Dietary Fats and Cardio-Metabolic Outcomes in a Cohort of Italian Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Cardio-Metabolic Outcomes
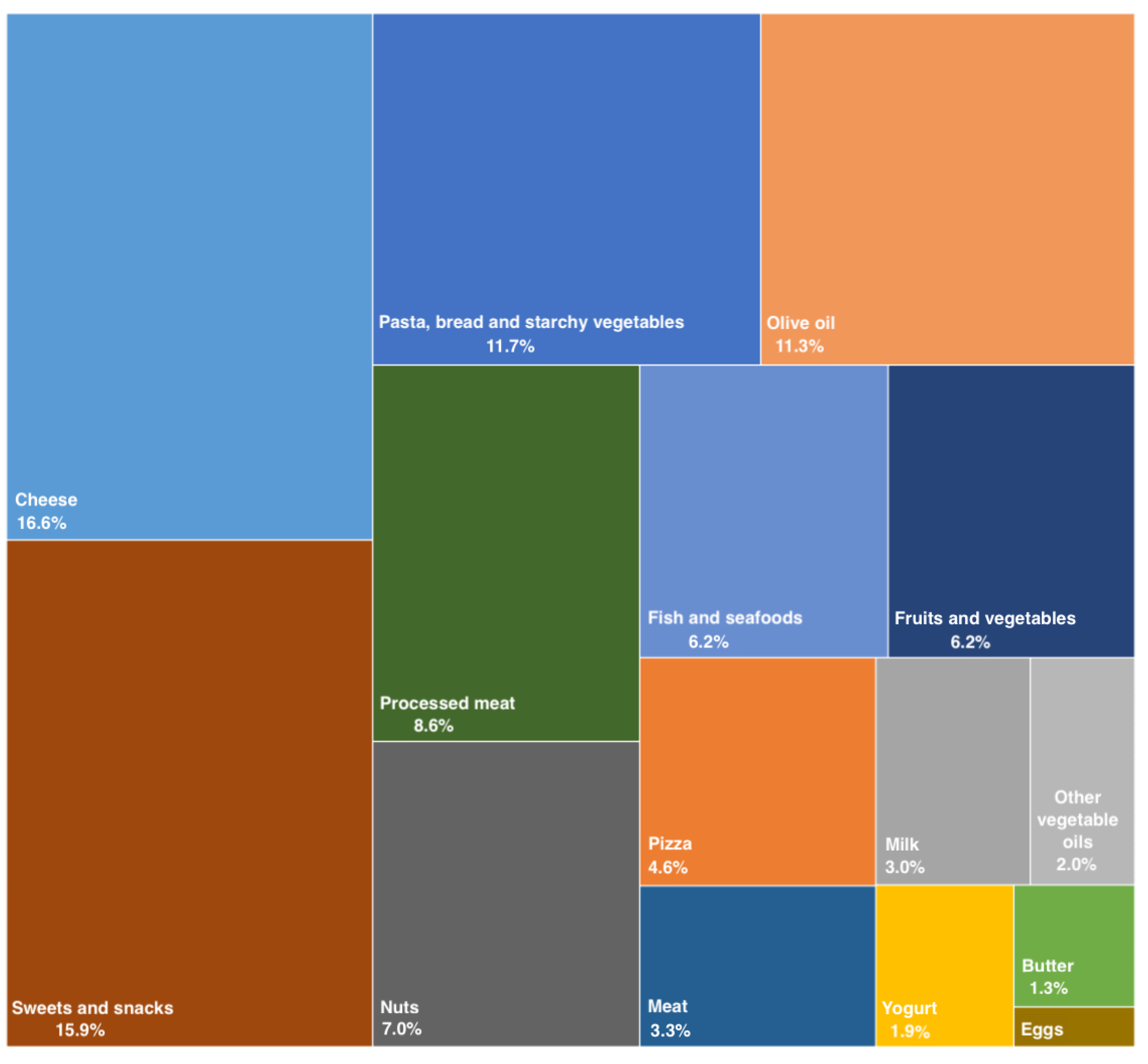
2.4. Dietary Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Monti, D.; Ostan, R.; Borelli, V.; Castellani, G.; Franceschi, C. Inflammaging and human longevity in the omics era. Mech. Ageing Dev. 2017, 165, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Levings, M.K. Immune regulation in obesity-associated adipose inflammation. J. Immunol. 2013, 191, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, K.D.; Murphy, S.; Xu, J.; Arias, E. Mortality in the united states, 2016. NCHS Data Brief 2017, 293, 1–8. [Google Scholar]
- Mozaffarian, D.; Ludwig, D.S. The 2015 US dietary guidelines: Lifting the ban on total dietary fat. JAMA 2015, 313, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G. Are there any concerns about dairy food consumption and cardiovascular health? Int. J. Food Sci. Nutr. 2021, 72, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Tieri, M.; Ghelfi, F.; Titta, L.; Marventano, S.; Lafranconi, A.; Gambera, A.; Alonzo, E.; Sciacca, S.; Buscemi, S.; et al. Dairy foods and health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020, 71, 138–151. [Google Scholar] [CrossRef]
- Tieri, M.; Ghelfi, F.; Vitale, M.; Vetrani, C.; Marventano, S.; Lafranconi, A.; Godos, J.; Titta, L.; Gambera, A.; Alonzo, E.; et al. Whole grain consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2020, 71, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; La Vignera, S.; Condorelli, R.A.; Godos, J.; Marventano, S.; Tieri, M.; Ghelfi, F.; Titta, L.; Lafranconi, A.; Gambera, A.; et al. Total, red and processed meat consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2022, 73, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.M.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.G.; Møller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [CrossRef]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.L. The science of fatty acids and inflammation. Adv. Nutr. 2015, 6, 293S–301S. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Grosso, G.; Marventano, S.; D’Urso, M.; Mistretta, A.; Galvano, F. The Mediterranean healthy eating, ageing, and lifestyle (MEAL) study: Rationale and study design. Int. J. Food Sci. Nutr. 2017, 68, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a food frequency questionnaire for use in Italian adults living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef]
- Marventano, S.; Mistretta, A.; Platania, A.; Galvano, F.; Grosso, G. Reliability and relative validity of a food frequency questionnaire for Italian adults living in Sicily, Southern Italy. Int. J. Food Sci. Nutr. 2016, 67, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 8, CD011737. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Teicholz, N.; Magkos, F.; Bier, D.M.; Brenna, J.T.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; Yusuf, S.; et al. Dietary Saturated Fats and Health: Are the U.S. Guidelines Evidence-Based? Nutrients 2021, 13, 3305. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Iggman, D.; Rosqvist, F.; Larsson, A.; Arnlöv, J.; Beckman, L.; Rudling, M.; Risérus, U. Role of dietary fats in modulating cardiometabolic risk during moderate weight gain: A randomized double-blind overfeeding trial (LIPOGAIN study). J. Am. Heart Assoc. 2014, 3, e001095. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.N.; Capettini, L.S.A.; Lemos, V.S.; Santos, R.A.S.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Akpa, M.M.; Point, F.; Sawadogo, S.; Radenne, A.; Mounier, C. Inhibition of insulin and T3-induced fatty acid synthase by hexanoate. Lipids 2010, 45, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Asakawa, M.; Yamamoto, M.; Matsuda, M.; Maki, A.; Matsumoto, Y. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Ann. Surg. 2003, 237, 246–255. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zhang, X.; Xu, Q.; Zhang, Y.; Xue, C.; Guo, C. Medium-chain fatty acids decrease serum cholesterol via reduction of intestinal bile acid reabsorption in C57BL/6J mice. Nutr. Metab. 2018, 15, 37. [Google Scholar] [CrossRef]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Dabadie, H.; Peuchant, E.; Bernard, M.; LeRuyet, P.; Mendy, F. Moderate intake of myristic acid in sn-2 position has beneficial lipidic effects and enhances DHA of cholesteryl esters in an interventional study. J. Nutr. Biochem. 2005, 16, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, V.; Kumar, N.; Ai, W.; Gopal, T.; Bhatt, S.; Harris, E.N.; Talmon, G.A.; Desouza, C.V. Myristic Acid Supplementation Aggravates High Fat Diet-Induced Adipose Inflammation and Systemic Insulin Resistance in Mice. Biomolecules 2022, 12, 739. [Google Scholar] [CrossRef]
- Zock, P.L.; de Vries, J.H.; Katan, M.B. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arterioscler. Thromb. 1994, 14, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Li, Y.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Willett, W.C.; Hu, F.B.; Sun, Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: Two prospective longitudinal cohort studies. BMJ 2016, 355, i5796. [Google Scholar] [CrossRef]
- Turpeinen, A.M.; Wübert, J.; Aro, A.; Lorenz, R.; Mutanen, M. Similar effects of diets rich in stearic acid or trans-fatty acids on platelet function and endothelial prostacyclin production in humans. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 316–322. [Google Scholar] [CrossRef][Green Version]
- Hunter, J.E.; Zhang, J.; Kris-Etherton, P.M. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. Am. J. Clin. Nutr. 2010, 91, 46–63. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary fats and cardiovascular disease: A presidential advisory from the american heart association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Snetselaar, L.; Bailey, R.; Sabaté, J.; Van Horn, L.; Schneeman, B.; Bahnfleth, C.; Kim, J.H.; Spahn, J.; Butera, G.; Terry, N.; et al. Types of Dietary Fat and Cardiovascular Disease: A Systematic Review [Internet]; USDA Nutrition Evidence Systematic Review: Alexandria, VA, USA, 2020. [Google Scholar]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: A systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef] [PubMed]
- DuBroff, R.; de Lorgeril, M. Fat or fiction: The diet-heart hypothesis. BMJ Evid. Based Med. 2021, 26, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Rett, B.S.; Whelan, J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011, 8, 36. [Google Scholar] [CrossRef]
- Miura, K.; Stamler, J.; Nakagawa, H.; Elliott, P.; Ueshima, H.; Chan, Q.; Brown, I.J.; Tzoulaki, I.; Saitoh, S.; Dyer, A.R.; et al. The International Study of Macro-Micronutrients and Blood Pressure Study. Hypertension 2008, 52, 408–414. [Google Scholar] [CrossRef]
- Zhang, X.; Ritonja, J.A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 Polyunsaturated Fatty Acids Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2022, 11, e025071. [Google Scholar] [CrossRef]
- Mori, T.A. Omega-3 fatty acids and hypertension in humans. Clin. Exp. Pharmacol. Physiol. 2006, 33, 842–846. [Google Scholar] [CrossRef]
- Guo, X.-F.; Li, K.-L.; Li, J.-M.; Li, D. Effects of EPA and DHA on blood pressure and inflammatory factors: A meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 3380–3393. [Google Scholar] [CrossRef]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef]
- Salmerón, J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rimm, E.B.; Willett, W.C. Dietary fat intake and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2001, 73, 1019–1026. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Wanders, A.J.; Blom, W.A.M.; Zock, P.L.; Geleijnse, J.M.; Brouwer, I.A.; Alssema, M. Plant-derived polyunsaturated fatty acids and markers of glucose metabolism and insulin resistance: A meta-analysis of randomized controlled feeding trials. BMJ Open Diabetes Res. Care 2019, 7, e000585. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Marklund, M.; Imamura, F.; Tintle, N.; Ardisson Korat, A.V.; de Goede, J.; Zhou, X.; Yang, W.-S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef]
- Julibert, A.; Bibiloni, M.D.M.; Tur, J.A. Dietary fat intake and metabolic syndrome in adults: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 887–905. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Cernigliaro, A.; Cincione, R.I.; Buscemi, S.; Libra, M.; Galvano, F.; Grosso, G. Total nut, tree nut, and peanut consumption and metabolic status in southern italian adults. Int. J. Environ. Res. Public Health 2021, 18, 1847. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A. Fatty acids and cardiovascular risk. evidence, lack of evidence, and diligence. Nutrients 2020, 12, 3782. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Crespo, M.C.; López de Las Hazas, M.C.; Visioli, F.; Dávalos, A. Olive oil consumption and its repercussions on lipid metabolism. Nutr. Rev. 2020, 78, 952–968. [Google Scholar] [CrossRef]
- Delgado, G.E.; Krämer, B.K.; Lorkowski, S.; März, W.; von Schacky, C.; Kleber, M.E. Individual omega-9 monounsaturated fatty acids and mortality—The Ludwigshafen Risk and Cardiovascular Health Study. J. Clin. Lipidol. 2017, 11, 126–135.e5. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Yamasaki, Y.; Takeo, J.; Miyahara, H.; Sebe, M.; Bando, M.; Tanba, Y.; Mishima, Y.; Takeji, K.; Ueshima, N.; et al. Long-chain monounsaturated fatty acids improve endothelial function with altering microbial flora. Transl. Res. 2021, 237, 16–30. [Google Scholar] [CrossRef]
| Total Fats | p-Value | ||||
|---|---|---|---|---|---|
| Q1 (n = 448) | Q2 (n = 496) | Q3 (n = 506) | Q4 (n = 486) | ||
| Total fats (g), mean (SD) | 36.2 (4.8) | 49.7 (3.9) | 64.3 (4.3) | 97.8 (31.6) | |
| Male | 181 (40.4) | 219 (44.2) | 212 (41.9) | 192 (29.5) | 0.477 |
| Female | 267 (59.6) | 277 (55.8) | 294 (58.1) | 294 (60.5) | |
| Age, mean (SD) | 49.2 (18.2) | 50.0 (17.5) | 49.0 (17.8) | 45.6 (16.8) | 0.001 |
| Educational level, n (%) | |||||
| Low | 152 (33.9) | 171 (34.5) | 169 (33.4) | 205 (42.2) | <0.001 |
| Medium | 146 (32.6) | 193 (38.9) | 211 (41.7) | 170 (35.0) | |
| High | 150 (33.5) | 132 (26.6) | 126 (24.9) | 111 (22.8) | |
| Smoking status, n (%) | |||||
| Non-smoker | 309 (69.0) | 309 (62.3) | 290 (57.3) | 287 (59.1) | 0.001 |
| Current smoker | 96 (21.4) | 125 (25.2) | 129 (25.5) | 115 (23.7) | |
| Former smoker | 43 (9.6) | 62 (12.5) | 87 (17.2) | 84 (17.2) | |
| Physical activity level, n (%) | |||||
| Low | 87 (19.7) | 83 (18.7) | 54 (13.6) | 105 (23.6) | <0.001 |
| Medium | 214 (48.4) | 194 (43.8) | 227 (57.0) | 221 (49.7) | |
| High | 141 (31.9) | 166 (37.5) | 117 (29.4) | 119 (26.7) | |
| BMI categories, n (%) | |||||
| Normal | 224 (51) | 221 (46) | 213 (45.8) | 193 (46.6) | 0.610 |
| Overweight | 137 (31.2) | 172 (35.8) | 169 (36.3) | 152 (36.7) | |
| Obese | 78 (17.8) | 87 (18.1) | 83 (17.8) | 69 (16.7) | |
| Mediterranean diet adherence, n (%) | |||||
| Low | 283 (63.2) | 259 (52.2) | 279 (55.1) | 230 (47.3) | <0.001 |
| Medium | 140 (31.3) | 172 (34.7) | 181 (35.8) | 198 (40.7) | |
| High | 25 (5.6) | 65 (13.1) | 46 (9.1) | 58 (11.9) | |
| OR (95% CI) | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Hypertension | ||||
| Total fats | ||||
| Energy-adjusted | 1 | 1.01 (0.78, 1.32) | 0.65 (0.49, 0.86) | 0.51 (0.36, 0.73) |
| Multivariate-adjusted | 1 | 0.92 (0.66, 1.29) | 0.53 (0.36, 0.76) | 0.57 (0.35, 0.91) |
| SFA | ||||
| Energy-adjusted | 1 | 1.01 (0.78, 1.32) | 0.73 (0.55, 0.97) | 0.68 (0.48, 0.96) |
| Multivariate-adjusted | 1 | 0.94 (0.68, 1.32) | 0.66 (0.46, 0.97) | 0.55 (0.34, 0.89) |
| MUFA | ||||
| Energy-adjusted | 1 | 1.04 (0.80, 1.35) | 0.68 (0.51, 0.91) | 0.55 (0.38, 0.78) |
| Multivariate-adjusted | 1 | 0.99 (0.72, 1.39) | 0.61 (0.42, 0.88) | 0.65 (0.41, 1.04) |
| PUFA | ||||
| Energy-adjusted | 1 | 0.85 (0.65, 1.11) | 0.86 (0.64, 1.15) | 0.74 (0.51, 1.09) |
| Multivariate-adjusted | 1 | 0.83 (0.60, 1.17) | 0.83 (0.56, 1.22) | 0.71 (0.43, 1.18) |
| Type-2 diabetes | ||||
| Total fats | ||||
| Energy-adjusted | 1 | 0.45 (0.27, 0.76) | 0.53 (0.31, 0.89) | 0.81 (0.44, 1.50) |
| Multivariate-adjusted | 1 | 0.39 (0.21, 0.74) | 0.27 (0.12, 0.61) | 0.95 (0.41, 2.19) |
| SFA | ||||
| Energy-adjusted | 1 | 0.46 (0.27, 0.77) | 0.63 (0.38, 1.04) | 0.70 (0.38, 1.30) |
| Multivariate-adjusted | 1 | 0.57 (0.31, 1.07) | 0.52 (0.26, 1.03) | 0.49 (0.20, 1.18) |
| MUFA | ||||
| Energy-adjusted | 1 | 0.40 (0.23, 0.68) | 0.63 (0.38, 1.06) | 0.86 (0.46, 1.59) |
| Multivariate-adjusted | 1 | 0.30 (0.15, 0.58) | 0.47 (0.22, 0.97) | 0.79 (0.33, 1.86) |
| PUFA | ||||
| Energy-adjusted | 1 | 0.99 (0.59, 1.65) | 1.19 (0.70, 2.04) | 0.87 (0.42, 1.81) |
| Multivariate-adjusted | 1 | 1.14 (0.60, 2.17) | 0.84 (0.41, 1.76) | 0.47 (0.18, 1.24) |
| Dyslipidemias | ||||
| Total fats | ||||
| Energy-adjusted | 1 | 0.79 (0.57, 1.10) | 0.69 (0.48, 0.99) | 0.87 (0.56, 1.36) |
| Multivariate-adjusted | 1 | 0.66 (0.44, 1.01) | 0.54 (0.33, 0.87) | 0.59 (0.33, 1.06) |
| SFA | ||||
| Energy-adjusted | 1 | 0.84 (0.60, 1.17) | 0.89 (0.62, 1.27) | 0.97 (0.63, 1.50) |
| Multivariate-adjusted | 1 | 0.79 (0.52, 1.21) | 0.73 (0.46, 1.18) | 0.66 (0.37, 1.19) |
| MUFA | ||||
| Energy-adjusted | 1 | 0.96 (0.69, 1.34) | 0.81 (0.56, 1.17) | 0.79 (0.50, 1.25) |
| Multivariate-adjusted | 1 | 0.88 (0.58, 1.34) | 0.75 (0.46, 1.22) | 0.60 (0.34, 1.09) |
| PUFA | ||||
| Energy-adjusted | 1 | 0.91 (0.65, 1.28) | 0.89 (0.61, 1.29) | 1.05 (0.65, 1.70) |
| Multivariate-adjusted | 1 | 1.10 (0.72, 1.69) | 0.67 (0.41, 1.09) | 0.69 (0.37, 1.28) |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| Hypertension | Type-2 Diabetes | Dyslipidemias | ||||||||||
| SFA | ||||||||||||
| C4-C10 | 1 | 0.98 (0.68, 1.42) | 0.97 (0.62, 1.05) | 1.42 (0.87, 2.30) | 1 | 0.99 (0.49, 2.00) | 0.61 (0.25, 1.50) | 0.25 (0.09, 0.72) | 1 | 0.69 (0.43, 1.12) | 0.49 (0.27, 0.89) | 0.43 (0.23, 0.82) |
| C12:0 | 1 | 1.35 (0.90, 2.01) | 1.10 (0.68, 1.80) | 1.05 (0.60, 1.86) | 1 | 0.58 (0.26, 1.50) | 0.82 (0.32, 2.09) | 1.56 (0.54, 4.52) | 1 | 0.81 (0.47, 1.38) | 0.82 (0.44, 1.51) | 0.80 (0.40, 1.60) |
| C14:0 | 1 | 1.76 (1.12, 2.76) | 2.36 (1.26, 4.24) | 1.62 (0.73, 3.60) | 1 | 1.20 (0.52, 2.78) | 2.63 (0.74, 9.04) | 2.96 (0.64, 13.59) | 1 | 1.06 (0.59, 1.91) | 1.77 (0.79, 3.97) | 1.47 (0.54, 4.00) |
| C16:0 | 1 | 0.80 (0.44, 1.45) | 1.15 (0.50, 2.65) | 1.09 (0.37, 3.19) | 1 | 0.83 (0.28, 2.42) | 0.92 (0.17, 4.88) | 1.16 (0.14, 9.68) | 1 | 0.66 (0.32, 1.39) | 0.80 (0.28, 2.27) | 0.60 (0.16, 2.28) |
| C18:0 | 1 | 0.69 (0.38, 1.26) | 0.28 (0.12, 0.64) | 0.41 (0.15, 1.12) | 1 | 0.49 (0.16, 1.51) | 0.19 (0.04, 0.97) | 0.26 (0.03, 1.93) | 1 | 1.29 (0.62, 2.71) | 0.95 (0.34, 2.62) | 1.67 (0.49, 5.75) |
| C20:0 | 1 | 0.81 (0.57, 1.14) | 1.11 (0.75, 1.65) | 0.59 (0.35, 1.01) | 1 | 0.81 (0.43, 1.49) | 1.29 (0.61, 2.73) | 1.35 (0.45, 4.03) | 1 | 0.88 (0.57, 1.34) | 1.02 (0.61, 1.68) | 0.80 (0.40, 1.60) |
| C22:0 | 1 | 1.12 (0.80, 1.57) | 1.10 (0.76, 1.58) | 0.64 (0.40, 1.01) | 1 | 0.88 (0.48, 1.62) | 0.86 (0.41, 1.79) | 0.97 (0.36, 2.64) | 1 | 0.68 (0.45, 1.04) | 1.01 (0.63, 1.60) | 0.54 (0.29, 1.01) |
| MUFA | ||||||||||||
| C14:1 | 1 | 0.67 (0.48, 0.93) | 0.53 (0.36, 0.76) | 0.57 (0.37, 0.88) | 1 | 0.60 (0.31, 1.16) | 0.99 (0.49, 1.98) | 2.03 (0.91, 4.56) | 1 | 1.13 (0.74, 1.73) | 0.94 (0.58, 1.53) | 1.47 (0.85, 2.56) |
| C16:1 | 1 | 1.27 (0.88, 1.85) | 0.94 (0.58, 1.55) | 1.28 (0.68, 2.40) | 1 | 0.65 (0.30, 1.37) | 0.95 (0.36, 2.51) | 1.62 (0.48, 5.46) | 1 | 0.65 (0.40, 1.05) | 0.65 (0.35, 1.21) | 0.83 (0.38, 1.81) |
| C18:1 | 1 | 0.96 (0.67, 1.38) | 0.72 (0.45, 1.14) | 0.52 (0.30, 0.92) | 1 | 0.30 (0.15, 0.63) | 0.20 (0.07, 0.53) | 0.21 (0.07, 0.67) | 1 | 0.91 (0.57, 1.46) | 0.72 (0.38, 1.34) | 0.54 (0.26, 1.14) |
| C20:1 | 1 | 1.01 (0.70, 1.47) | 1.81 (1.11, 2.97) | 1.20 (0.60, 2.41) | 1 | 1.99 (0.92, 4.28) | 2.71 (1.02, 7.18) | 1.73 (0.40, 7.47) | 1 | 1.22 (0.75, 1.99) | 1.32 (0.70, 2.48) | 3.35 (1.33, 8.42) |
| C22:1 | 1 | 1.36 (0.96, 1.95) | 1.08 (0.67, 1.71) | 0.86 (0.44, 1.68) | 1 | 1.54 (0.73, 3.22) | 0.62 (0.24, 1.59) | 1.41 (0.34, 5.81) | 1 | 1.29 (0.80, 2.07) | 0.59 (0.32, 1.09) | 0.50 (0.20, 1.24) |
| PUFA | ||||||||||||
| C18:2 | 1 | 0.63 (0.42, 0.94) | 0.56 (0.35, 0.91) | 0.33 (0.18, 0.60) | 1 | 0.90 (0.43, 1.85) | 0.49 (0.20, 1.25) | 0.32 (0.10, 0.97) | 1 | 0.93 (0.56, 1.54) | 0.69 (0.37, 1.28) | 0.61 (0.29, 1.29) |
| C18:3 | 1 | 1.13 (0.76, 1.67) | 1.13 (0.70, 1.84) | 1.38 (0.77, 2.46) | 1 | 0.97 (0.47, 2.00) | 1.15 (0.48, 2.76) | 1.47 (0.55, 3.95) | 1 | 0.89 (0.54, 1.47) | 1.19 (0.65, 2.18) | 0.93 (0.46, 1.87) |
| C20:4 | 1 | 0.93 (0.67, 1.29) | 0.87 (0.60, 1.26) | 1.14 (0.73, 1.78) | 1 | 1.30 (0.70, 2.39) | 1.23 (0.62, 2.45) | 0.97 (0.43, 2.18) | 1 | 0.85 (0.55, 1.31) | 0.85 (0.52, 1.38) | 1.21 (0.69, 2.11) |
| C20:5 | 1 | 0.80 (0.48, 1.33) | 1.00 (0.47, 2.14) | 0.30 (0.10, 0.89) | 1 | 1.08 (0.40, 2.89) | 0.40 (0.09, 1.78) | 0.95 (0.10, 9.34) | 1 | 0.76 (0.39, 1.50) | 0.67 (0.24, 1.83) | 1.05 (0.27, 4.13) |
| C22:6 | 1 | 0.33 (0.12, 0.92) | 0.51 (0.21, 1.26) | 0.60 (0.29, 1.24) | 1 | 0.38 (0.04, 3.46) | 0.55 (0.07, 4.16) | 0.86 (0.15, 4.83) | 1 | 0.64 (0.17, 2.38) | 1.20 (0.38, 3.78) | 0.79 (0.32, 1.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currenti, W.; Godos, J.; Alanazi, A.M.; Grosso, G.; Cincione, R.I.; La Vignera, S.; Buscemi, S.; Galvano, F. Dietary Fats and Cardio-Metabolic Outcomes in a Cohort of Italian Adults. Nutrients 2022, 14, 4294. https://doi.org/10.3390/nu14204294
Currenti W, Godos J, Alanazi AM, Grosso G, Cincione RI, La Vignera S, Buscemi S, Galvano F. Dietary Fats and Cardio-Metabolic Outcomes in a Cohort of Italian Adults. Nutrients. 2022; 14(20):4294. https://doi.org/10.3390/nu14204294
Chicago/Turabian StyleCurrenti, Walter, Justyna Godos, Amer M. Alanazi, Giuseppe Grosso, Raffaele Ivan Cincione, Sandro La Vignera, Silvio Buscemi, and Fabio Galvano. 2022. "Dietary Fats and Cardio-Metabolic Outcomes in a Cohort of Italian Adults" Nutrients 14, no. 20: 4294. https://doi.org/10.3390/nu14204294
APA StyleCurrenti, W., Godos, J., Alanazi, A. M., Grosso, G., Cincione, R. I., La Vignera, S., Buscemi, S., & Galvano, F. (2022). Dietary Fats and Cardio-Metabolic Outcomes in a Cohort of Italian Adults. Nutrients, 14(20), 4294. https://doi.org/10.3390/nu14204294








