Effect of Heat-Treated Garlic (Allium sativum L.) on Growth Parameters, Plasma Lipid Profile and Histological Changes in the Ileum of Atherogenic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Research Material
Garlic and Its Treatment
2.2. Bioactive Compounds in Fresh and Thermally Processed Garlic (Allium sativum L.)
2.2.1. Garlic Extraction
2.2.2. Total Polyphenols
2.2.3. Flavonoids
2.2.4. Flavanols
2.2.5. Antioxidant Activity
2.3. Animal Study
2.3.1. Experimental Design
2.3.2. Animal Sacrifice
2.4. Sampling and Measurements
2.4.1. Basic Experimental Data
2.4.2. Plasma Lipid Profile
2.4.3. Preparation of the Ileum Tissue Samples for Morphometric Analysis in Light Microscopy
2.4.4. Light Microscope Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Compounds in Fresh and Thermally Processed Garlic (Allium sativum L.)
3.2. Animal Study
3.2.1. Feed Intake and Diet Performance Indices in Rats Supplemented with Freeze-Dried Fresh and Thermally Processed Garlic
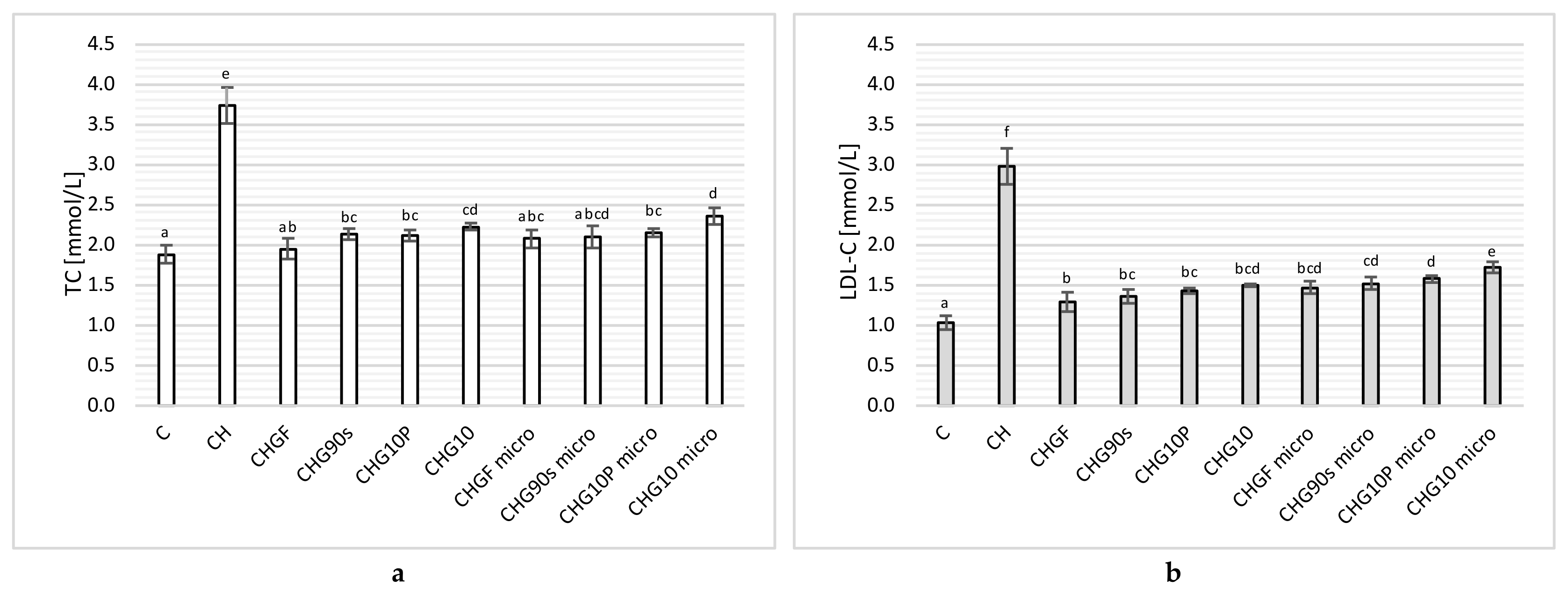
3.2.2. Lipid Profile in Rats Supplemented with Freeze-Dried Fresh and Thermally Processed Garlic
3.2.3. Morphometric Analysis of the Rats’ Ileum Fed Control- and Atherogenic Diet Supplemented with Freeze-Dried Fresh and Thermally Processed Garlic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive compounds. J. Nutr. 2001, 131, 955–962. [Google Scholar] [CrossRef]
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Santhosha, S.G.; Prakash, J.; Prabhavathi, S.N. Bioactive components of garlic and their physiological role in health maintenance: A review. Food Biosci. 2013, 3, 59–74. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review. Food Chem. 2016, 211, 41–50. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Rybczyńska-Tkaczyk, K.; Gaweł-Bęben, K.; Świeca, M.; Karaś, M.; Jakubczyk, A.; Matysiak, M.; Binduga, U.E.; Gmiński, J. Characterization of active compounds of different garlic (Allium sativum L.) cultivars. Polish J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]
- Petrovic, V.; Nepal, A.; Olaisen, C.; Bachke, S.; Hira, J.; Sogaard, C.K.; Rost, L.M.; Misund, K.; Andreassen, T.; Melo, T.M.; et al. Anti-cancer potential of homemade fresh garlic extract is related to increased endoplasmic reticulum stress. Nutrients 2018, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Rouhani, M.H.; Azadbakht, L. The effect of garlic intake on glycemic control in humans: A systematic review and meta-analysis. Progr. Nutr. 2017, 19, 10–18. [Google Scholar] [CrossRef]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef]
- Lawal, B.; Shittu, O.K.; Oibiokpa, F.I.; Mohammed, H.; Umar, S.I.; Haruna, G.M. Antimicrobial evaluation, acute and sub-acute toxicity studies of Allium sativum. J. Acute Dis. 2016, 5, 296–301. [Google Scholar] [CrossRef]
- Banerjee, S.K.; Maulik, S.K. Effect of garlic on cardiovascular disorders: A review. Nutr. J. 2002, 19, 1–4. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Locatelli, D.A.; Gonzálezc, R.E.; Cavagnaro, P.F.; Alejandra, B.; Camargo, A.B. Analytical methods for bioactive sulfur compounds in Allium: An integrated review and future directions. J. Food Comps. Anal. 2017, 61, 4–19. [Google Scholar] [CrossRef]
- Augusti, K.T. Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L). Indian J. Exp. Biol. 1996, 34, 634–640. [Google Scholar]
- Aslani, N.; Entezari, M.H.; Askari, G.; Maghsoudi, Z.; Maracy, M.R. Effect of garlic and lemon juice mixture on lipid profile and some cardiovascular risk factors in people 30-60 years old with moderate hyperlipidaemia: A randomized clinical trial. Int. J. Prev. Med. 2016, 29, 95. [Google Scholar] [CrossRef]
- Warshafsky, S.; Kamer, R.S.; Sivak, S.L. Effect of garlic on total serum cholesterol. A meta-analysis. Ann. Intern. Med. 1993, 119, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Toben, C.; Fakler, P. Effect of garlic on serum lipids: An updated meta-analysis. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef]
- Ried, K. Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis. Exp. Ther. Med. 2019, 19, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Leontowicz, M.; Leontowicz, H.; Najman, K.; Namiesnik, J.; Park, Y.S.; Jung, S.T.; Kang, S.G.; Trakhtenberg, S. Supplementation of garlic lowers lipids and increases antioxidant capacity in plasma of rats. Nutr. Res. 2006, 26, 362–368. [Google Scholar] [CrossRef]
- Gorinstein, S.; Drzewiecki, J.; Leontowicz, H.; Leontowicz, M.; Najman, K.; Katrich, E.; Barasch, D.; Yamamoto, K.; Trakhtenberg, S. Raw and boiled garlic enhances plasma antioxidant activity and improves plasma lipid metabolism in cholesterol—fed rats. Life Sci. 2006, 78, 655–663. [Google Scholar] [CrossRef]
- Elmahdi, B.; Maha, M.K.; Afaf, I.A. The effect of fresh crushed garlic bulbs (Allium sativum) on plasma lipids in hypercholesterolemic rats. Res. J. Anim. Vet. Sci. 2008, 3, 15–19. [Google Scholar]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Jastrzębski, Z.; Najman, K.; Tashma, Z.; Katrich, E.; Heo, B.G.; Cho, J.Y.; Park, Y.J.; et al. The influence of raw and processed garlic and onions on plasma classical and non–classical atherosclerosis indices: Investigations in vitro and in vivo. Phytother. Res. 2010, 24, 706–714. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Najman, K.; Bielecki, W.; Ham, K.S.; Kang, S.G.; Paredes-Lopez, O.; Martinez-Ayala, A.L.; Trakhtenberg, S. Aorta and liver changes in rats fed cholesterol-containing and raw vegetables-supplemented diets: Experiments in vitro and in vivo. J. Agric. Food Chem. 2011, 59, 7441–7451. [Google Scholar] [CrossRef]
- Sobenin, A.; Priyanishnikov, V.V.; Kunnova, L.M.; Rabinovich, Y.A.; Martirosyan, D.M.; Orekhov, A.N. The effects of time-released garlic powder tablets on multifunctional cardiovascular risk in patients with coronary artery disease. Lipids Health Dis. 2010, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Olaiya, O.G.; Ailenosi, S.S.; Adelaja, A.; Eniola, K. Effects of aqueous extracts of garlic and vitamin C on the kidney of albino rats. Asian J. Exp. Biol. Sci. 2011, 2, 455–461. [Google Scholar]
- Banerjee, S.K.; Maulik, M.; Manchandra, S.C.; Dinda, A.K.; Das, T.K.; Maulik, S.K. Garlic-induced alteration in rat liver and Sidney morphology and associated changes in endogenous antioxidant status. Food Chem. Toxicol. 2001, 39, 793–797. [Google Scholar] [CrossRef]
- Joseph, P.K.; Ramesha, R.; Sundaresh, C.S. Toxic effects of garlic extract and garlic oil in rats. Indian J. Exp. Biol. 1989, 27, 977–979. [Google Scholar] [PubMed]
- Nakagawa, S.; Masamoto, K.; Sumiyoshi, H.; Kunihiro, K.; Fuwa, T. Effect of raw and extracted aged garlic on growth of young rats and their organ after peroral administration. Toxicol. Sci. 1980, 5, 91–112. [Google Scholar] [CrossRef]
- Omotoso, G.O.; Muonagolu, J.; Enaibe, B.U. Histological evaluation of the jejunum and ileum of rats after administration of high dose garlic aqueous exctract. Int. J. Health Sci. 2012, 6, 111–116. [Google Scholar] [CrossRef]
- Hoshino, T.; Kashimoto, N.; Kasuga, S. Effects of garlic preparations on the gastrointestinal mucosa. J. Nutr. 2001, 131, 1109–1113. [Google Scholar] [CrossRef]
- Najman, K.; Leontowicz, H.; Leontowicz, M. The influence of plants from Alliaceae family on morphological parameters of intestine in atherogenic rats. Nutrients 2021, 13, 3876. [Google Scholar] [CrossRef]
- David, A.V.A.; Satyanarayana, N.; Parasuraman, S.; Bharathi, S.; Arulmoli, R. Ameliorative effect of quercetin on methotrexate induced toxicity in Sprague-Dawley rats: A histopathological study. Indian J. Pharm. Educ. Res. 2016, 50, 200–2008. [Google Scholar] [CrossRef]
- Riad, N.H.A.; Fares, N.H.; Mostafa, O.M.S.; Mahmoud, Y.I. The effect of garlic on murine Schistosomiasis Mansoni: A histological and ultrastructural study on the ileum. Res. J. Med. Sci. 2008, 3, 188–201. [Google Scholar]
- Arija, I.; Viveros, A.; Brenes, A.; Canales, R.; Pizarro, M.; Castaño, M. Histological alterations in the intestinal epithelium caused by the inclusion of full-fat sunflower kernels in broiler chicken diets. Poult. Sci. 2000, 79, 1332–1334. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Feucht, W.; Polster, J. Nuclei of plants as a sink for flavanols. J. Biosci. 2001, 56, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Kim, H.K.; Ye, S.H.; Lim, T.S.; Ha, T.Y.; Kwon, J.H. Physiological activities of garlic extracts as affected by habitat and solvents. J. Med. Food 2005, 8, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Drzewiecki, J.; Cvikrova, M.; Martincova, O.; Katrich, E.; Trakhtenberg, S. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J. Agric. Food Chem. 2008, 56, 4418–4426. [Google Scholar] [CrossRef]
- Gorinstein, S.; Jastrzębski, Z.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Park, Y.S.; Heo, B.G.; Cho, J.Y.; Bae, J.H. Comparative control of the bioactivity of some frequently consumed vegetables subjected to different processing conditions. Food Control. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Im, M.H.; Park, Y.S.; Ham, K.S.; Kang, S.G.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Gorinstein, S. Effects of cooking on the bioactivity of lotus roots and white onions. Int. J. Food Prop. 2012, 15, 49–59. [Google Scholar] [CrossRef]
- Locatelli, D.A.; Altamirano, J.C.; González, R.E.; Camargo, A.B. Home-cooked garlic remains a healthy food. J. Funct. Foods 2015, 16, 1–8. [Google Scholar] [CrossRef]
- Lawson, L.D.; Gardner, C.D. Composition, stability and bioavailability of garlic products used in a clinical trial. J. Agric. Food Chem. 2005, 53, 6254–6261. [Google Scholar] [CrossRef]
- Zeng, T.; Guo, F.F.; Zhang, C.L.; Zhao, S.; Dou, D.D.; Gao, X.C.; Xie, K.Q.; Yu, L.; Xie, K.Q. The anti-fatty liver effects of garlic oil on acute ethanol-exposed mice. Chem. Biol. Interact. 2008, 176, 234–242. [Google Scholar] [CrossRef]
- Reinhart, K.M.; Talati, R.; White, C.M.; Coleman, C.I. The impact of garlic on lipid parameters: A systematic review and metaanalysis. Nutr. Res. Rev. 2009, 22, 39–48. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Islami, M.R.; Karam, G.R.A.; Khaksari, M.; Lotfi, A.S.; Hajizadeh, M.R.; Mirzaee, M.R. Study of the effects of raw garlic consumption on the level of lipids and other blood biochemical factors in hyperlipidemic individuals. Pak. J. Pharm. Sci. 2006, 19, 295–298. [Google Scholar] [PubMed]
- Gebhardt, R.; Beck, H.; Wagner, K.G. Inhibition of cholesterol biosynthesis by allicin and ajoene in rat hepatocytes and HepG2 cellls. Biochim. Biophys. Acta 1994, 1213, 57–62. [Google Scholar] [CrossRef]
- Gebhardt, R.; Beck, H. Differential inhibitory effects of garlic-derived organosulfur compounds on cholesterol biosynthesis in primary rat hepatocyte culture. Lipids 1996, 31, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yeh, Y.Y. S-alk(en)yl cysteines of garlic inhibit cholesterol synthesis by deactivating HMG-CoA reductase in cultured rat hepatocytes. J. Nutr. 2002, 132, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Rahman, E.; Donia, S.S.; Naguib, Y.M. Garlic improves altered vascular reactivity and plasma lipids in high cholesterol-fed rats. Menoufia Med. J. 2013, 26, 35–43. [Google Scholar] [CrossRef]
- Nayaked, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Najman, K.; Sadowska, A.; Hallmann, E. Evaluation of bioactive and physicochemical properties of white and black garlic (Allium sativum L.) from conventional and organic cultivation. Appl. Sci. 2021, 11, 874. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Selim, H.M. Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition 2016, 32, 849–854. [Google Scholar] [CrossRef]
- Yang, Q.; Hu, Q.; Yamato, O.; Lee, K.W.; Maede, Y.; Yoshihara, T. Organosulfur compounds from garlic (Allium sativum) oxidizing canine erythrocytes. Z. Naturforsch. C J. Biosci. 2003, 58, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Kodera, Y. Dietary Tolerance/Absorption/Metabolism of Garlic. In Nutraceuticals: Designer Foods III Garlic, Soy and Licorice; Trumbell, C.T., Lanchance, P., Eds.; Food and Nutrition Press: Thousand Oaks, CA, USA, 1997; pp. 95–103. [Google Scholar]
- Bajaj-Elliott, M.; Poulsom, R.; Pender, S.L.F.; Wathen, N.C.; MacDonald, T.T. Interactions between stromal cell-derived keratinocyte growth factor and epithelial transforming growth factor in immune-mediated crypt cell hyperplasia. J. Clin. Investig. 1998, 102, 1473–1480. [Google Scholar] [CrossRef]
- Harris, J.C.; Plummer, S.; Turner, M.P.; Lloyd, D. The microaerophillic flagellate Giardia intestinalis: Allium sativum (garlic) is an effective antigiardial. Microbiology 2000, 146, 3119–3127. [Google Scholar] [CrossRef]
- Rahmy, T.R.; Hemmaid, K.Z. Prophylactic action of garlic on the histological and histochemical patterns of hepatic and gastric tissues in rats injected with a snake venom. J. Nat. Toxins. 2001, 10, 137–165. [Google Scholar]
- Goda, T.; Takase, S. Effect of dietary fat content on microvillus in rat jejunum. J. Nutr. Sci. Vitaminol. 1994, 40, 127–136. [Google Scholar] [CrossRef]


| Abbreviation | Garlic Heat Treatment |
|---|---|
| GF | Freeze-dried fresh garlic |
| G90s | Freeze-dried 90 s blanched garlic |
| G10P | Freeze-dried 10 min pan fried without fat garlic |
| G10 | Freeze-dried 10 min boiled in water garlic |
| GF micro | Freeze-dried fresh and microwaved garlic |
| G90s micro | Freeze-dried 90 s blanched and microwaved garlic |
| G10P micro | Freeze-dried 10 min pan fried without fat and microwaved garlic |
| G10 micro | Freeze-dried 10 min boiled in water and microwaved garlic |
| Abbreviation | Experimental Group |
|---|---|
| C | Control group |
| CH | Control group with 1% of cholesterol |
| CHGF | Group with 1% of cholesterol and freeze-dried fresh garlic addition |
| CHG90s | Group with 1% of cholesterol and freeze-dried 90 s. blanched garlic addition |
| CHG10P | Group with 1% of cholesterol and freeze-dried 10 min pan fried without fat garlic addition |
| CHG10 | Group with 1% of cholesterol and freeze-dried 10 min boiled in water garlic addition |
| CHGF micro | Group with 1% of cholesterol and freeze-dried fresh and microwaved garlic addition |
| CHG90s micro | Group with 1% of cholesterol and freeze-dried 90 s blanched and microwaved garlic addition |
| CHG10P micro | Group with 1% of cholesterol and freeze-dried 10 min pan fried without fat and microwaved garlic addition |
| CHG10 micro | Group with 1% of cholesterol and freeze-dried 10 min boiled in water and microwaved garlic addition |
| Sample | Total Polyphenols [mg GAE/g d.m.] | Flavonoids [mg CE/g d.m.] | Flavanols [µg CE/g d.m.] | ABTS [µmol TE/g d.m.] |
|---|---|---|---|---|
| GF | 19.40 ± 1.12 c | 3.37 ± 0.31 c | 6.71 ± 0.41 f | 47.73 ± 1.69 c |
| G90s | 16.11 ± 1.11 b | 3.15 ± 0.19 c | 5.16 ± 0.38 d | 38.77 ± 1.61 b |
| G10P | 15.98 ± 0.98 b | 2.94 ± 0.18 bc | 4.29 ± 0.31 c | 38.92 ± 1.21 b |
| G10 | 13.52 ± 0.81 a | 1.84 ± 0.12 a | 3.99 ± 0.20 b | 30.43 ± 1.19 a |
| GF micro | 18.43 ± 0.77 c | 3.20 ± 0.25 c | 5.88 ± 0.36 e | 44.43 ± 1.35 c |
| G90s micro | 15.53 ± 0.89 b | 2.37 ± 0.21 b | 5.06 ± 0.39 d | 35.01 ± 1.51 b |
| G10P micro | 15.11 ± 0.78 b | 2.29 ± 0.19 b | 3.86 ± 0.39 b | 37.76 ± 1.20 b |
| G10 micro | 12.05 ± 0.81 a | 1.76 ± 0.12 a | 3.06 ± 0.21 a | 27.16 ± 1.19 a |
| Group | Feed Intake [g] | Cholesterol Intake [g] | Garlic Intake [mg] | BWG [g BW] | FER [g/g BW] | SI-L [% BW] |
|---|---|---|---|---|---|---|
| C | 590 ± 68 ab | - | - | 178.2 ± 27.0 bcd | 3.33 ± 0.34 a | 3.65 ± 0.37 a |
| CH | 501 ± 36 a | 5.0 ± 0.4 a | - | 134.9 ± 26.2 ab | 3.80 ± 0.61 a | 3.70 ± 0.38 a |
| CHGF | 481 ± 42 a | 4.8 ± 0.4 a | 579 ± 50 a | 113.9 ± 27.0 a | 4.32 ± 0.54 a | 3.26 ± 0.31 a |
| CHG90s | 501 ± 20 a | 5.0 ± 0.2 a | 602 ± 28 a | 127.4 ± 14.6 ab | 3.96 ± 0.34 a | 3.44 ± 0.37 a |
| CHG10P | 531 ± 23 a | 5.3 ± 0.2 a | 642 ± 31 a | 143.2 ± 18.6 abc | 3.74 ± 0.37 a | 3.48 ± 0.63 a |
| CHG10 | 525 ± 42 a | 5.2 ± 0.4 a | 635 ± 53 a | 136.2 ± 31.0 ab | 3.98 ± 0.76 a | 3.42 ± 0.66 a |
| CHGF micro | 661 ± 17 b | 6.6 ± 0.2 b | 746 ± 21 b | 198.0 ± 23.9 cd | 3.38 ± 0.38 a | 3.68 ± 0.35 a |
| CHG90s micro | 665 ± 11 b | 6.6 ± 0.1 b | 752 ± 14 b | 203.7 ± 22.7 d | 3.29 ± 0.34 a | 3.86 ± 0.16 a |
| CHG10P micro | 666 ± 13 b | 6.7 ± 0.1 b | 752 ± 16 b | 206.7 ± 28.0 d | 3.27 ± 0.45 a | 3.48 ± 0.46 a |
| CHG10 micro | 663 ± 18 b | 6.6 ± 0.2 b | 745 ± 20 b | 197.8 ± 13.1 cd | 3.36 ± 0.14 a | 3.55 ± 0.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najman, K.; Sadowska, A.; Buczak, K.; Leontowicz, H.; Leontowicz, M. Effect of Heat-Treated Garlic (Allium sativum L.) on Growth Parameters, Plasma Lipid Profile and Histological Changes in the Ileum of Atherogenic Rats. Nutrients 2022, 14, 336. https://doi.org/10.3390/nu14020336
Najman K, Sadowska A, Buczak K, Leontowicz H, Leontowicz M. Effect of Heat-Treated Garlic (Allium sativum L.) on Growth Parameters, Plasma Lipid Profile and Histological Changes in the Ileum of Atherogenic Rats. Nutrients. 2022; 14(2):336. https://doi.org/10.3390/nu14020336
Chicago/Turabian StyleNajman, Katarzyna, Anna Sadowska, Krzysztof Buczak, Hanna Leontowicz, and Maria Leontowicz. 2022. "Effect of Heat-Treated Garlic (Allium sativum L.) on Growth Parameters, Plasma Lipid Profile and Histological Changes in the Ileum of Atherogenic Rats" Nutrients 14, no. 2: 336. https://doi.org/10.3390/nu14020336
APA StyleNajman, K., Sadowska, A., Buczak, K., Leontowicz, H., & Leontowicz, M. (2022). Effect of Heat-Treated Garlic (Allium sativum L.) on Growth Parameters, Plasma Lipid Profile and Histological Changes in the Ileum of Atherogenic Rats. Nutrients, 14(2), 336. https://doi.org/10.3390/nu14020336






