Green Tea Catechins Modulate Skeletal Development with Effects Dependent on Dose, Time, and Structure in a down Syndrome Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Ethical Statement
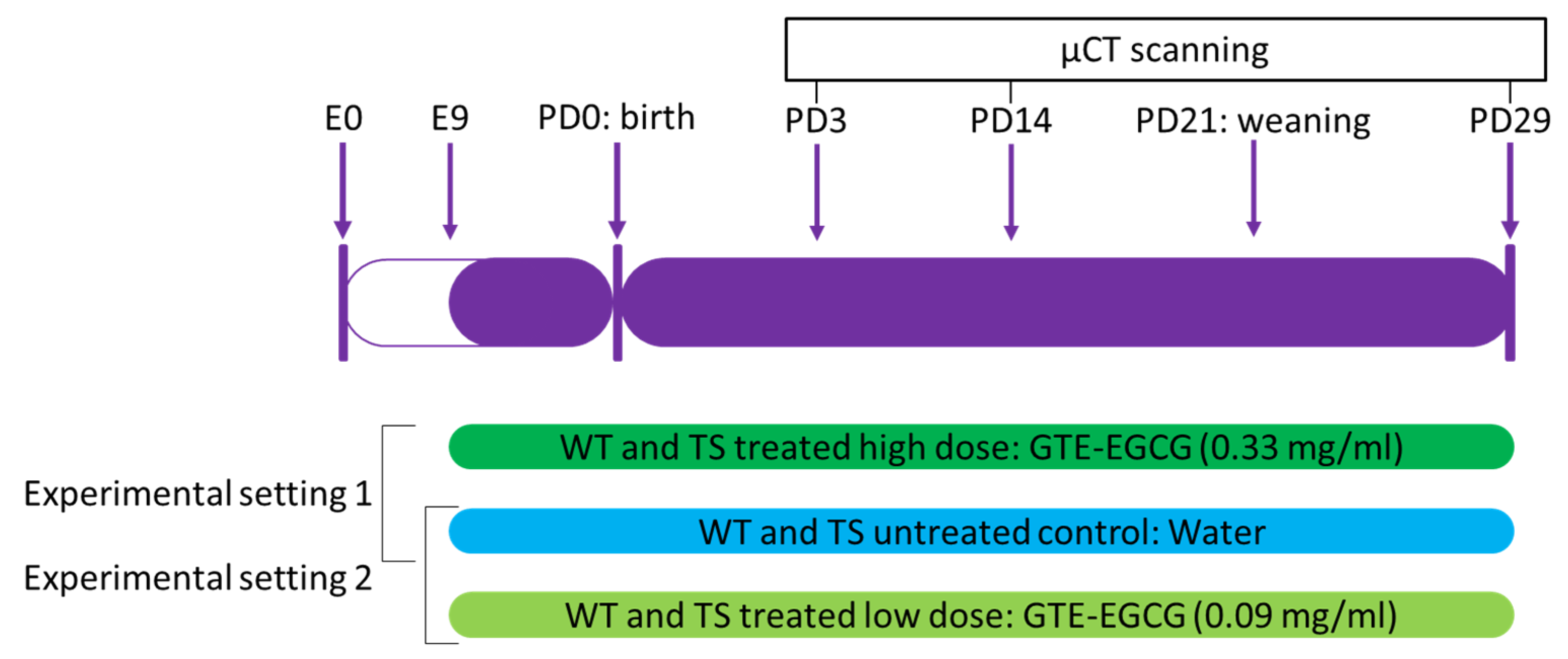
2.2. Experimental Design and Treatment
2.3. In Vivo Micro-Computed Tomography (µCT)
2.4. Image Data Processing
2.5. Shape Analysis of Craniofacial Structures
2.6. Shape Analysis of Postcranial Structures
2.7. Bone Mineral Density (BMD)
2.8. Statistical Analyses
3. Results
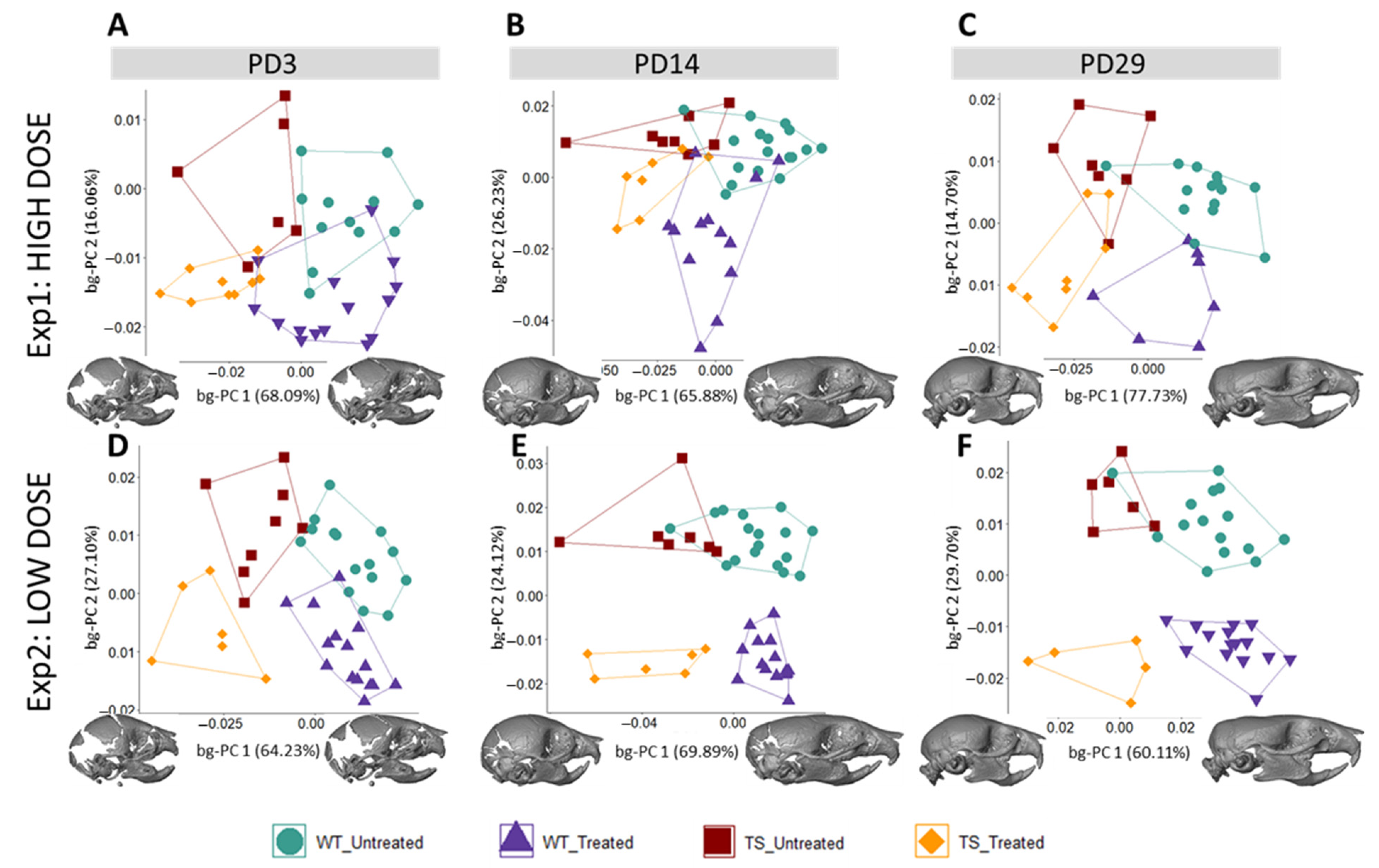
3.1. Facial Shape in Ts65Dn Mice Partly Rescued by Low-Dose GTE-EGCG Treatment
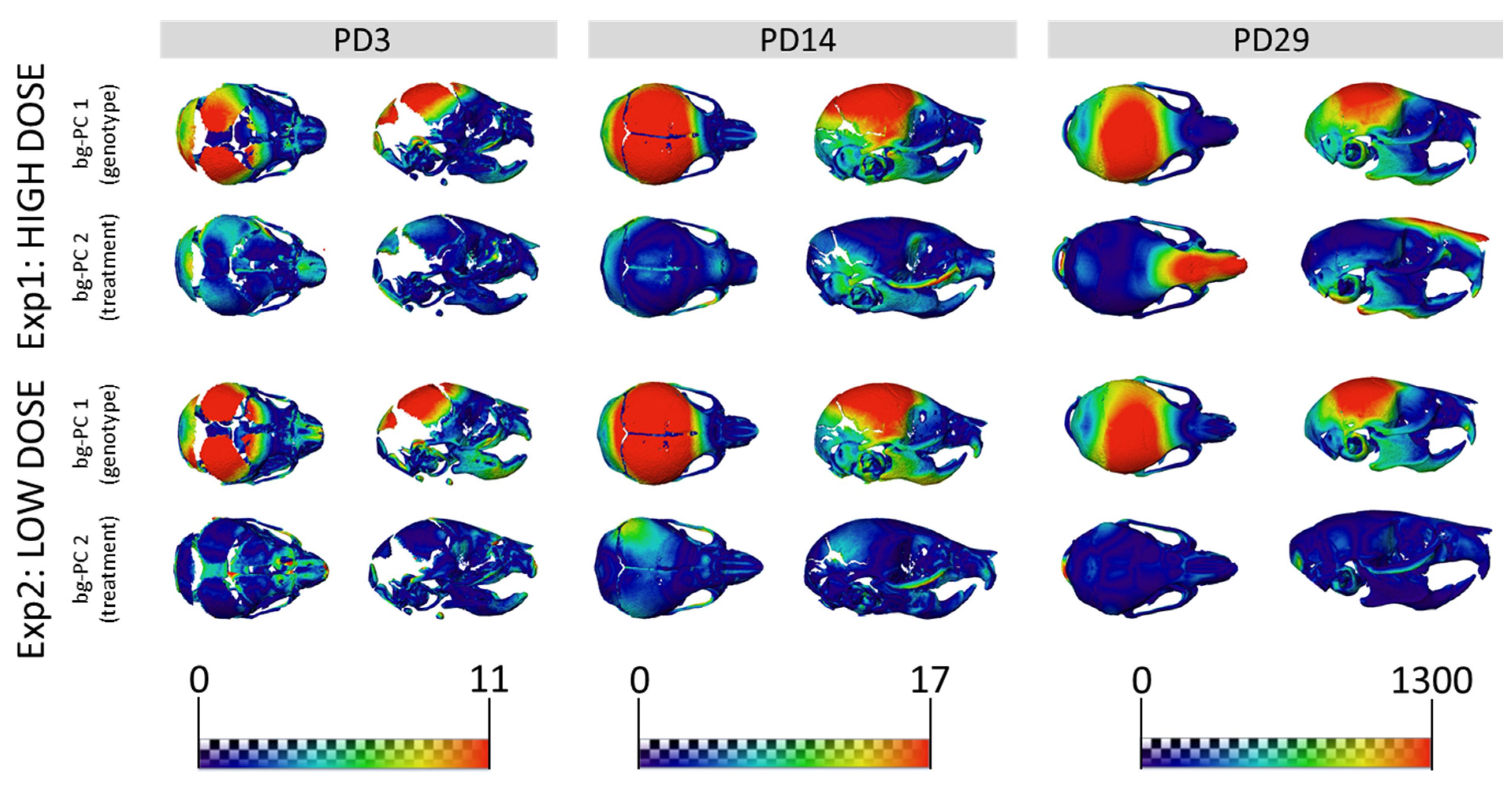
3.2. Skull Shape Associated with DS Is Not Rescued by GTE-EGCG Treatments in Ts65Dn Mice
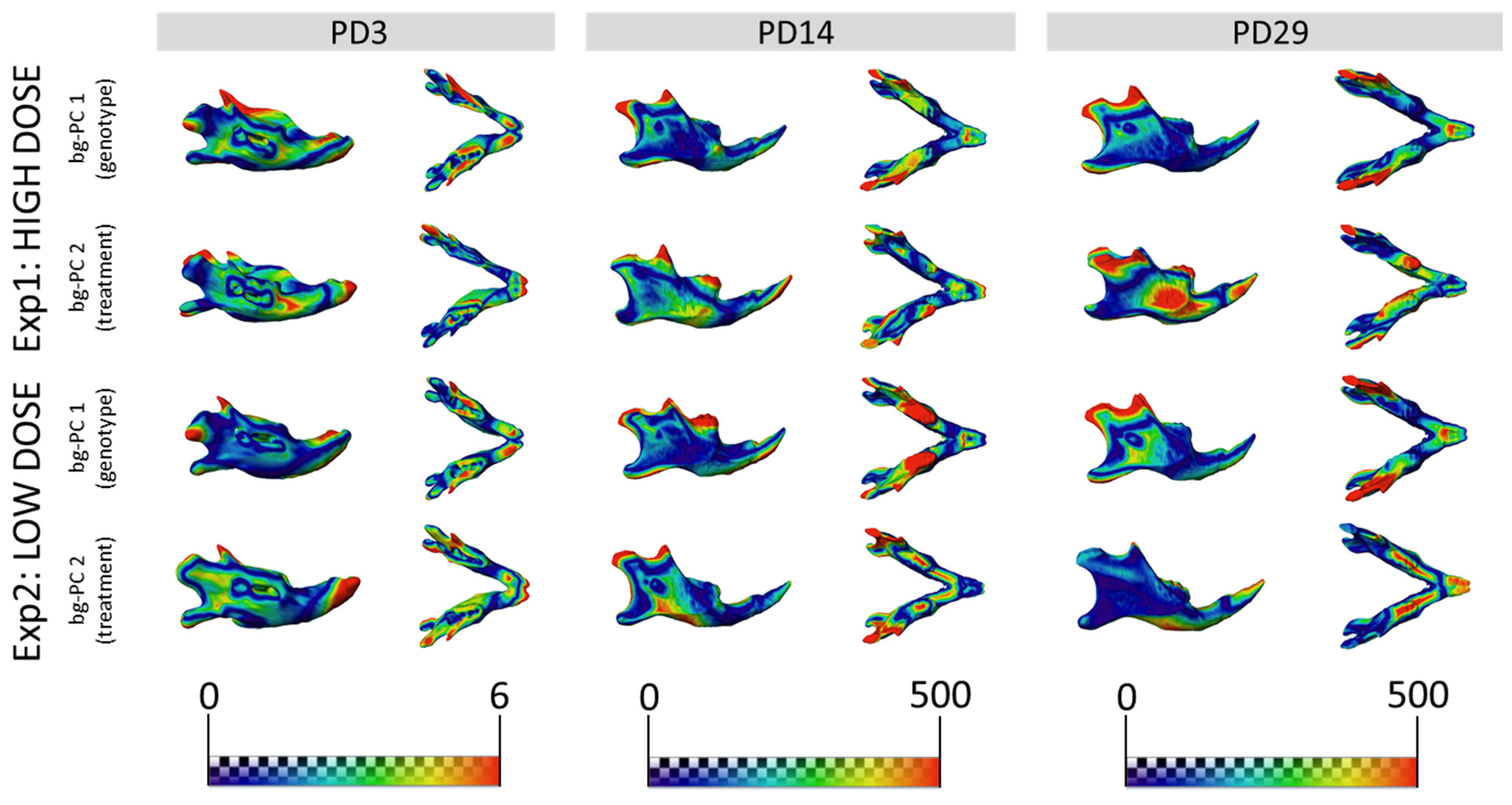
3.3. GTE-EGCG Treatments Modulated Mandibular Shape without Rescuing Genotype Alterations
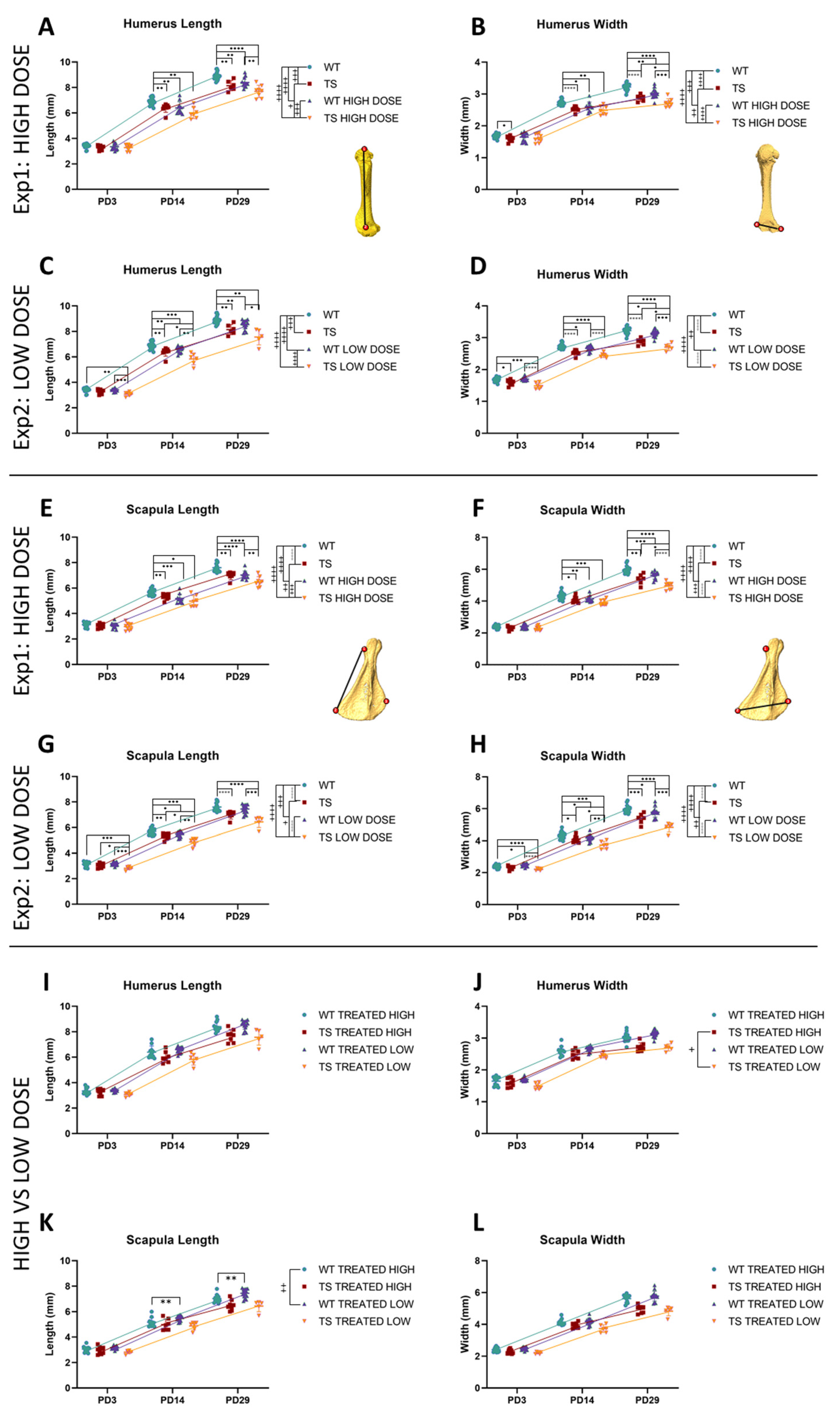
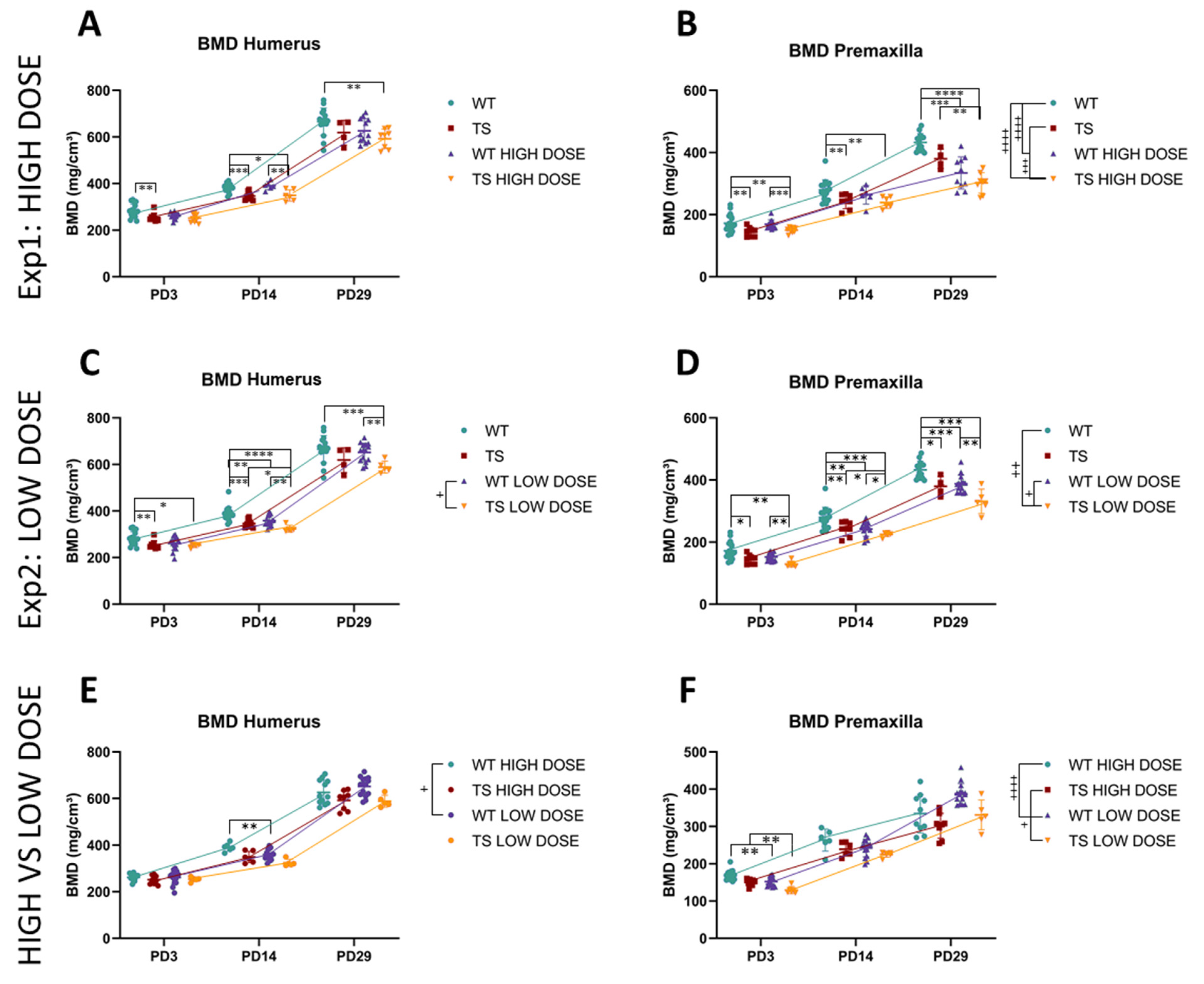
3.4. Upper Limb Alterations Associated with DS Not Rescued by GTE-EGCG Treatments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roper, R.J.; Reeves, R.H. Understanding the basis for Down syndrome phenotypes. PLoS Genet. 2006, 2, e50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.R.; Roper, R.J. Current Analysis of Skeletal Phenotypes in Down Syndrome. Curr. Osteoporos. Rep. 2021, 19, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Richtsmeier, J.T.; Baxter, L.L.; Reeves, R.H. Parallels of craniofacial maldevelopment in down syndrome and Ts65Dn mice. Dev. Dyn. 2000, 217, 137–145. [Google Scholar] [CrossRef]
- Suri, S.; Tompson, B.D.; Cornfoot, L. Cranial base, maxillary and mandibular morphology in Down syndrome. Angle Orthod. 2010, 80, 861–869. [Google Scholar] [CrossRef]
- Starbuck, J.M.; Llambrich, S.; Gonzàlez, R.; Albaigès, J.; Sarlé, A.; Wouters, J.; González, A.; Sevillano, X.; Sharpe, J.; De La Torre, R.; et al. Green tea extracts containing epigallocatechin-3-gallate modulate facial development in Down syndrome. Sci. Rep. 2021, 11, 4715. [Google Scholar] [CrossRef]
- Blazek, J.D.; Abeysekera, I.; Li, J.; Roper, R.J. Rescue of the abnormal skeletal phenotype in Ts65Dn Down syndrome mice using genetic and therapeutic modulation of trisomic Dyrk1a. Hum. Mol. Genet. 2015, 24, 5687–5696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, C.L.; Hussamy, D.J.; McIntire, D.D.; Twickler, D.M.; Dashe, J.S. Femur length parameters in fetuses with Down syndrome. J. Matern. -Fetal Neonatal Med. 2020, 33, 2516–2521. [Google Scholar] [CrossRef]
- de Moraes, M.E.; Tanaka, J.L.; de Moraes, L.C.; Filho, E.M.; de Melo Castilho, J.C. Skeletal age of individuals with Down syndrome. Spec. Care Dent. 2008, 28, 101–106. [Google Scholar] [CrossRef]
- Longo, D.; DeFigueiredo, D.; Cicero, S.; Sacchini, C.; Nicolaides, K.H. Femur and humerus length in trisomy 21 fetuses at 11-14 weeks of gestation. Ultrasound Obstet. Gynecol. 2004, 23, 143–147. [Google Scholar] [CrossRef]
- Keeling, J.W.; Hansen, B.F.; Kjaer, I. Pattern of malformations in the axial skeleton in human trisomy 21 fetuses. Am. J. Med. Genet. 1997, 68, 466–471. [Google Scholar] [CrossRef]
- Carfì, A.; Liperoti, R.; Fusco, D.; Giovannini, S.; Brandi, V.; Vetrano, D.L.; Meloni, E.; Mascia, D.; Villani, E.R.; Manes Gravina, E.; et al. Bone mineral density in adults with Down syndrome. Osteoporos. Int. 2017, 28, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- LaCombe, J.M.; Roper, R.J. Skeletal dynamics of Down syndrome: A developing perspective. Bone 2020, 133, 115215. [Google Scholar] [CrossRef]
- McElyea, S.D.; Starbuck, J.M.; Tumbleson-Brink, D.M.; Harrington, E.; Blazek, J.D.; Ghoneima, A.; Kula, K.; Roper, R.J. Influence of prenatal EGCG treatment and Dyrk1a dosage reduction on craniofacial features associated with Down syndrome. Hum. Mol. Genet. 2016, 25, 4856–4869. [Google Scholar] [CrossRef] [Green Version]
- Dierssen, M.; de Lagrán, M.M. DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A): A gene with dosage effect during development and neurogenesis. Sci. World J. 2006, 6, 1911–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arron, J.R.; Winslow, M.M.; Polleri, A.; Chang, C.-P.; Wu, H.; Gao, X.; Neilson, J.R.; Chen, L.; Heit, J.J.; Kim, S.K.; et al. NFAT dysregulation by increased dosage of DSCR1 and DYRK1A on chromosome 21. Nature 2006, 441, 595. Available online: https://www.nature.com/articles/nature04678#supplementary-information (accessed on 4 June 2021). [CrossRef]
- Lee, Y.; Ha, J.; Kim, H.J.; Kim, Y.-S.; Chang, E.-J.; Song, W.-J.; Kim, H.-H. Negative Feedback Inhibition of NFATc1 by DYRK1A Regulates Bone Homeostasis. J. Biol. Chem. 2009, 284, 33343–33351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Torre, R.; De Sola, S.; Pons, M.; Duchon, A.; de Lagran, M.M.; Farré, M.; Fitó, M.; Benejam, B.; Langohr, K.; Rodriguez, J.; et al. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol. Nutr. Food Res. 2014, 58, 278–288. [Google Scholar] [CrossRef] [PubMed]
- García-Cerro, S.; Martínez Fernández, P.; Sánchez, V.; Corrales, A.; Flórez, J.; Vidal, R.; Rueda, N.; Arbones, M.; Martínez-Cué, C. Overexpression of Dyrk1A Is Implicated in Several Cognitive, Electrophysiological and Neuromorphological Alterations Found in a Mouse Model of Down Syndrome. PLoS ONE 2014, 9, e106572. [Google Scholar] [CrossRef] [Green Version]
- Guedj, F.; Sébrié, C.; Rivals, I.; Ledru, A.; Paly, E.; Bizot, J.C.; Smith, D.; Rubin, E.; Gillet, B.; Arbones, M.; et al. Green tea polyphenols rescue of brain defects induced by overexpression of DYRK1A. PLoS ONE 2009, 4, e4606. [Google Scholar] [CrossRef] [Green Version]
- Llambrich, S.; González, R.; Albaigès, J.; Wouters, J.; Marain, F.; Himmelreich, U.; Sharpe, J.; Dierssen, M.; Gsell, W.; Martínez-Abadías, N.; et al. Multimodal in vivo Imaging of the Integrated Postnatal Development of Brain and Skull and Its Co-modulation With Neurodevelopment in a Down Syndrome Mouse Model. Front. Med. 2022, 9, 815739. [Google Scholar] [CrossRef]
- de la Torre, R.; de Sola, S.; Hernandez, G.; Farré, M.; Pujol, J.; Rodriguez, J.; Espadaler, J.M.; Langohr, K.; Cuenca-Royo, A.; Principe, A.; et al. Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down’s syndrome (TESDAD): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 801–810. [Google Scholar] [CrossRef]
- Souchet, B.; Duchon, A.; Gu, Y.; Dairou, J.; Chevalier, C.; Daubigney, F.; Nalesso, V.; Créau, N.; Yu, Y.; Janel, N.; et al. Prenatal treatment with EGCG enriched green tea extract rescues GAD67 related developmental and cognitive defects in Down syndrome mouse models. Sci. Rep. 2019, 9, 3914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catuara-Solarz, S.; Espinosa-Carrasco, J.; Erb, I.; Langohr, K.; Gonzalez, J.R.; Notredame, C.; Dierssen, M. Combined Treatment with Environmental Enrichment and (-)-Epigallocatechin-3-Gallate Ameliorates Learning Deficits and Hippocampal Alterations in a Mouse Model of Down Syndrome. eNeuro 2016, 3, ENEURO.0103-16.2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noll, C.; Kandiah, J.; Moroy, G.; Gu, Y.; Dairou, J.; Janel, N. Catechins as a Potential Dietary Supplementation in Prevention of Comorbidities Linked with Down Syndrome. Nutrients 2022, 14, 2039. [Google Scholar] [CrossRef]
- Abeysekera, I.; Thomas, J.; Georgiadis, T.M.; Berman, A.G.; Hammond, M.A.; Dria, K.J.; Wallace, J.M.; Roper, R.J. Differential effects of Epigallocatechin-3-gallate containing supplements on correcting skeletal defects in a Down syndrome mouse model. Mol. Nutr. Food Res. 2016, 60, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Sebastiani, G.; Almeida-Toledano, L.; Serra-Delgado, M.; Navarro-Tapia, E.; Sailer, S.; Valverde, O.; Garcia-Algar, O.; Andreu-Fernández, V. Therapeutic Effects of Catechins in Less Common Neurological and Neurodegenerative Disorders. Nutrients 2021, 13, 2232. [Google Scholar] [CrossRef]
- Jamal, R.; LaCombe, J.; Patel, R.; Blackwell, M.; Thomas, J.R.; Sloan, K.; Wallace, J.M.; Roper, R.J. Increased dosage and treatment time of Epigallocatechin-3-gallate (EGCG) negatively affects skeletal parameters in normal mice and Down syndrome mouse models. PLoS ONE 2022, 17, e0264254. [Google Scholar] [CrossRef]
- Stringer, M.; Abeysekera, I.; Thomas, J.; LaCombe, J.; Stancombe, K.; Stewart, R.J.; Dria, K.J.; Wallace, J.M.; Goodlett, C.R.; Roper, R.J. Epigallocatechin-3-gallate (EGCG) consumption in the Ts65Dn model of Down syndrome fails to improve behavioral deficits and is detrimental to skeletal phenotypes. Physiol. Behav. 2017, 177, 230–241. [Google Scholar] [CrossRef]
- Goodlett, C.R.; Stringer, M.; LaCombe, J.; Patel, R.; Wallace, J.M.; Roper, R.J. Evaluation of the therapeutic potential of Epigallocatechin-3-gallate (EGCG) via oral gavage in young adult Down syndrome mice. Sci. Rep. 2020, 10, 10426. [Google Scholar] [CrossRef]
- Blazek, J.D.; Malik, A.M.; Tischbein, M.; Arbones, M.L.; Moore, C.S.; Roper, R.J. Abnormal mineralization of the Ts65Dn Down syndrome mouse appendicular skeleton begins during embryonic development in a Dyrk1a-independent manner. Mech. Dev. 2015, 136, 133–142. [Google Scholar] [CrossRef]
- Reeves, R.H.; Irving, N.G.; Moran, T.H.; Wohn, A.; Kitt, C.; Sisodia, S.S.; Schmidt, C.; Bronson, R.T.; Davisson, M.T. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat. Genet. 1995, 11, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Davisson, M.T.; Schmidt, C.; Akeson, E.C. Segmental trisomy of murine chromosome 16: A new model system for studying Down syndrome. Prog. Clin. Biol. Res. 1990, 360, 263–280. [Google Scholar]
- Blazek, J.D.; Gaddy, A.; Meyer, R.; Roper, R.J.; Li, J. Disruption of bone development and homeostasis by trisomy in Ts65Dn Down syndrome mice. Bone 2011, 48, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Herault, Y.; Delabar, J.M.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Yu, E.; Brault, V. Rodent models in Down syndrome research: Impact and future opportunities. Dis. Model. Mech. 2017, 10, 1165–1186. [Google Scholar] [CrossRef] [Green Version]
- Duchon, A.; Raveau, M.; Chevalier, C.; Nalesso, V.; Sharp, A.J.; Herault, Y. Identification of the translocation breakpoints in the Ts65Dn and Ts1Cje mouse lines: Relevance for modeling Down syndrome. Mamm Genome 2011, 22, 674–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinholdt, L.G.; Ding, Y.; Gilbert, G.J.; Czechanski, A.; Solzak, J.P.; Roper, R.J.; Johnson, M.T.; Donahue, L.R.; Lutz, C.; Davisson, M.T. Molecular characterization of the translocation breakpoints in the Down syndrome mouse model Ts65Dn. Mamm Genome 2011, 22, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Choy, K.W.; Pang, C.P.; Rogers, M.S. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2006, 22, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Chan, K.P.; Rogers, M.S.; Choy, K.W.; Pang, C.P. Pharmacokinetic studies of green tea catechins in maternal plasma and fetuses in rats. J. Pharm. Sci. 2006, 95, 1372–1381. [Google Scholar] [CrossRef]
- Ramachandran, B.; Jayavelu, S.; Murhekar, K.; Rajkumar, T. Repeated dose studies with pure Epigallocatechin-3-gallate demonstrated dose and route dependant hepatotoxicity with associated dyslipidemia. Toxicol. Rep. 2016, 3, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Shaw, P.R.; Klein, J.A.; Aziz, N.M.; Haydar, T.F. Longitudinal neuroanatomical and behavioral analyses show phenotypic drift and variability in the Ts65Dn mouse model of Down syndrome. Dis. Model. Mech. 2020, 13, dmm046243. [Google Scholar] [CrossRef]
- James Rohlf, F.; Marcus, L.F. A revolution morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Evolution and development of shape: Integrating quantitative approaches. Nat. Rev. Genet. 2010, 11, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis; Wiley: Chichester, UK, 1998. [Google Scholar]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hallgrimsson, B.; Percival, C.J.; Green, R.; Young, N.M.; Mio, W.; Marcucio, R. Morphometrics, 3D Imaging, and Craniofacial Development. Curr. Top. Dev. Biol. 2015, 115, 561–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardini, A.; O’Higgins, P.; Rohlf, F.J. Seeing Distinct Groups Where There are None: Spurious Patterns from Between-Group PCA. Evol. Biol. 2019, 46, 303–316. [Google Scholar] [CrossRef]
- Bookstein, F.L. Pathologies of Between-Groups Principal Components Analysis in Geometric Morphometrics. Evol. Biol. 2019, 46, 271–302. [Google Scholar] [CrossRef] [Green Version]
- Cardini, A.; Polly, P.D. Cross-validated Between Group PCA Scatterplots: A Solution to Spurious Group Separation? Evol. Biol. 2020, 47, 85–95. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 3.6.2; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kassambara, A. Package ‘ggpubr: ‘ggplot2’ Based Publication Ready Plots’, R package version 0.1.6; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.H.; Chen, C.C.; Wang, S.J.; Yeh, S.H. Bone mineral density in children with Down’s syndrome detected by dual photon absorptiometry. Nucl. Med. Commun. 1992, 13, 773–775. [Google Scholar] [CrossRef]
- Fischer-Brandies, H.; Schmid, R.G.; Fischer-Brandies, E. Craniofacial development in patients with Down’s syndrome from birth to 14 years of age. Eur. J. Orthod. 1986, 8, 35–42. [Google Scholar] [CrossRef]
- Vicente, A.; Bravo-González, L.-A.; López-Romero, A.; Muñoz, C.S.; Sánchez-Meca, J. Craniofacial morphology in down syndrome: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 19895. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.R.; LaCombe, J.; Long, R.; Lana-Elola, E.; Watson-Scales, S.; Wallace, J.M.; Fisher, E.M.C.; Tybulewicz, V.L.J.; Roper, R.J. Interaction of sexual dimorphism and gene dosage imbalance in skeletal deficits associated with Down syndrome. Bone 2020, 136, 115367. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Reeves, R.H.; Richtsmeier, J.T. Effects of aneuploidy on skull growth in a mouse model of Down syndrome. J. Anat. 2007, 210, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dutka, T.; Reeves, R.H.; Richtsmeier, J.T. Chronic up-regulation of sonic hedgehog has little effect on postnatal craniofacial morphology of euploid and trisomic mice. Dev. Dyn. 2016, 245, 114–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazuki, Y.; Gao, F.J.; Li, Y.; Moyer, A.J.; Devenney, B.; Hiramatsu, K.; Miyagawa-Tomita, S.; Abe, S.; Kazuki, K.; Kajitani, N.; et al. A non-mosaic transchromosomic mouse model of Down syndrome carrying the long arm of human chromosome 21. eLife 2020, 9, e56223. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, N.; Redhead, Y.; Vidal-García, M.; Lo Vercio, L.; Liu, W.; Fisher, E.M.C.; Hallgrímsson, B.; Tybulewicz, V.L.J.; Schnabel, J.A.; Green, J.B.A. A landmark-free morphometrics pipeline for high-resolution phenotyping: Application to a mouse model of Down syndrome. Development 2021, 148, dev188631. [Google Scholar] [CrossRef]
- Lana-Elola, E.; Cater, H.; Watson-Scales, S.; Greenaway, S.; Müller-Winkler, J.; Gibbins, D.; Nemes, M.; Slender, A.; Hough, T.; Keskivali-Bond, P.; et al. Comprehensive phenotypic analysis of the Dp1Tyb mouse strain reveals a broad range of Down syndrome-related phenotypes. Dis. Model. Mech. 2021, 14, dmm.049157. [Google Scholar] [CrossRef]
- Fowler, T.W.; McKelvey, K.D.; Akel, N.S.; Vander Schilden, J.; Bacon, A.W.; Bracey, J.W.; Sowder, T.; Skinner, R.A.; Swain, F.L.; Hogue, W.R.; et al. Low Bone Turnover and Low BMD in Down Syndrome: Effect of Intermittent PTH Treatment. PLoS ONE 2012, 7, e42967. [Google Scholar] [CrossRef] [Green Version]
- Stagni, F.; Giacomini, A.; Guidi, S.; Ciani, E.; Bartesaghi, R. Timing of therapies for Down syndrome: The sooner, the better. Front. Behav. Neurosci. 2015, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Mah, Y.-J.; Song, J.S.; Kim, S.-O.; Lee, J.-H.; Jeon, M.; Jung, U.-W.; Moon, S.J.; Kim, J.-H.; Choi, H.-J. The effect of epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both in vitro and in vivo. Arch. Oral Biol. 2014, 59, 539–549. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Kang, L.; Wang, C.-Z.; Huang, H.H.; Cheng, T.-L.; Huang, H.-T.; Lee, M.-J.; Lin, Y.-S.; Ho, M.-L.; Wang, G.-J.; et al. (-)-Epigallocatechin-3-Gallate (EGCG) Enhances Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Molecules 2018, 23, 3221. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Kang, L.; Wang, C.Z.; Huang, P.J.; Huang, H.T.; Lin, S.Y.; Chou, S.H.; Lu, C.C.; Shen, P.C.; Lin, Y.S.; et al. (-)-Epigallocatechin-3-Gallate Decreases Osteoclastogenesis via Modulation of RANKL and Osteoprotegrin. Molecules 2019, 24, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.R.; Sloan, K.; Cave, K.; Wallace, J.M.; Roper, R.J. Skeletal Deficits in Male and Female down Syndrome Model Mice Arise Independent of Normalized Dyrk1a Expression in Osteoblasts. Genes 2021, 12, 1729. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, C.N.; Allen, J.R.; Baumann, D.D.; Deitz, S.L.; Blazek, J.D.; Newbauer, A.; Darrah, A.; Long, B.C.; Young, B.; Clement, M.; et al. Non-trisomic homeobox gene expression during craniofacial development in the Ts65Dn mouse model of Down syndrome. Am. J. Med. Genet. Part A 2013, 161, 1866–1874. [Google Scholar] [CrossRef] [Green Version]
- Roper, R.J.; VanHorn, J.F.; Cain, C.C.; Reeves, R.H. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog. Mech. Dev. 2009, 126, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.A.; Sussan, T.E.; Reeves, R.H.; Richtsmeier, J.T. Complex contributions of Ets2 to craniofacial and thymus phenotypes of trisomic “Down syndrome” mice. Am. J. Med. Genet. Part A 2009, 149A, 2158–2165. [Google Scholar] [CrossRef] [Green Version]
- Stringer, M.; Abeysekera, I.; Dria, K.J.; Roper, R.J.; Goodlett, C.R. Low dose EGCG treatment beginning in adolescence does not improve cognitive impairment in a Down syndrome mouse model. Pharmacol. Biochem. Behav. 2015, 138, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, S.E. Down syndrome and the complexity of genome dosage imbalance. Nat. Rev. Genet. 2017, 18, 147–163. [Google Scholar] [CrossRef]
- Lana-Elola, E.; Watson-Scales, S.D.; Fisher, E.M.C.; Tybulewicz, V.L.J. Down syndrome: Searching for the genetic culprits. Dis. Models Mech. 2011, 4, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Primers 2020, 6, 9. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar] [CrossRef]
- Scholl, C.; Lepper, A.; Lehr, T.; Hanke, N.; Schneider, K.L.; Brockmöller, J.; Seufferlein, T.; Stingl, J.C. Population nutrikinetics of green tea extract. PLoS ONE 2018, 13, e0193074. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Taylor, L.S.; Mauer, L.J. Degradation kinetics of catechins in green tea powder: Effects of temperature and relative humidity. J. Agric. Food Chem. 2011, 59, 6082–6090. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Fernández, V.; Almeida Toledano, L.; Pizarro, N.; Navarro-Tapia, E.; Gómez-Roig, M.D.; de la Torre, R.; García-Algar, Ó. Bioavailability of Epigallocatechin Gallate Administered with Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llambrich, S.; González-Colom, R.; Wouters, J.; Roldán, J.; Salassa, S.; Wouters, K.; Van Bulck, V.; Sharpe, J.; Callaerts-Vegh, Z.; Vande Velde, G.; et al. Green Tea Catechins Modulate Skeletal Development with Effects Dependent on Dose, Time, and Structure in a down Syndrome Mouse Model. Nutrients 2022, 14, 4167. https://doi.org/10.3390/nu14194167
Llambrich S, González-Colom R, Wouters J, Roldán J, Salassa S, Wouters K, Van Bulck V, Sharpe J, Callaerts-Vegh Z, Vande Velde G, et al. Green Tea Catechins Modulate Skeletal Development with Effects Dependent on Dose, Time, and Structure in a down Syndrome Mouse Model. Nutrients. 2022; 14(19):4167. https://doi.org/10.3390/nu14194167
Chicago/Turabian StyleLlambrich, Sergi, Rubèn González-Colom, Jens Wouters, Jorge Roldán, Sara Salassa, Kaat Wouters, Vicky Van Bulck, James Sharpe, Zsuzsanna Callaerts-Vegh, Greetje Vande Velde, and et al. 2022. "Green Tea Catechins Modulate Skeletal Development with Effects Dependent on Dose, Time, and Structure in a down Syndrome Mouse Model" Nutrients 14, no. 19: 4167. https://doi.org/10.3390/nu14194167
APA StyleLlambrich, S., González-Colom, R., Wouters, J., Roldán, J., Salassa, S., Wouters, K., Van Bulck, V., Sharpe, J., Callaerts-Vegh, Z., Vande Velde, G., & Martínez-Abadías, N. (2022). Green Tea Catechins Modulate Skeletal Development with Effects Dependent on Dose, Time, and Structure in a down Syndrome Mouse Model. Nutrients, 14(19), 4167. https://doi.org/10.3390/nu14194167





