [Google Scholar] [CrossRef]
- Kataoka, H.; Yang, K.; Rock, K.L. The xanthine oxidase inhibitor Febuxostat reduces tissue uric acid content and inhibits injury-induced inflammation in the liver and lung. Eur. J. Pharmacol. 2015, 746, 174–179. [Google Scholar] [CrossRef]
- Yin, W.; Zhou, Q.L.; Ouyang, S.X.; Chen, Y.; Gong, Y.T.; Liang, Y.M. Uric acid regulates NLRP3/IL-1β signaling pathway and further induces vascular endothelial cells injury in early CKD through ROS activation and K+ efflux. BMC Nephrol. 2019, 20, 319. [Google Scholar] [CrossRef]
- Xie, H.; Sun, J.; Chen, Y.; Zong, M.; Xu, D.; Wang, Y. (-)-Epigallocatechin-3-gallate protects against uric acid-induced endothelial dysfunction in human umbilical vein endothelial cells. Pharmacogn. Mag. 2019, 15, 487. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, Y.; Hao, Z.; Bi, H.; Liu, Y.; Yang, X.; Xia, Y. Protective effect of uric acid on ox-LDL-induced HUVECs injury via Keap1-Nrf2-ARE pathway. J. Immunol. Res. 2021, 2021, 5151168. [Google Scholar] [CrossRef]
- Corry, D.B.; Eslami, P.; Yamamoto, K.; Nyby, M.D.; Makino, H.; Tuck, M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J. Hypertens. 2008, 26, 269–275. [Google Scholar] [CrossRef]
- Yu, M.-A.; Sánchez-Lozada, L.G.; Johnson, R.J.; Kang, D.-H. Oxidative stress with an activation of the renin–angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 2010, 28, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hong, Q.; Zhang, X.; Xiao, W.; Wang, L.; Cui, S.; Feng, Z.; Lv, Y.; Cai, G.; Chen, X.; et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun. Signal. 2017, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Wang, L.; Huang, Z.; Feng, Z.; Cui, S.; Fu, B.; Cai, G.; Chen, X.; Wu, D. High Concentrations of Uric Acid and Angiotensin II Act Additively to Produce Endothelial Injury. Mediators Inflamm. 2020, 2020, 8387654. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Duan, X.M.; Liu, Y.; Yu, J.; Tang, Y.L.; Liu, Z.L.; Jiang, S.; Zhang, C.P.; Liu, J.Y.; Xu, J.X. Uric Acid Induces Endothelial Dysfunction by Activating the HMGB1/RAGE Signaling Pathway. Biomed Res. Int. 2017, 2017, 4391920. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Bernard, L.M. The renal renin-angiotensin system. Adv. Physiol. Educ. 2009, 33, 270–274. [Google Scholar] [CrossRef]
- Fountain, J.H.; Lappin, S.L. Physiology, Renin Angiotensin System. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Bernstein, K.E.; Berk, B.C. The Biology of Angiotensin II Receptors. Am. J. Kidney Dis. 1993, 22, 745–754. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z.; Patel, M.P.; Chappell, M.; Butany, J.; Backx, P.H.; Tsushima, R.G.; Scholey, J.W.; Khokha, R.; Penninger, J.M. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 2007, 75, 29–39. [Google Scholar] [CrossRef]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000, 87, e1–e9. [Google Scholar] [CrossRef]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A human homolog of angiotensin-converting enzyme: Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Penninger, J.M. Recombinant human angiotensin-converting enzyme 2 as a new renin-angiotensin system peptidase for heart failure therapy. Curr. Heart Fail. Rep. 2011, 8, 176–183. [Google Scholar] [CrossRef]
- Chinnaiyan, K.M.; Alexander, D.; McCullough, P.A. Role of angiotensin II in the evolution of diastolic heart failure. J. Clin. Hypertens. 2005, 7, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Paneni, F.; Ciavarella, G.M.; De Biase, L.; Palano, F.; Baldini, R.; Quarta, G.; Tocci, G.; Benedetto, U.; Ferrucci, A.; et al. Evaluation of systolic properties in hypertensive patients with different degrees of diastolic dysfunction and normal ejection fraction. Am. J. Hypertens. 2009, 22, 437–443. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miteva, K.; Van Linthout, S.; Pappritz, K.; Müller, I.; Spillmann, F.; Haag, M.; Stachelscheid, H.; Ringe, J.; Sittinger, M.; Tschöpe, C. Human Endomyocardial Biopsy Specimen-Derived Stromal Cells Modulate Angiotensin II-Induced Cardiac Remodeling. Stem Cells Transl. Med. 2016, 5, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Meng, Y.; Pan, M.; Zheng, B.; Chen, Y.; Li, W.; Yang, Q.; Zheng, Z.; Sun, N.; Zhang, Y.; Li, X. Autophagy Attenuates Angiotensin II-Induced Pulmonary Fibrosis by Inhibiting Redox Imbalance-Mediated NOD-Like Receptor Family Pyrin Domain Containing 3 Inflammasome Activation. Antioxid. Redox Signal. 2019, 30, 520–541. [Google Scholar] [CrossRef]
- Nehme, A.; Zouein, F.A.; Zayeri, Z.D.; Zibara, K. An Update on the Tissue Renin Angiotensin System and Its Role in Physiology and Pathology. J. Cardiovasc. Dev. Dis. 2019, 6, 14. [Google Scholar] [CrossRef]
- Ideu, S.; Kueppers, F.; Lippmann, M.; Rosen, H.; Niederman, M.; Fein, A. Angiotensin Converting Enzyme in Bronchoalveolar Lavage in ARDS *. Chest 1987, 91, 52–56. [Google Scholar]
- Orfanos, S.E.; Armaganidis, A.; Glynos, C.; Psevdi, E.; Kaltsas, P.; Sarafidou, P.; Catravas, J.D.; Dafni, U.G.; Langleben, D.; Roussos, C. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lung injury. Circulation 2000, 102, 2011–2018. [Google Scholar] [CrossRef]
- Liu, L.; Qiu, H.B.; Yang, Y.; Wang, L.; Ding, H.M.; Li, H.P. Losartan, an antagonist of AT1 receptor for angiotensin II, attenuates lipopolysaccharide-induced acute lung injury in rat. Arch. Biochem. Biophys. 2009, 481, 131–136. [Google Scholar] [CrossRef]
- Gu, H.; Xie, Z.; Li, T.; Zhang, S.; Lai, C.; Zhu, P.; Wang, K.; Han, L.; Duan, Y.; Zhao, Z.; et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016, 6, 19840. [Google Scholar] [CrossRef]
- Ye, R.; Liu, Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp. Mol. Pathol. 2020, 113, 104350. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lu, A.; Lu, X.; Zhang, L.; Fang, H.; Zhou, L.; Yang, T. Activation of Renal (Pro)Renin Receptor Contributes to High Fructose-Induced Salt Sensitivity. Hypertension 2017, 69, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Kim, I.K. Maternal high-fructose intake induces hypertension through activating histone codes on the (pro)renin receptor promoter. Biochem. Biophys. Res. Commun. 2020, 527, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gu, J.; Lv, H.; Li, H.; Cheng, Y.; Liu, Y.; Jiang, Y. Uric acid induced inflammatory responses in endothelial cells via up-regulating(pro)renin receptor. Biomed. Pharmacother. 2019, 109, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Takeuchi, H.; Fukunaga, K.; Hirano, Y.; Suda, K.; Hagiwara, T.; Miyasho, T.; Yamada, S.; Nakamura, R.; Takahashi, T.; et al. Attenuation of lipopolysaccharide-induced acute lung injury after (pro)renin receptor blockade. Exp. Lung Res. 2005, 41, 199–207. [Google Scholar] [CrossRef]
- Seong, H.Y.; Cho, H.M.; Kim, M.; Kim, I. Maternal high-fructose intake induces multigenerational activation of the renin-angiotensin-aldosterone system. Hypertension 2019, 74, 518–525. [Google Scholar] [CrossRef]
- Bundalo, M.M.; Zivkovic, M.D.; Romic, S.D.; Tepavcevic, S.N.; Koricanac, G.B.; Djuric, T.M.; Stankovic, A.D. Fructose-rich diet induces gender-specific changes in expression of the renin-angiotensin system in rat heart and upregulates the ACE/AT1R axis in the male rat aorta. JRAAS—J. Renin-Angiotensin-Aldosterone Syst. 2016, 17, 147032031664291. [Google Scholar] [CrossRef]
- Camelo, L.; Marinho, T.D.S.; Águila, M.B.; Souza-Mello, V.; Barbosa-da-Silva, S. Intermittent fasting exerts beneficial metabolic effects on blood pressure and cardiac structure by modulating local renin-angiotensin system in the heart of mice fed high-fat or high-fructose diets. Nutr. Res. 2019, 63, 51–62. [Google Scholar] [CrossRef]
- Froogh, G.; Kandhi, S.; Duvvi, R.; Le, Y.; Weng, Z.; Alruwaili, N.; Ashe, J.O.; Sun, D.; Huang, A. The contribution of chymase-dependent formation of ANG II to cardiac dysfunction in metabolic syndrome of young rats: Roles of fructose and EETs. Am. J. Physiol. Circ. Physiol. 2020, 318, H985–H993. [Google Scholar] [CrossRef]
- Kim, M.; Do, G.Y.; Kim, I. Activation of the renin-angiotensin system in high fructose-induced metabolic syndrome. Korean J. Physiol. Pharmacol. 2020, 24, 318–328. [Google Scholar] [CrossRef]
- Dhar, I.; Dhar, A.; Wu, L.; Desai, K.M. Increased Methylglyoxal Formation with Upregulation of Renin Angiotensin System in Fructose Fed Sprague Dawley Rats. PLoS ONE 2013, 8, e74212. [Google Scholar] [CrossRef] [PubMed]
- Mukohda, M.; Yamawaki, H.; Okada, M.; Hara, Y. Methylglyoxal Augments Angiotensin II–Induced Contraction in Rat Isolated Carotid Artery. J. Pharmacol. Sci. 2010, 114, 390–398. [Google Scholar] [CrossRef]
- Medeiros, M.L.; Oliveira, A.L.; de Oliveira, M.G.; Mónica, F.Z.; Antunes, E. Methylglyoxal Exacerbates Lipopolysaccharide-Induced Acute Lung Injury via RAGE-Induced ROS Generation: Protective Effects of Metformin. J. Inflamm. Res. 2021, 14, 6477–6489. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, A.G.; Гeopгиeвич, A.A.; Savateeva-Lyubimova, T.N.; Hикoлaeвнa, С.-Л.Т.; Muzhikyan, A.A.; Apтyшoвич, М.A. The effect methylglyoxal on acute lung injury induced by influenza A(H1N1)PDM09 in mice. Med. Acad. J. 2019, 19, 65–72. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, J.; Lim, J.; Cho, S.; An, S.; Lee, M.; Yoon, N.; Seo, M.; Lim, S.; Park, S. Soluble RAGE attenuates AngII-induced endothelial hyperpermeability by disrupting HMGB1-mediated crosstalk between AT1R and RAGE. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Fujita, M.; Okuda, H.; Tsukamoto, O.; Asano, Y.; Liao, Y.; Hirata, A.; Kim, J.; Miyatsuka, T.; Takashima, S.; Minamino, T.; et al. Blockade of angiotensin II receptors reduces the expression of receptors for advanced glycation end products in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, e138–e139. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Srikrishna, G.; Freeze, H.H. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 2009, 11, 615–628. [Google Scholar] [CrossRef]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef]
- Buckley, S.T.; Ehrhardt, C. The Receptor for Advanced Glycation End Products (RAGE) and the lung. J. Biomed. Biotechnol. 2010, 2010, 917108. [Google Scholar] [CrossRef]
- Uchida, T.; Shirasawa, M.; Ware, L.B.; Kojima, K.; Hata, Y.; Makita, K.; Mednick, G.; Matthay, Z.A.; Matthay, M.A. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am. J. Respir. Crit. Care Med. 2006, 173, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Jabaudon, M.; Futier, E.; Roszyk, L.; Chalus, E.; Guerin, R.; Petit, A.; Mrozek, S.; Perbet, S.; Cayot-Constantin, S.; Chartier, C.; et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients*. Crit. Care Med. 2011, 39, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Ware, L.B.; Eisner, M.D.; Parsons, P.E.; Thompson, B.T.; Wickersham, N.; Matthay, M.A. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008, 63, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Nakazato, K.; Sugimoto, K.; Yoshihisa, A.; Yamaki, T.; Kunii, H.; Suzuki, H.; Saitoh, S.; Takeishi, Y. Plasma Levels of Receptor for Advanced Glycation End-Products and High-Mobility Group Box 1 in Patients With Pulmonary Hypertension. Int. Heart J. 2016, 57, 234–240. [Google Scholar] [CrossRef]
- Caraher, E.J.; Kwon, S.; Haider, S.H.; Crowley, G.; Lee, A.; Ebrahim, M.; Zhang, L.; Chen, L.C.; Gordon, T.; Liu, M.; et al. Receptor for advanced glycation endproducts and World Trade Center particulate induced lung function loss: A case-cohort study and murine model of acute particulate exposure. PLoS ONE 2017, 12, e0184331. [Google Scholar] [CrossRef]
- Achouiti, A.; van der Meer, A.J.; Florquin, S.; Yang, H.; Tracey, K.J.; van ’t Veer, C.; de Vos, A.F.; van der Poll, T. High-mobility group box 1 and the receptor for advanced glycation end products contribute to lung injury during Staphylococcus aureus pneumonia. Crit. Care 2013, 17, R296. [Google Scholar] [CrossRef]
- Weber, D.J.; Gracon, A.S.A.; Ripsch, M.S.; Fisher, A.J.; Cheon, B.M.; Pandya, P.H.; Vittal, R.; Capitano, M.L.; Kim, Y.; Allette, Y.M.; et al. The HMGB1-RAGE axis mediates traumaticbrain injury—Induced pulmonary dysfunction in lung transplantation. Sci. Transl. Med. 2014, 6, 252ra124. [Google Scholar] [CrossRef]
- Blondonnet, R.; Audard, J.; Belville, C.; Clairefond, G.; Lutz, J.; Bouvier, D.; Roszyk, L.; Gross, C.; Lavergne, M.; Fournet, M.; et al. RAGE inhibition reduces acute lung injury in mice. Sci. Rep. 2017, 7, 7208. [Google Scholar] [CrossRef]
- Gomez-Ojeda, A.; Elizarraraz-Morrill, R.; Luevano-Contreras, C.; Del, L.; Ibarra-Reynoso, R.; Uribarri, J.; Garay-Sevilla, M.E. Free Fructose Intake Decreases Soluble RAGE Receptor (sRAGE) and Glyoxal and Methylglyoxal Urinary Excretion on Healthy Volunteers. Curr. Dev. Nutr. 2020, 4, 631. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Zheng, M.; Hao, Y.; Hou, L.; Zhang, S. Effect of pioglitazone on insulin resistance in fructose-drinking rats correlates with AGEs/RAGE inhibition and block of NAPDH oxidase and NF kappa B activation. Eur. J. Pharmacol. 2010, 629, 153–158. [Google Scholar] [CrossRef]
- Cannizzaro, L.; Rossoni, G.; Savi, F.; Altomare, A.; Marinello, C.; Saethang, T.; Carini, M.; Payne, D.M.; Pisitkun, T.; Aldini, G.; et al. Regulatory landscape of AGE-RAGE-oxidative stress axis and its modulation by PPARγ activation in high fructose diet-induced metabolic syndrome. Nutr. Metab. 2017, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Jaiswal, N.; Maurya, C.K.; Sharma, A.; Ahmad, I.; Ahmad, S.; Gupta, A.P.; Gayen, J.R.; Tamrakar, A.K. Fructose-induced AGEs-RAGE signaling in skeletal muscle contributes to impairment of glucose homeostasis. J. Nutr. Biochem. 2019, 71, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Sotokawauchi, A.; Matsui, T.; Higashimoto, Y.; Yamagishi, S.I. Fructose causes endothelial cell damage via activation of advanced glycation end products–receptor system. Diabetes Vasc. Dis. Res. 2019, 16, 556–561. [Google Scholar] [CrossRef]
- Jaiswal, N.; Maurya, C.K.; Arha, D.; Avisetti, D.R.; Prathapan, A.; Raj, P.S.; Raghu, K.G.; Kalivendi, S.V.; Tamrakar, A.K. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis 2015, 20, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gong, J.; Wang, M. Phloretin and its methylglyoxal adduct: Implications against advanced glycation end products-induced inflammation in endothelial cells. Food Chem. Toxicol. 2019, 129, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.L.; de Oliveira, M.G.; Tavares, E.G.; Mello, G.C.; Anhê, G.F.; Mónica, F.Z.; Antunes, E. Long-term methylglyoxal intake aggravates murine Th2-mediated airway eosinophil infiltration. Int. Immunopharmacol. 2020, 81, 106254. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Srivastava, S.K. Aldose reductase: Congenial and injurious profiles of an enigmatic enzyme. Biochem. Med. Metab. Biol. 1992, 48, 91–121. [Google Scholar] [CrossRef]
- Alim, Z.; Beydemir, Ş. Effects of some anti-neoplastic drugs on sheep liver sorbitol dehydrogenase. Arch. Physiol. Biochem. 2012, 118, 244–252. [Google Scholar] [CrossRef]
- Reddy, A.B.M.; Ramana, K.V. Aldose Reductase Inhibition: Emerging Drug Target for the Treatment of Cardiovascular Complications. Recent Pat. Cardiovasc. Drug Discov. 2010, 5, 25–32. [Google Scholar] [CrossRef]
- Sonowal, H.; Saxena, A.; Ramana, K.V. Contribution of Aldose Reductase-Mediated Oxidative Stress Signaling in Inflammatory Lung Diseases. In Oxidative Stress in Lung Diseases; Springer: Singapore, 2019; pp. 225–246. [Google Scholar]
- Ravindranath, T.M.; Mong, P.Y.; Ananthakrishnan, R.; Li, Q.; Quadri, N.; Schmidt, A.M.; Ramasamy, R.; Wang, Q. Novel Role for Aldose Reductase in Mediating Acute Inflammatory Responses in the Lung. J. Immunol. 2009, 183, 8128–8137. [Google Scholar] [CrossRef]
- Yadav, U.C.S.; Naura, A.S.; Aguilera-Aguirre, L.; Boldogh, I.; Boulares, H.A.; Calhoun, W.J.; Ramana, K.V.; Srivastava, S.K. Aldose Reductase Inhibition Prevents Allergic Airway Remodeling through PI3K/AKT/GSK3β Pathway in Mice. PLoS ONE 2013, 8, e57442. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, Y.; Lu, Y.; Yang, J. Amelioration of bleomycininduced pulmonary fibrosis of rats by an aldose reductase inhibitor, epalrestat. Korean J. Physiol. Pharmacol. 2015, 19, 401–411. [Google Scholar] [CrossRef]
- García-Arroyo, F.E.; Tapia, E.; Blas-Marron, M.G.; Gonzaga, G.; Silverio, O.; Cristóbal, M.; Osorio, H.; Arellano-Buendía, A.S.; Zazueta, C.; Aparicio-Trejo, O.E.; et al. Vasopressin mediates the renal damage induced by limited fructose rehydration in recurrently dehydrated rats. Int. J. Biol. Sci. 2017, 13, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Roncal-Jimenez, C.A.; Lanaspa-Garcia, M.A.; Oppelt, S.A.; Kuwabara, M.; Jensen, T.; Milagres, T.; Andres-Hernando, A.; Ishimoto, T.; Garcia, G.E.; et al. Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J. Neurophysiol. 2017, 117, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Herman, B.A.; Ferguson, K.M.; Fernandez, J.V.B.; Kauffman, S.; Spicher, J.T.; King, R.J.; Halterman, J.A. NFAT5 is differentially expressed in sprague-dawley rat tissues in response to high salt and high fructose diets. Genet. Mol. Biol. 2019, 42, 452–464. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Ishimoto, T.; Li, N.; Cicerchi, C.; Orlicky, D.J.; Ruzycki, P.; Rivard, C.; Inaba, S.; Roncal-Jimenez, C.A.; Bales, E.S.; et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 2013, 4, 2434. [Google Scholar] [CrossRef]
- Pal, P.B.; Sonowal, H.; Shukla, K.; Srivastava, S.K.; Ramana, K.V. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-α1/mTOR pathway. J. Mol. Endocrinol. 2019, 63, 11–25. [Google Scholar] [CrossRef]
- Dowling, R.J.O.; Topisirovic, I.; Fonseca, B.D.; Sonenberg, N. Dissecting the role of mTOR: Lessons from mTOR inhibitors. Biochim. Biophys. Acta 2010, 1804, 433–439. [Google Scholar] [CrossRef]
- Dazert, E.; Hall, M.N. mTOR signaling in disease. Curr. Opin. Cell Biol. 2011, 23, 744–755. [Google Scholar] [CrossRef]
- Üstün, S.; Lassnig, C.; Preitschopf, A.; Mikula, M.; Müller, M.; Hengstschläger, M.; Weichhart, T. Effects of the mTOR inhibitor everolimus and the PI3K/mTOR inhibitor NVP-BEZ235 in murine acute lung injury models. Transpl. Immunol. 2015, 33, 45–50. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, J.; Wu, Y.F.; Lou, J.; Mao, Y.Y.; Shen, H.H.; Chen, Z.H. MTOR and autophagy in regulation of acute lung injury: A review and perspective. Microbes Infect. 2014, 16, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Fei, Q.; Streicher, A.; Zhang, W.; Isabelle, C.; Patel, P.; Lam, H.C.; Pinilla-Vera, M.; Amador-Munoz, D.; Barragan-Bradford, D.; et al. Mechanosensitive activation of mTORC1 mediates ventilator induced lung injury during the acute respiratory distress syndrome. bioRxiv 2020. [Google Scholar] [CrossRef]
- Romero, Y.; Bueno, M.; Ramirez, R.; Álvarez, D.; Sembrat, J.C.; Goncharova, E.A.; Rojas, M.; Selman, M.; Mora, A.L.; Pardo, A. mTORC activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell 2016, 15, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, E.M.; Tian, Y.; Nigdelioglu, R.; Witt, L.J.; Cetin-Atalay, R.; Meliton, A.Y.; Woods, P.S.; Kimmig, L.M.; Sun, K.A.; Gökalp, G.A.; et al. TGF-b promotes metabolic reprogramming in lung fibroblasts via mTORC1-dependent ATF4 activation. Am. J. Respir. Cell Mol. Biol. 2020, 63, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Mitani, A.; Ito, K.; Vuppusetty, C.; Barnes, P.J.; Mercado, N. Restoration of corticosteroid sensitivity in chronic obstructive pulmonary disease by inhibition of mammalian target of rapamycin. Am. J. Respir. Crit. Care Med. 2016, 193, 143–153. [Google Scholar] [CrossRef]
- Li, X.; Shan, C.; Wu, Z.; Yu, H.; Yang, A.; Tan, B. Emodin alleviated pulmonary inflammation in rats with LPS-induced acute lung injury through inhibiting the mTOR/HIF-1α/VEGF signaling pathway. Inflamm. Res. 2020, 69, 365–373. [Google Scholar] [CrossRef]
- Hu, Y.; Lou, J.; Mao, Y.Y.; Lai, T.W.; Liu, L.Y.; Zhu, C.; Zhang, C.; Liu, J.; Li, Y.Y.; Zhang, F.; et al. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy 2016, 12, 2286–2299. [Google Scholar] [CrossRef]
- Ma, C.; Zhu, L.; Wang, J.; He, H.; Chang, X.; Gao, J.; Shumin, W.; Yan, T. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. J. Ethnopharmacol. 2015, 168, 349–355. [Google Scholar] [CrossRef]
- Qu, L.; Chen, C.; He, W.; Chen, Y.; Li, Y.; Wen, Y.; Zhou, S.; Jiang, Y.; Yang, X.; Zhang, R.; et al. Glycyrrhizic acid ameliorates LPS-induced acute lung injury by regulating autophagy through the PI3K/AKT/mTOR pathway. Am. J. Transl. Res. 2019, 11, 2042–2055. [Google Scholar]
- Wu, K.; Tian, R.; Huang, J.; Yang, Y.; Dai, J.; Jiang, R.; Zhang, L. Metformin alleviated endotoxemia-induced acute lung injury via restoring AMPK-dependent suppression of mTOR. Chem. Biol. Interact. 2018, 291, 1–6. [Google Scholar] [CrossRef]
- Sangüesa, G.; Roglans, N.; Baena, M.; Velázquez, A.M.; Laguna, J.C.; Alegret, M. mTOR is a key protein involved in the metabolic effects of simple sugars. Int. J. Mol. Sci. 2019, 20, 1117. [Google Scholar] [CrossRef] [PubMed]
- Sangüesa, G.; Montañés, J.C.; Baena, M.; Sánchez, R.M.; Roglans, N.; Alegret, M.; Laguna, J.C. Chronic fructose intake does not induce liver steatosis and inflammation in female Sprague–Dawley rats, but causes hypertriglyceridemia related to decreased VLDL receptor expression. Eur. J. Nutr. 2019, 58, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- David, J.; Dardevet, D.; Mosoni, L.; Savary-Auzeloux, I.; Polakof, S. Impaired Skeletal Muscle Branched-Chain Amino Acids Catabolism Contributes to Their Increased Circulating Levels in a Non-Obese Insulin-Resistant Fructose-Fed Rat Model. Nutrients 2019, 11, 355. [Google Scholar] [CrossRef]
- Mathiyazhagan, J.; Kodiveri Muthukaliannan, G. The role of mTOR and oral intervention of combined Zingiber officinale—Terminalia chebula extract in type 2 diabetes rat models. J. Food Biochem. 2020, 44, 13250. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Blagih, J.; Zani, F.; Rees, A.; Hill, D.G.; Jenkins, B.J.; Bull, C.J.; Moreira, D.; Bantan, A.I.M.; Cronin, J.G.; et al. Fructose reprogrammes glutamine-dependent oxidative metabolism to support LPS-induced inflammation. Nat. Commun. 2021, 12, 1209. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ma, C.; Wu, H.; Ma, Y.; Liu, Z.; Zhong, P.; Jin, C.; Ning, W.; Wu, X.; Zhang, Y.; et al. Fructose Induces Pulmonary Fibrotic Phenotype Through Promoting Epithelial-Mesenchymal Transition Mediated by ROS-Activated Latent TGF-β1. Front. Nutr. 2022, 9, 1004. [Google Scholar] [CrossRef]
- WHO EMRO. Nutrition Advice for Adults during the COVID-19 Outbreak. Available online: http://www.emro.who.int/nutrition/news/nutrition-advice-for-adults-during-the-COVID-19-outbreak.html (accessed on 10 May 2022).
- De Faria Coelho-Ravagnani, C.; Corgosinho, F.C.; Sanches, F.L.F.Z.; Prado, C.M.M.; Laviano, A.; Mota, J.F. Dietary recommendations during the COVID-19 pandemic. Nutr. Rev. 2021, 79, 382–393. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.H.; Yaroch, A.L.; Blanck, H.M. Reported Changes in Eating Habits Related to Less Healthy Foods and Beverages during the COVID-19 Pandemic among US Adults. Nutrients 2022, 14, 526. [Google Scholar] [CrossRef]
- Sánchez, E.; Lecube, A.; Bellido, D.; Monereo, S.; Malagón, M.M.; Tinahones, F.J. Leading Factors for Weight Gain during COVID-19 Lockdown in a Spanish Population: A Cross-Sectional Study. Nutrients 2021, 13, 894. [Google Scholar] [CrossRef]
- Drieskens, S.; Berger, N.; Vandevijvere, S.; Gisle, L.; Braekman, E.; Charafeddine, R.; De Ridder, K.; Demarest, S. Short-term impact of the COVID-19 confinement measures on health behaviours and weight gain among adults in Belgium. Arch. Public Health 2021, 79, 22. [Google Scholar] [CrossRef]
- Pradeilles, R.; Pareja, R.; Creed-Kanashiro, H.M.; Griffiths, P.L.; Holdsworth, M.; Verdezoto, N.; Eymard-Duvernay, S.; Landais, E.; Stanley, M.; Rousham, E.K. Diet and food insecurity among mothers, infants, and young children in Peru before and during COVID-19: A panel survey. Matern. Child Nutr. 2022, 18, e13343. [Google Scholar] [CrossRef] [PubMed]
- Webber, B.J.; Lang, M.A.; Stuever, D.M.; Escobar, J.D.; Bylsma, V.F.H.; Wolff, G.G. Peer Reviewed: Health-Related Behaviors and Odds of COVID-19 Hospitalization in a Military Population. Prev. Chronic Dis. 2021, 18, 210222. [Google Scholar] [CrossRef] [PubMed]
- Abdulah, D.M.; Hassan, A.B. Relation of Dietary Factors with Infection and Mortality Rates of COVID-19 across the World. J. Nutr. Health aging 2020, 24, 1011–1018. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Nejad, M.M.; Bagheri, H.; Mousavi, S.H.; Salahshour, F. Is prior use of renin-angiotensin system (RAS) inhibitors associated with more favourable outcome in COVID-19 hospitalized patients ? Front. Emerg. Med. 2022, 6, e34. [Google Scholar]
- Wang, H.Y.; Peng, S.; Ye, Z.; Li, P.; Li, Q.; Shi, X.; Zeng, R.; Yao, Y.; He, F.; Li, J.; et al. Renin-angiotensin system inhibitor is associated with the reduced risk of all-cause mortality in COVID-19 among patients with/without hypertension. Front. Med. 2021, 16, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Liu, X.; Wei, Y.; Li, X.; Zheng, B.; Gong, Q.; Dong, L.; Zhong, J. Laboratory Predictors of COVID-19 Mortality: A Retrospective Analysis from Tongji Hospital in Wuhan. Mediators Inflamm. 2021, 2021, 6687412. [Google Scholar] [CrossRef]
- Chen, B.; Lu, C.; Gu, H.Q.; Li, Y.; Zhang, G.; Lio, J.; Luo, X.; Zhang, L.; Hu, Y.; Lan, X.; et al. Serum Uric Acid Concentrations and Risk of Adverse Outcomes in Patients With COVID-19. Front. Endocrinol. 2021, 12, 439. [Google Scholar] [CrossRef]
- Gaztanaga, J.; Ramasamy, R.; Schmidt, A.M.; Fishman, G.; Schendelman, S.; Thangavelu, K.; Perfetti, R.; Katz, S.D. A pilot open-label study of aldose reductase inhibition with AT-001 (caficrestat) in patients hospitalized for COVID-19 infection: Results from a registry-based matched-control analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102328. [Google Scholar] [CrossRef]
- Justin Coyle, D.; Efehi Igbinomwanhia, M.M.; Alejandro Sanchez-Nadales, M.; Sorin Danciu, M.M.; Chae Chu, M.; Nishit Shah, M. A Recovered Case of COVID-19 Myocarditis and ARDS Treated With Corticosteroids, Tocilizumab, and Experimental AT-001. Case Rep. 2020, 2, 1331–1336. [Google Scholar] [CrossRef]
- Lim, A.; Radujkovic, A.; Weigand, M.A.; Merle, U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann. Intensive Care 2021, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Saputra, G.N.R.; Yudhawati, R.; Fitriah, M. Association of soluble receptor for advanced glycation end-products (sRAGE) serum on COVID-19 severity: A cross-sectional study. Ann. Med. Surg. 2022, 74, 103303. [Google Scholar] [CrossRef] [PubMed]
- Yalcin Kehribar, D.; Cihangiroglu, M.; Sehmen, E.; Avci, B.; Capraz, A.; Yildirim Bilgin, A.; Gunaydin, C.; Ozgen, M. The receptor for advanced glycation end product (RAGE) pathway in COVID-19. Biomarkers 2021, 26, 114–118. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Díazcouder, A.; González-Ramírez, J.; Sanchez, F.; Leija-Martínez, J.J.; Martínez-Coronilla, G.; Amezcua-Guerra, L.M.; Sánchez-Muñoz, F. Negative Effects of Chronic High Intake of Fructose on Lung Diseases. Nutrients 2022, 14, 4089. https://doi.org/10.3390/nu14194089
Hernández-Díazcouder A, González-Ramírez J, Sanchez F, Leija-Martínez JJ, Martínez-Coronilla G, Amezcua-Guerra LM, Sánchez-Muñoz F. Negative Effects of Chronic High Intake of Fructose on Lung Diseases. Nutrients. 2022; 14(19):4089. https://doi.org/10.3390/nu14194089
Chicago/Turabian StyleHernández-Díazcouder, Adrián, Javier González-Ramírez, Fausto Sanchez, José J. Leija-Martínez, Gustavo Martínez-Coronilla, Luis M. Amezcua-Guerra, and Fausto Sánchez-Muñoz. 2022. "Negative Effects of Chronic High Intake of Fructose on Lung Diseases" Nutrients 14, no. 19: 4089. https://doi.org/10.3390/nu14194089
APA StyleHernández-Díazcouder, A., González-Ramírez, J., Sanchez, F., Leija-Martínez, J. J., Martínez-Coronilla, G., Amezcua-Guerra, L. M., & Sánchez-Muñoz, F. (2022). Negative Effects of Chronic High Intake of Fructose on Lung Diseases. Nutrients, 14(19), 4089. https://doi.org/10.3390/nu14194089







