Phenolic Acids as Antidepressant Agents
Abstract
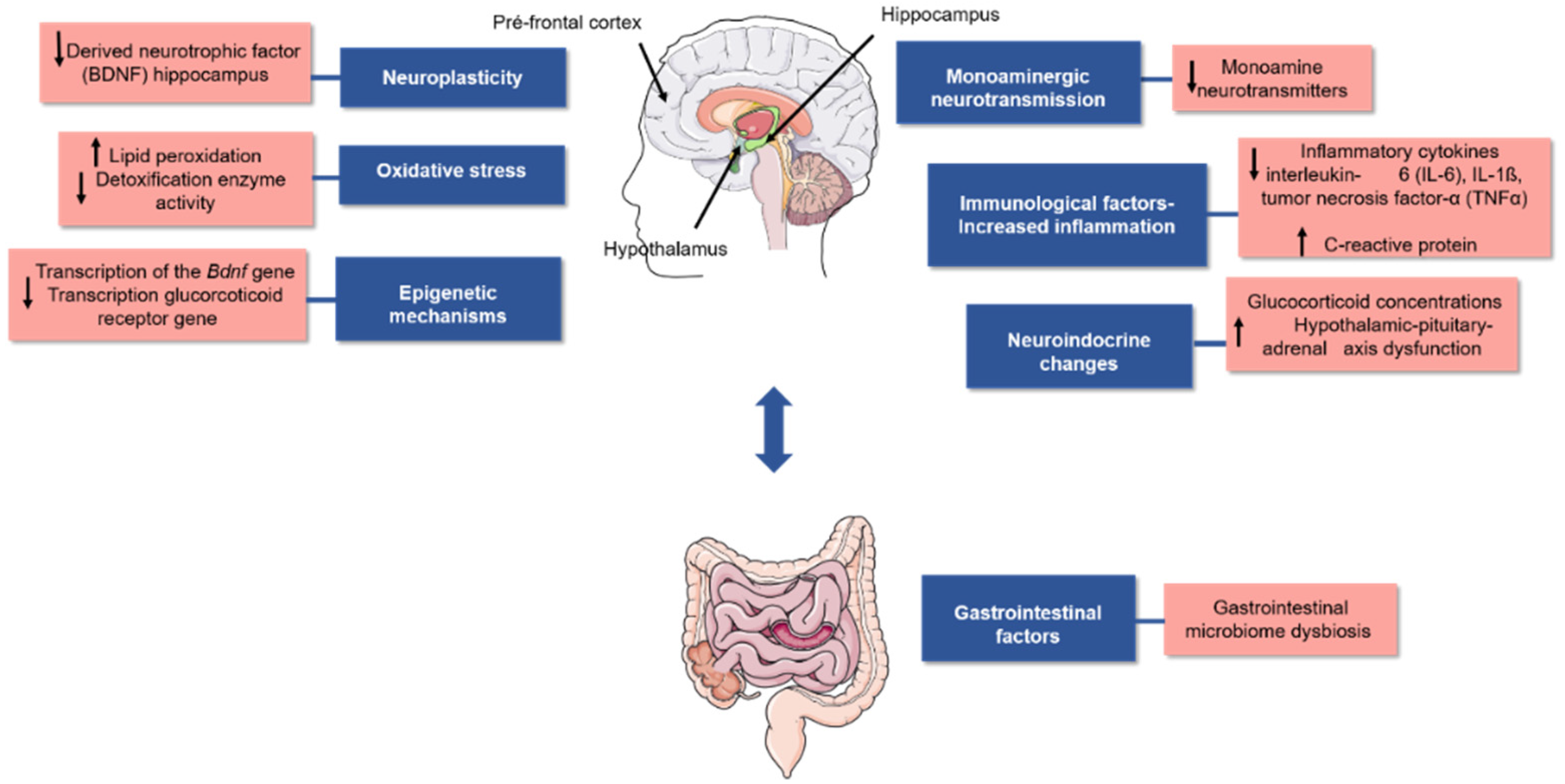
:1. Introduction
2. Phenolic Acids
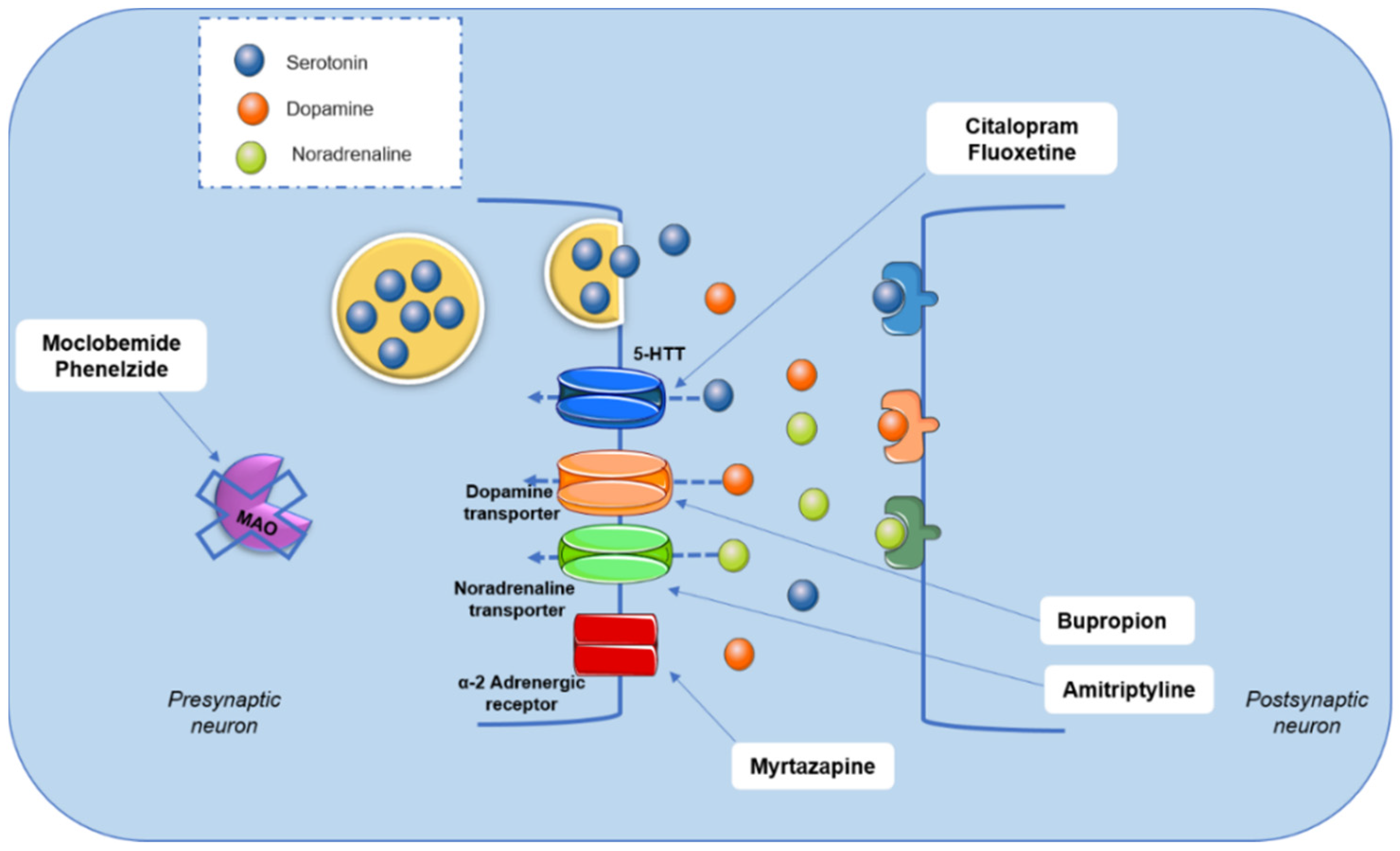
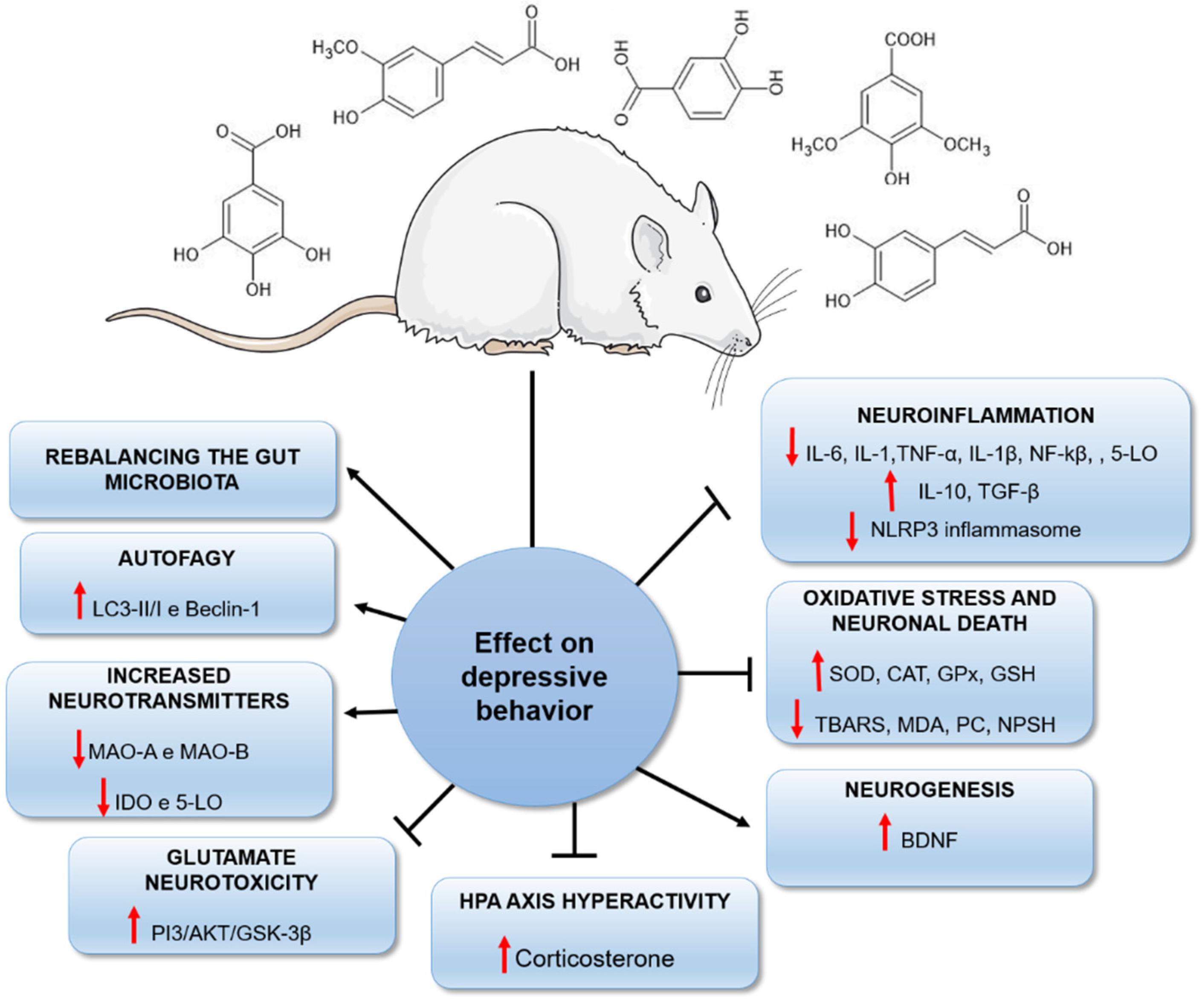
2.1. Antidepressant Effects of Phenolic Acids
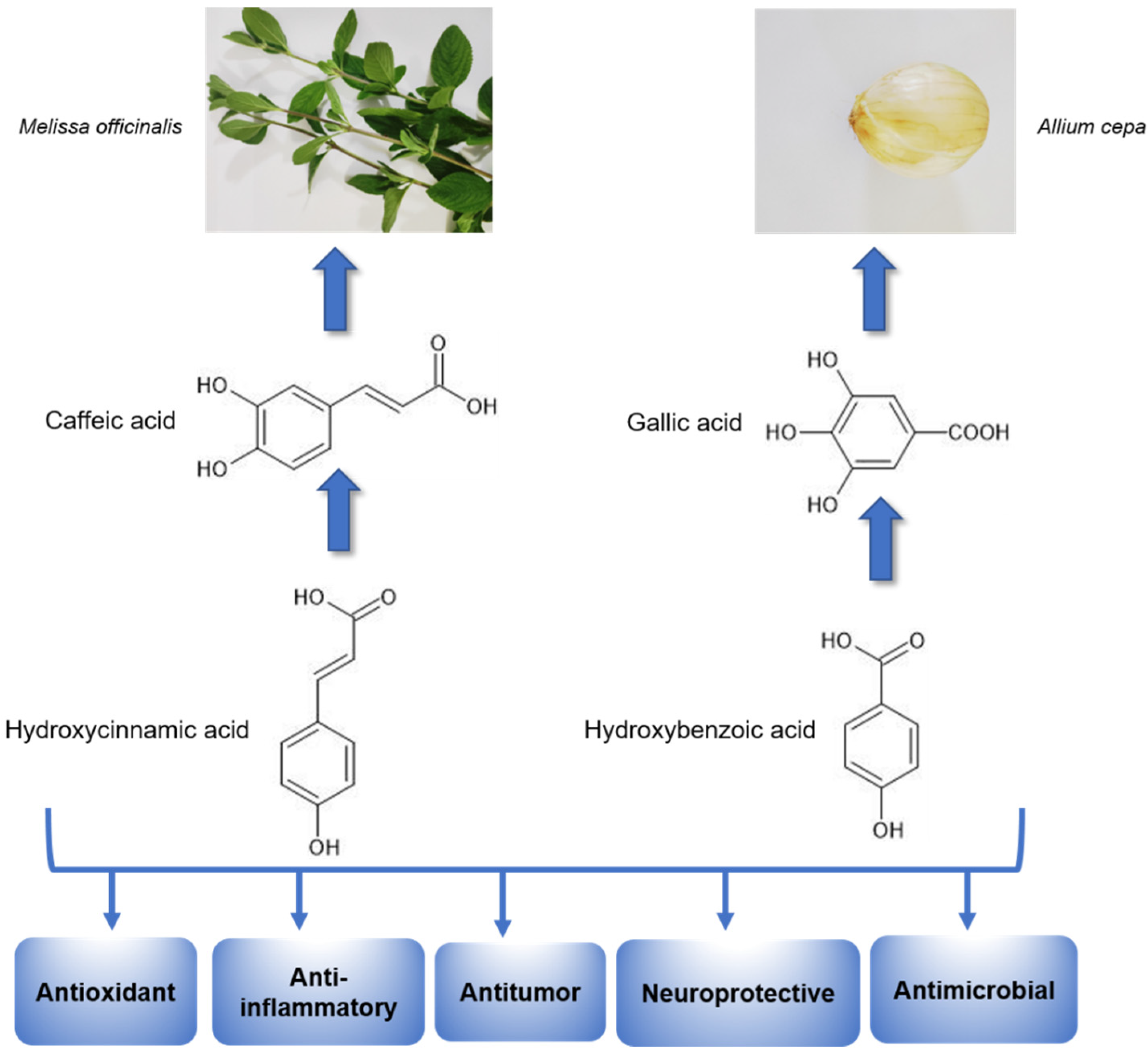
2.2. Antidepressant Potential of Medicinal Plants Rich in Phenolic Acids
3. Nutraceutical Perspectives
| Phenolic Acid | Behavioral Analysis | Animal | Dose (per kg) | Time from Treatment | Effects | Reference |
|---|---|---|---|---|---|---|
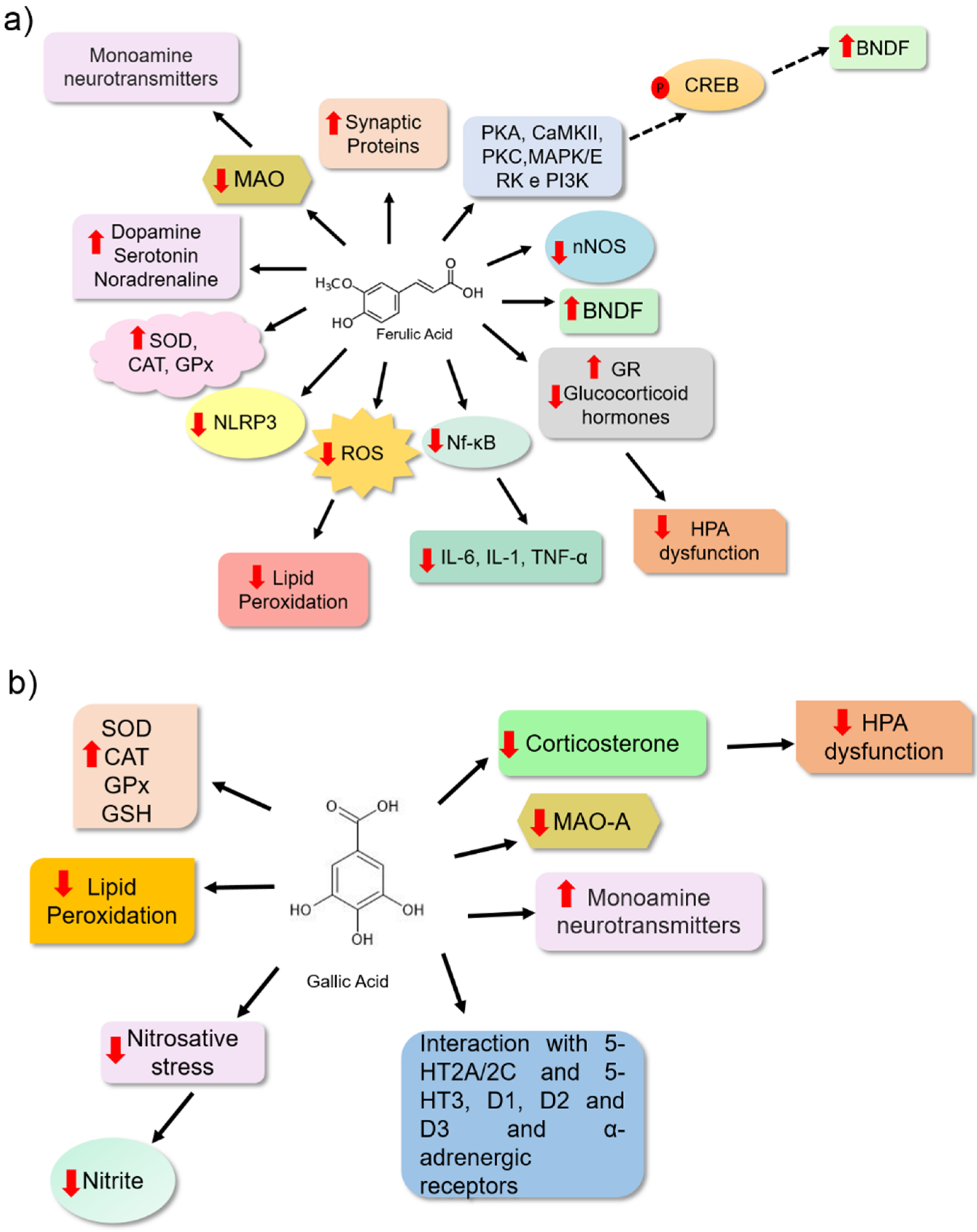
| Ferulic Acid | Sucrose preference and forced swim tests | Male Sprague Dawley Rats | 12.5, 25, and 50 mg | 28 days | It reduced the concentration of proinflammatory cytokines IL-6, IL-1β, and TNF-α in the hippocampus, reduced expression of neuronal nitric oxide synthase (nNOS), increased IL-10, reduced ACTH, corticosterone in the hippocampus and increased GR expression. | [47] |
| Ferulic Acid | Tail suspension test | Male Swiss mice | 1 mg | 7 days | It reduced markers of oxidative stress (MDA, nitrite, and PC) in the brains of the animals and increased NPSH levels. | [58] |
| Ferulic Acid | Sucrose preference and forced swim tests | ICR male mice | 20 and 40 mg | 28 days | It increased the concentration of BDNF, and of the synaptic proteins PSD95, inapsin I in the prefrontal cortex and hippocampus | [60] |
| Ferulic Acid | Tail suspension and sucrose preference tests | ICR male mice | 20, 40, and 80 mg | 28 days | It reduced mRNA expression of IL-1β, IL-6, and TNF-α and reduced mRNA expression and protein levels of CD11b, protein levels of NF-κB and IL-1β inhibited the NLRP3 inflammasome in the prefrontal cortex. | [66] |
| Ferulic Acid | Thermal hyperalgesia, mechanical allodynia, tail suspension, and forced swimming | ICR male mice | 5, 10, 20, 40, and 80 mg | 30 min before the test | It increased noradrenaline, 5-HT and dopamine in the hippocampus and frontal cortex; reduced lipid peroxidation levels, nitrite, IL-1β, TNF-α in the frontal cortex and hippocampus; increased SOD activity, GSH levels and reduced levels of the neuromodulator substance P, NF-κβ p65, and caspase-3. | [59] |
| Ferulic Acid | Forced swim test | Male Sprague Dawley Rats | 25 and 50 mg | 24, 5 and 1 h before the test | It inhibited monoamine reuptake, reduced CRH, ACTH concentrations and increased 5-HT in plasma, prefrontal cortex, and hippocampus of rats. | [135] |
| Ferulic Acid | Forced swim and tail suspension tests | Male ICR mice | 10, 20, 40, and 80 mg | 30 min before the test | It increased serotonin and noradrenaline levels in the hippocampus, frontal cortex, and hypothalamus, and inhibited monoamine oxidase-A (MAO-A) activity in the frontal cortex and hippocampus. | [55] |
| Ferulic Acid | Forced swim and tail suspension tests | Male Swiss mice | 0.001, 0.01, 0.1, 1, and 10 mg | 60 min before the test | Interacted with the serotonergic system. | [14] |
| Ferulic Acid | Tail suspension and forced swim tests | Male Swiss mice | 0.01, 0.1, 1, and 10 mg | 21 days | It increased SOD, CAT, and GSH-Px activities in the cerebral cortex, decreased TBARS levels in animals subjected to stress. | [136] |
| Ferulic Acid | Tail suspension test | Male Swiss mice | 0.01 mg | 30 min before the test | It activated the PKA, CaMKII and PKC, MAPK/ERK, and PI3K signaling pathways. | [61] |
| Gallic acid | Forced swim test | Male Sprague Dawley Rats | 50 and 100 mg | 28 days | It reduced MDA levels and increased CAT and GPx activity in the brain homogenates of the animals. | [68] |
| Gallic acid | Tail suspension and forced swim tests | BALB/c mice | 25 and 50 mg | 7 days | It reduced TBARS levels and increased SOD activity and GSH levels. | [69] |
| Gallic acid | Tail suspension and modified forced swim tests | Male BALB/c mice | 30 and 60 mg | 24, 5 and 1 h before the tests | It increased the levels of serotonin and catecholamines in the synaptic clefts of the central nervous system. It also had its effect related to α-adrenergic, 5-HT2A/2C, and 5-HT3 serotoninergic and D1, D2, and D3 dopaminergic receptors. | [18] |
| Gallic acid | Forced swim and sucrose preference tests | Male Swiss mice | 5, 10, 20 mg | 21 days | It reduced MAO-A activity, reduced nitrite and malondialdehyde levels in plasma. In addition, it reduced the corticosterone content in the plasma of the mice. It increased the levels of reduced glutathione and catalase activity. | [70] |
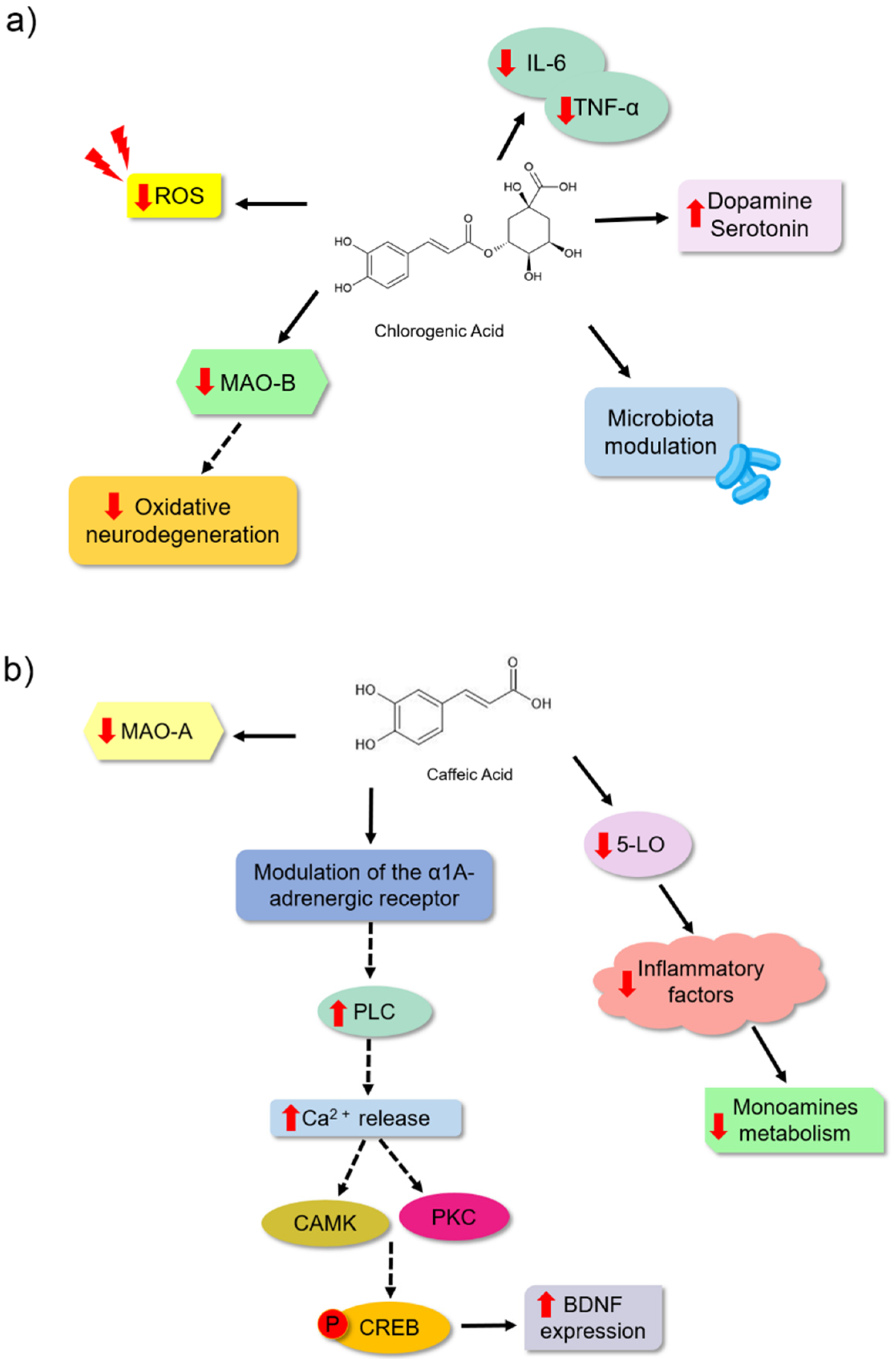
| Chlorogenic acid | Tail suspension and forced swim tests | ICR male mice | 10 and 30 mg | 7 days | Inhibited the reduction in the number of neuronal dendritic spines, inhibited the enzyme MAO-B and ROS production in hippocampal astrocyte cultures of the animals. | [77] |
| Chlorogenic acid | Sucrose preference, forced swim and tail suspension tests | Wistar male | 500 mg | 14 days | Significantly reduced serum levels of the proinflammatory cytokines IL-6 and TNF-α; increased serum concentrations of the neurotransmitters serotonin and dopamine. Modified the structure of the intestinal microbial community of the animals. | [9] |
| Caffeic acid | Forced swim test | ICR male mice | 4 mg | 30 min before the test | Attenuated the reduction in BDNF mRNA expression levels in the frontal cortex and TrkB in the mouse amygdala. | [78] |
| Caffeic acid | Stress tests with conditioned fear and forced swim Forced swim test | ICR male mice and ddY mice | 4 mg | 30 min before the test | It modulated the α1A adrenergic receptor. | [80] |
| Caffeic acid | Forced swim test | ICR male mice | 1–4 mg | 30 min before the test | It slightly reduced the activity of MAO-A. | [79] |
| Caffeic acid | Forced swim test | Male Sprague Dawley Rats | 10 and 30 mg | 21 days | It modulated NE and 5-HT synthesis and affected the metabolism of other neurotransmitters through inhibition of the inflammatory 5-Lipoxygenase (5-LO) pathway. | [82] |
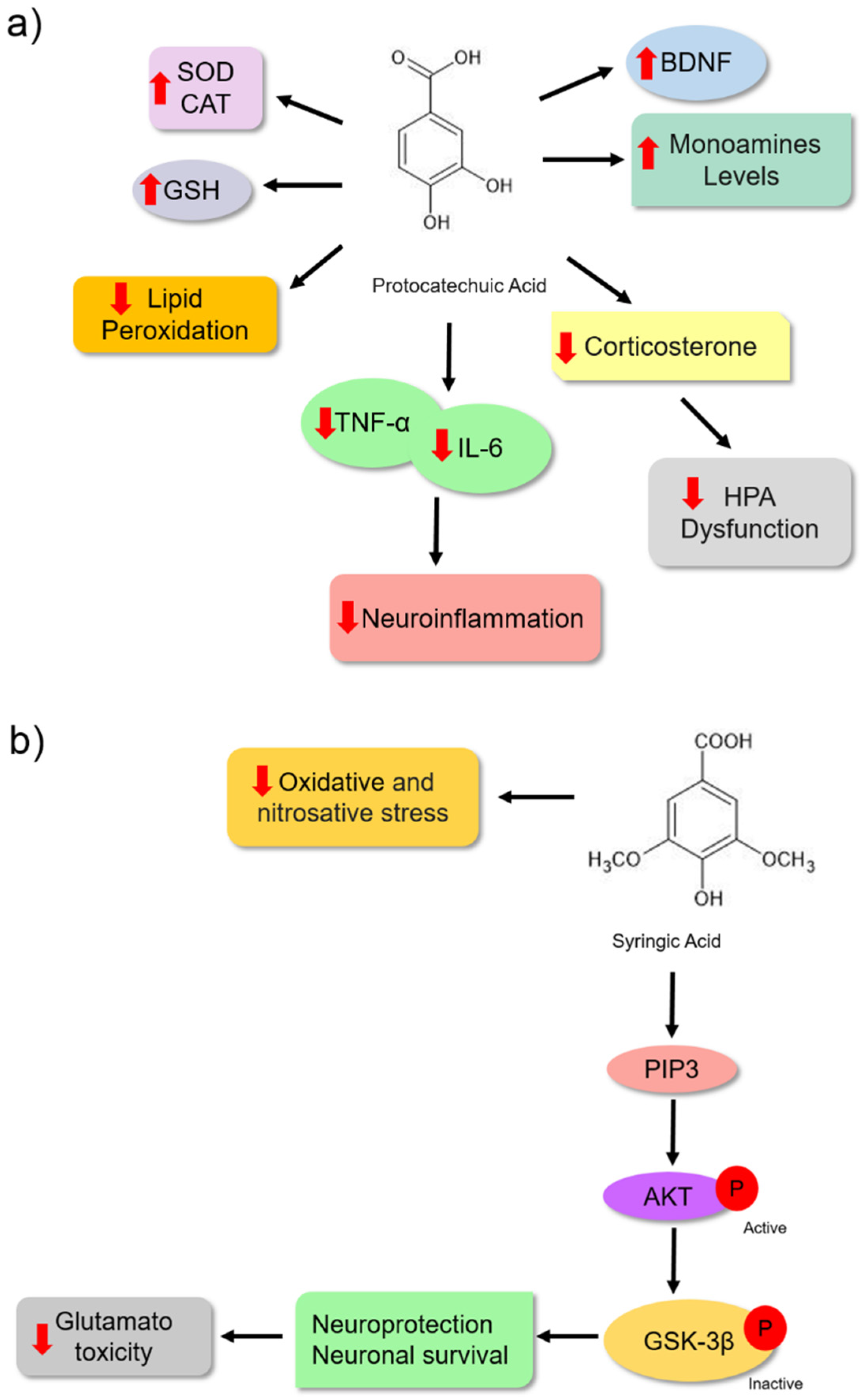
| Protocatechuic Acid | Forced swim test | Swiss albino mice | 100 and 200 mg | 8 hours and 40 min | Reduced serum corticosterone levels, MDA formation in hippocampus and cerebral cortex; restored SOD and CAT activities in hippocampus and cerebral cortex. | [84] |
| Protocatechuic Acid | Forced swim test | Wistar rats of both sexes | 100 and 200 mg | 14 days | It increased the levels of 5-HT, DA, and NE, prevented the reduction in BDNF, prevented the elevation of TNF-α and IL-6 levels, reduced MDA levels and increased CAT activity and GSH content in the hippocampus and cerebral cortex; it reduced the serum corticosterone level in the animals. | [85] |
| Syringic acid | Forced swim and tail suspension tests | Male Swiss mice | 0.1, 1, 10 and 100 mg | Acute (1 time) 60 before the test Subchronic (7 days) | Reduced TBARS levels in serum. Neutralized nitrite production in the serum and brain, reduced protein carbonyl production, and reduced glutamate-induced toxicity in the hippocampus and cortex of the animals. | [90] |
| Syringic acid | Tail suspension test | Male Swiss mice | 1 mg | 7 days | It protected hippocampal and cerebrocortical slices against glutamate-induced damage, possibly through the PI3K/Akt/GSK-3β pathway. | [51] |
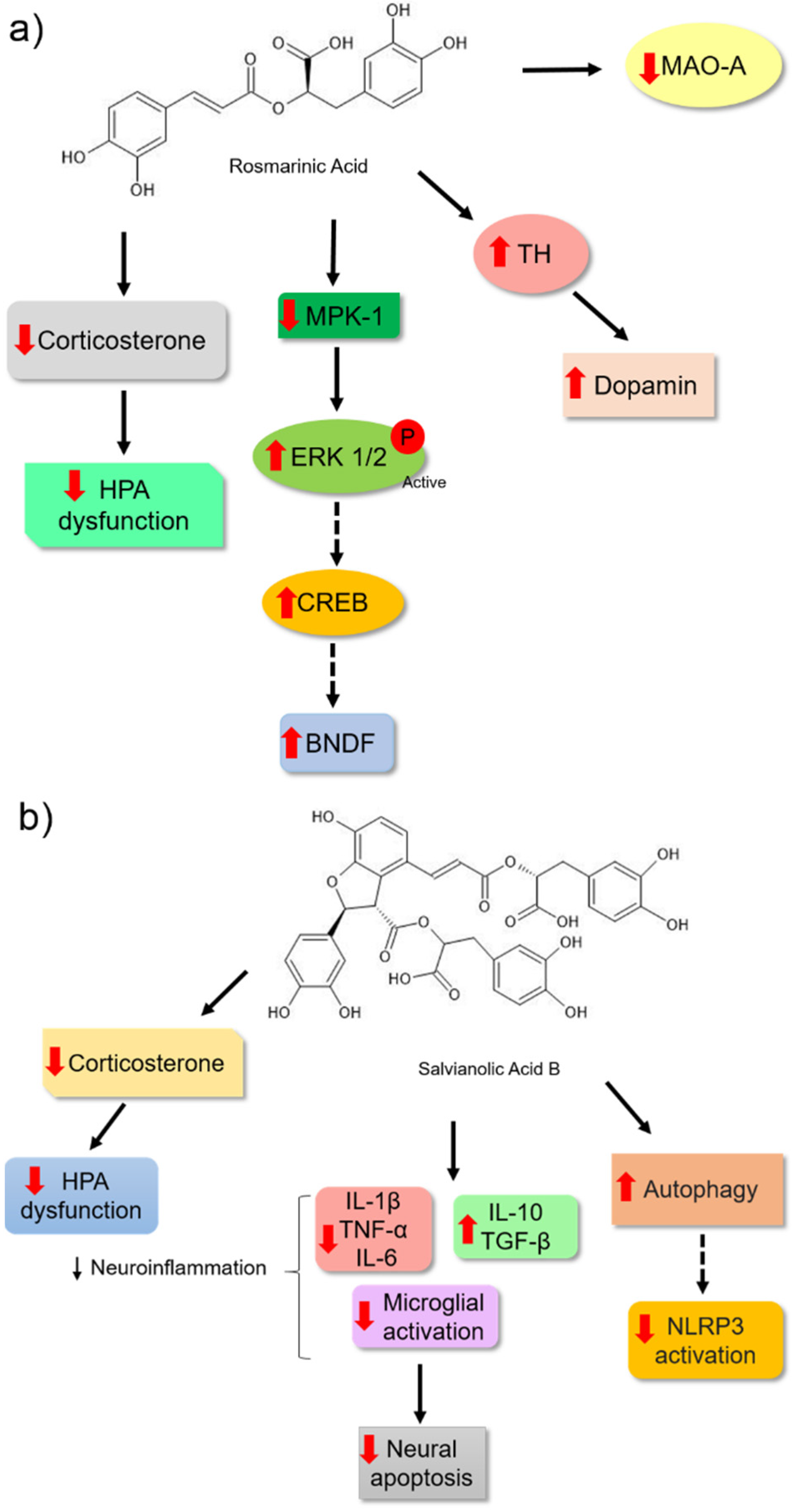
| Rosmarinic Acid | Forced swim test | ICR male mice | 1–4 mg | 30 min before the test | Slightly inhibited the activi- ty of monoamine oxidase-A | [79] |
| Rosmarinic Acid | Forced swim test andMorris water maze test | Male Sprague Dawley Rats | 5 and 10 mg | 14 days | It increased hippocampal expression of pERK1/2 and BDNF levels. | [94] |
| Rosmarinic Acid | Forced swim test | Male ddY mice | 1, 2, and 4 mg | 7 and 14 days | Positively modulated hippocampal neurogenesis. | [93] |
| Rosmarinic Acid | Tail suspension test | ICR male mice | 5 and 10 mg | 7 days | It reduced serum corticosterone levels, increased dopamine, reduced Mpk-1 mRNA expression and increased BDNF mRNA expression, increased tyrosine hydroxylase and pyruvate carboxylase expression. | [95] |
| Salvianolic acid B | Forced swim and sucrose preference tests | Male Sprague Dawley Rats | 20 mg | 14 days | It alleviated the increased expression of proinflammatory cytokines, IL-1β and IL-6, reduced the expression of Iba-1, restored the expression of autophagic biomarkers, including LC3-II/I and Beclin-1, in the rat hippocampus and reduced the expression of NLRP3, ASC, caspase-1 P20, components of the NLRP3 inflammasome. | [100] |
| Salvianolic acid B | Sucrose preference, forced swim and tail suspension tests | Male C57BL/6 mice | 20 mg | 21 days | It reduced the mRNA expression and protein levels of IL-1β and TNF-α and increased the expression of IL-10 and TGF-β in the hippocampus and cortex of mice. Reduced plasma levels of corticosterone. Prevented apoptosis in the hippocampus and cortex of mice and reduced microglia activation in these brain regions. | [98] |
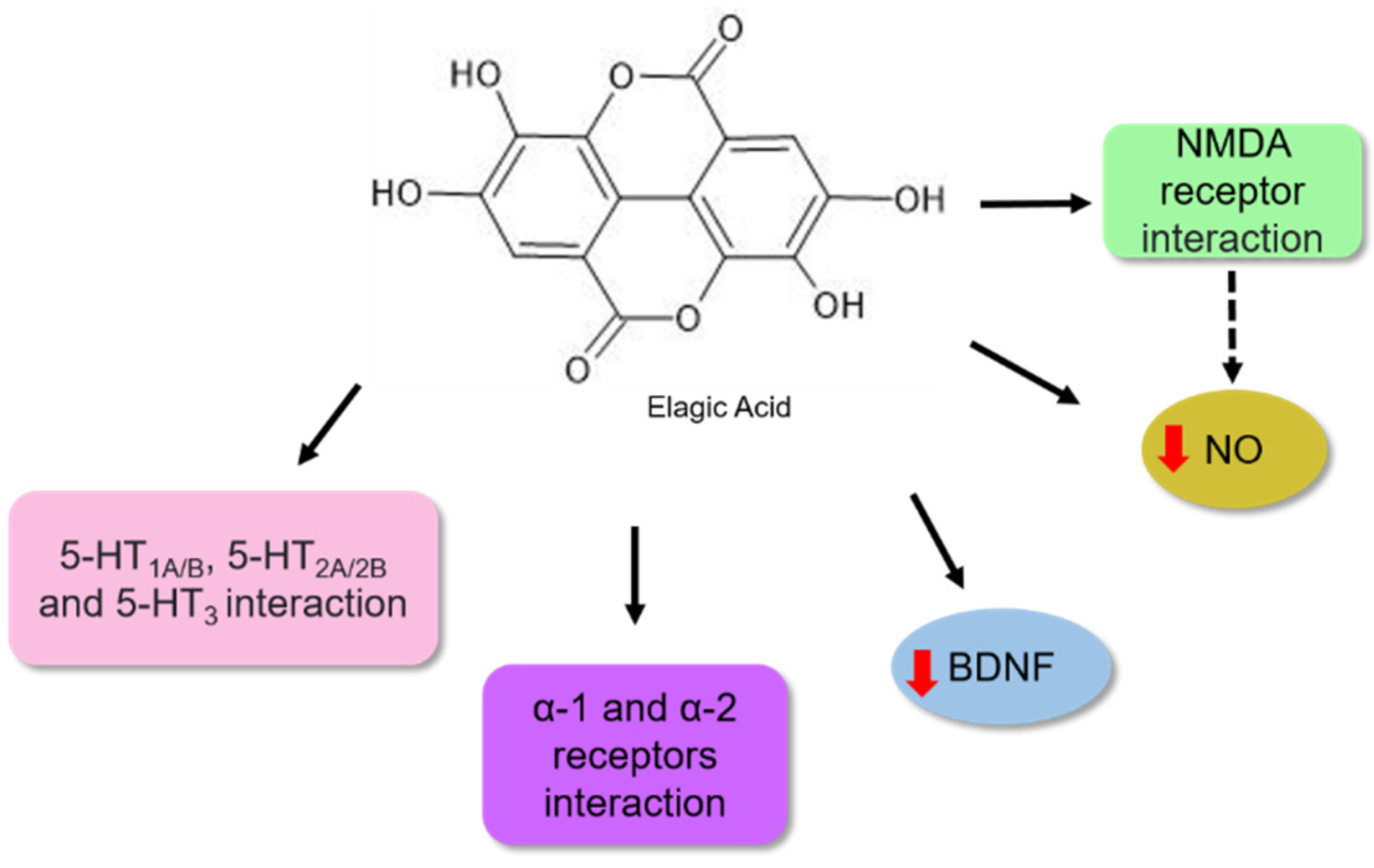
| Ellagic Acid | Forced swim test and splash test | NMRI male mice | 6.25, 12.5, 25, 50, and 100 mg | 60 min before the test | It significantly reduced the level of nitric oxide (NO) in the hippocampus, modulated the expression of NR2A and NR2B subunits of the NMDA-R receptor. | [107] |
| Ellagic Acid | Forced swim and tail suspension tests | Male BALB/c mice | 1, 2.5, and 5 mg | 14 days | Increased the levels of BDNF protein in the hippocampus of the animals. | [106] |
| Ellagic Acid | Forced swim and tail suspension tests | Albino mice | 25, 50, and 100 mg | Acute—30 before the tests Chronic—14 days | It modulated the monoaminergic and noradrenergic systems. | [105] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. 2017. Available online: https://apps.who.int/iris/handle/10665/254610 (accessed on 25 March 2020).
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef]
- Vismari, L.; Alves, G.J.; Palermo-Neto, J. Depression, antidepressants and imune system: A new look to an old problem. Rev. Psiquiatr. Clín. 2008, 35, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 319, 2299–2312. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar] [CrossRef]
- Liu, X.M.D.; Yan, Y.; Li, F.M.D.; Zhang, D.M.D. Fruit and vegetable consumption and the risk of depression: A meta-analysis. Nutrition 2016, 32, 296–302. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Zhou, N.; Ma, W.; Gu, X.; Chen, B.; Zeng, Y.; Yang, L.; Zhou, M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019, 10, 2947–2957. [Google Scholar] [CrossRef]
- Kennis, M.; Gerritsen, L.; van Dalen, M.; Williams, A.; Cuijpers, P.; Bockting, C. Prospective biomarkers of major depressive disorder: A systematic review and meta-analysis. Mol. Psychiatry 2020, 25, 321–338. [Google Scholar] [CrossRef] [Green Version]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Transtorno depressivo maior: Novas perspectivas clínicas, neurobiológicas e de tratamento. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef]
- Scapagnini, G.; Davinelli, S.; Drago, F.; De Lorenzo, A.; Oriani, G. Antioxidants as antidepressants: Fact or fiction? CNS Drugs 2012, 26, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef]
- Zeni, A.L.; Zomkowski, A.D.; Maraschin, M.; Rodrigues, A.L.; Tasca, C.I. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: Evidence for the involvement of the serotonergic system. Eur. J. Pharmacol. 2012, 679, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, S.W.; Kim, Y.K. Molecular Neurobiology and Promising New Treatment in Depression. Int. J. Mol. Sci. 2016, 17, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palhano-Fontes, F.; Barreto, D.; Onias, H.; Andrade, K.C.; Novaes, M.M.; Pessoa, J.A.; Mota-Rolim, S.A.; Osório, F.L.; Sanches, R.; Dos Santos, R.G.; et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychol. Med. 2019, 49, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.M. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim. Care Companion, J. Clin. Psychiatry 2001, 3, 22–27. [Google Scholar] [CrossRef]
- Can, Ö.D.; Turan, N.; Özkay, Ü.D.; Öztürk, Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017, 190, 110–117. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Fabrizi, C.; Fornai, F. Potential Antidepressant Effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidant 2020, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [CrossRef]
- Ncube, B.; Staden, J.V. Tilting Plant Metabolism for Improved Metabolite Biosynthesis and Enhanced Human Benefit. Molecules 2015, 20, 12698–12731. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Roy, A. A Review on the Alkaloids an Important Therapeutic Compound from Plants. Int. J. Plant Biotechnol. 2017, 3, 1–9. [Google Scholar]
- Saki, K.; Bahmani, M.; Rafieian-Kopaei, M. The effect of most important medicinal plants on two importnt psychiatric disorders (anxiety and depression)—A review. Asian Pac. J. Trop. Med. 2014, 7, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Diniz, L.R.L.; Souza, M.T.S.; Barboza, J.N.; Almeida, R.N.; Sousa, D.P. Antidepressant Potential of Cinnamic Acids: Mechanisms of Action and Perspectives in Drug Development. Molecules 2019, 24, 4469. [Google Scholar] [CrossRef] [Green Version]
- Sajkowska-Kozielewicz, J.J.; Kozielewicz, P.; Barnes, N.M.; Wawer, I.; Paradowska, K. Antioxidant, Cytotoxic, and Antiproliferative Activities and Total Polyphenol Contents of the Extracts of Geissospermum reticulatum Bark. Oxidative Med. Cell. Longev. 2016, 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Banerjee, J.; Das, A.; Sinha, M.; Saha, S. Biological Efficacy of Medicinal Plant Extracts in Preventing Oxidative Damage. Oxidative Med. Cell. Longev. 2018, 2018, 2. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic Acids of Plant Origin—A Review on Their Antioxidant Activity In Vitro (O/W Emulsion Systems) Along with Their in Vivo Health Biochemical Properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rienks, J.; Dobson, A.J.; Mishra, G.D. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: Results from a large community-based prospective study. Eur. J. Clin. Nutr. 2013, 67, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihrshahi, S.; Dobson, A.J.; Mishra, G.D. Fruit and vegetable consumption and prevalence and incidence of depressive symptoms in mid-age women: Results from the Australian longitudinal study on women’s health. Eur. J. Clin. Nutr. 2015, 69, 585–591. [Google Scholar] [CrossRef]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules 2018, 23, 999. [Google Scholar] [CrossRef] [Green Version]
- Igoumenidis, P.E.; Iosifidis, S.V.; Lopez-Quiroga, E.; Bakalis, S.; Karathanos, V.T. Absorption of Phenolic Acids in Rice Kernels after Boiling in Spearmint Aqueous Extracts of Different Concentrations. A Diffusion Study. J. Food Sci. 2019, 84, 2859–2867. [Google Scholar] [CrossRef]
- RusselL, W.; Duthie, G. Session 3: Influences of food constituents on gut health: Plant secondary metabolites and gut health: The case for phenolic acids. Proc. Nutr. Soc. 2011, 70, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic Potential of Dietary Phenolic Acids. Adv. Pharmacol. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug. Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, N.; Hou, N.; Dong, L.; Li, J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017, 46, 68–73. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, m.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna, J. Phytomed. 2019, 9, 574–586. [Google Scholar] [CrossRef]
- Yun, Z.; Rui, L.; Chenlin, F.; Xiaolin, L.; Shuai, H.; Lulu, W.; Zhihuab, L.; Jiandong, J.; Yanxing, H. Chlorogenic acid inhibits esophageal squamous cell carcinoma growth in vitro and in vivo by downregulating the expression of BMI1 and SOX2. Biomed. Pharmacother. 2020, 121, 109602. [Google Scholar] [CrossRef]
- Su, M.; Liu, F.; Luo, Z.; Wu, H.; Zhang, X.; Wang, D.; Zhu, Y.; Sun, Z.; Xu, W.; Miao, Y. The Antibacterial Activity and Mechanism of Chlorogenic Acid Against Foodborne Pathogen Pseudomonas aeruginosa. Foodborne Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Q.; Zuo, Z.; Chu, J.; Xiao, H.; Tariqjaved, M.; He, C. Protocatechuic acid (PCA) induced a better antiviral effect by immune enhancement in SPF chickens. Microb. Pathog. 2018, 114, 233–238. [Google Scholar] [CrossRef]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, Y.; Chen, Y.; Yue, Y.; Li, Y.; Xia, S.; Li, Y.; Deng, H.; Zhang, J.; Cao, Y. Ferulic Acid Improves Depressive-Like Behavior in Prenatally-Stressed Offspring Rats via Anti-Inflammatory Activity and HPA Axis. Int. J. Mol. Sci. 2019, 20, 493. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, M.; Matuo, T.; Tsuno, T.; Hosoda, A.; Nomura, E.; Taniguchi, H.; Sasaki, H.; Morishita, H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. BioFactors 2004, 21, 315–319. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Z.; Chen, X.; Lian, X.; Zhu, H.; Zheng, J.; Sun, L. The anticoagulant ability of ferulic acid and its applications for improving the blood compatibility of silk fibroin. Biomed. Mater. 2008, 3, 044106. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Li, H.; Tang, Y.; Yang, L.; Cao, S.; Qin, D. Antidepressant Potential of Chlorogenic Acid-Enriched Extract from Eucommia ulmoides Oliver Bark with Neuron Protection and Promotion of Serotonin Release through Enhancing Synapsin I Expression. Molecules 2016, 21, 260. [Google Scholar] [CrossRef] [Green Version]
- Dalmagro, A.P.; Camargo, A.; Severo Rodrigues, A.L.; Zeni, A.L.B. Involvement of PI3K/Akt/GSK-3β signaling pathway in the antidepressant-like and neuroprotective effects of Morus nigra and its major phenolic, syringic acid. Chem.-Biol. Interact. 2019, 314, 108843. [Google Scholar] [CrossRef]
- Monteiro, Á.B.; de Souza Rodrigues, C.K.; do Nascimento, E.P.; dos Santos Sales, V.; de Araújo Delmondes, G.; da Costa, M.H.N.; de Oliveira, V.A.P.; de Morais, L.P.; Boligon, A.A.; Barbosa, R.; et al. Anxiolytic and antidepressant-like effects of Annona coriacea (Mart.) and caffeic acid in mice. Food Chem. Toxicol. 2020, 136, 111049. [Google Scholar] [CrossRef] [PubMed]
- Costa De Melo, N.; Sánchez-Ortiz, B.L.; Dos Santos Sampaio, T.I.; Matias Pereira, A.C.; Pinheiro Da Silva Neto, F.L.; Ribeiro Da Silva, H.; Alves Soares Cruz, R.; Keita, H.; Soares Pereira, A.M.; Tavares Carvalho, J.C. Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio). Pharmaceuticals 2019, 12, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochemy 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Chen, J.; Lin, D.; Zhang, C.; Li, G.; Zhang, N.; Ruan, L.; Yan, Q.; Li, J.; Yu, X.; Xie, X.; et al. Antidepressant-like effects of ferulic acid: Involvement of serotonergic and norepinergic systems. Metab. Brain Dis. 2015, 30, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Patel, M. Targeting Oxidative Stress in Central Nervous System Disorders. Trends Pharmacol. Sci. 2016, 37, 768–778. [Google Scholar] [CrossRef] [Green Version]
- Zeni, A.L.B.; Camargo, A.; Dalmagro, A.P. Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 2017, 125, 131–136. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Shao, T.; Ruan, L.; Wang, L.; Sun, J.; Li, J.; Zhu, X.; O’donnell, J.M.; Pan, J. Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: Behavioral and neurobiological analyses. Metab. Brain Dis. 2013, 28, 571–583. [Google Scholar] [CrossRef]
- Liu, Y.M.; Hu, C.Y.; Shen, J.D.; Wu, S.H.; Li, Y.C.; Yi, L.T. Elevation of synaptic protein is associated with the antidepressant-like effects of ferulic acid in a chronic model of depression. Physiol. Behav. 2017, 169, 184–188. [Google Scholar] [CrossRef]
- Zeni, A.L.; Zomkowski, A.D.; Maraschin, M.; Rodrigues, A.L.; Tasca, C.I. Involvement of PKA, CaMKII, PKC, MAPK/ERK and PI3K in the acute antidepressant-like effect of ferulic acid in the tail suspension test. Pharmacol. Biochem. Behav. 2012, 103, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verduijn, J.; Milaneschi, Y.; Schoevers, R.A.; Van Hemert, A.M.; Beekman, A.T.F.; Penninx, B.W.J.H. Pathophysiology of major depressive disorder: Mechanisms involved in etiology are not associated with clinical progression. Transl. Psychiatry 2015, 5, e649. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.M.; Shen, J.D.; Xu, L.P.; Li, H.B.; Li, Y.C.; Yi, L.T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Samad, N.; Jabeen, S.; Imran, I.; Zulfiqar, I.; Bilal, K. Protective effect of gallic acid against arsenic-induced anxiety-/depression- like behaviors and memory impairment in male rats. Metab. Brain Dis. 2019, 34, 1091–1102. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Habtemariam, S.; Di Lorenzo, A.; Sureda, A.; Khanjani, S.; Nabavi, S.M.; Daglia, M. Post-Stroke Depression Modulation and in Vivo Antioxidant Activity of Gallic Acid and Its Synthetic Derivatives in a Murine Model System. Nutriens 2016, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Chhillar, R.; Dhingra, D. Antidepressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress. Fundam. Clin. Pharmacol. 2012, 27, 409–418. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.; Kiecolt-Glaser, J.K. Stress and depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic Acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Alternat. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.W.; Han, T.; Jung, J.; Song, Y.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Cho, S.; Kim, I.H.; Han, D.; et al. Chlorogenic Acid from Hawthorn Berry (Crataegus pinnatifida Fruit) Prevents Stress Hormone-Induced Depressive Behavior, through Monoamine Oxidase B-Reactive Oxygen Species Signaling in Hippocampal Astrocytes of Mice. Mol. Nutr. Food Res. 2018, 62, 1800029. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Yamada, T.; Masuya, J.; Matsushita, K.; Tahara, M.; Iimori, M.; Matsumiya, T. Caffeic acid attenuates the decrease in cortical BDNF mRNA expression. induced by exposure to forced swimming stress in mice. Eur. J. Pharmacol. 2006, 534, 115–121. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Inazu, M.; Egashira, T.; Matsumiya, T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 2002, 449, 261–267. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Miyamoto, J.; Masuya, J.; Iimori, M.; Matsumiya, T. Cafeic acid produces antidepressive- and/or anxiolytic-like e¡ects through indirect modulation of the a1A-adrenoceptor system in mice. Clin. Neurosci. Neuropathol. 2003, 14, 1067–1070. [Google Scholar] [CrossRef]
- Seki, K.; Yoshida, S.; Jaiswal, M.K. Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural. Regen. Res. 2018, 13, 1159–1169. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, L.; Yang, J.Q.; Luo, Y.; Cui, T.; Du, T.T.; Jiang, X.H. Evaluation on monoamine neurotransmitters changes in depression rats given with sertraline, meloxicam or/and caffeic acid. Genes Dis. 2019, 6, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. Int. Sch. Res. Not. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakare, V.N.; Dhakane, V.D.; Patel, B.M. Attenuation of acute restraint stress-induced depressive like behavior and hippocampal alterations with protocatechuic acid treatment in mice. Metab. Brain Dis. 2017, 32, 401–413. [Google Scholar] [CrossRef]
- Thakare, V.N.; Patil, R.R.; Suralkar, A.A.; Dhakane, V.D.; Patel, B.M. Protocatechuic acid attenuate depressive-like behavior in olfactory bulbectomized rat model: Behavioral and neurobiochemical investigations. Metab. Brain Dis. 2019, 34, 775–787. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, G.H.; Hwang, K.H. Monoamine oxidase and dopamine b-hydroxylase inhibitors from the fruits of Gardenia jasminoides. Biomol. Ther. 2012, 20, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewer, C.; Rauen, T. Electrogenic glutamate transporters in the CNS: Molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J. Membr. Biol. 2005, 203, 1–20. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef] [Green Version]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [Green Version]
- Dalmagro, A.P.; Camargo, A.; Zeni, A.L.B. Morus nigra and its major phenolic, syringic acid, have antidepressant-like and neuroprotective effects in mice. Metab. Brain Dis. 2017, 32, 1963–1973. [Google Scholar] [CrossRef]
- Jaworski, T.; Banach-Kasper, E.; Gralec, K. GSK-3β at the Intersection of Neuronal Plasticity and Neurodegeneration. Neural Plast. 2019, 2019, 4209475. [Google Scholar] [CrossRef] [Green Version]
- Alfieri, A.; Mann, G.E. Bioactive Nutraceuticals and Stroke. Bioact. Nutraceuticals Diet. Suppl. Neurol. Brain Dis. 2015, 2015, 365–379. [Google Scholar] [CrossRef]
- Ito, N.; Yabe, T.; Gamo, Y.; Nagai, T.; Oikawa, T.; Yamada, H.; Hanawa, T. Rosmarinic Acid from Perillae Herba Produces an Antidepressant-Like Effect in Mice through Cell Proliferation in the Hippocampus. Biol. Pharm. Bull. 2008, 31, 1376–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Liu, P.; Yang, F.; Zhang, Y.H.; Miao, D. Rosmarinic acid ameliorates depressive-like behaviors in rat model of CUS and Up-regulates BDNF levels in the hippocampus and hippocampal-derived astrocytes. Neurochem. Res. 2013, 38, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; El Omri, A.; Han, J.; Isoda, H. Antidepressant-like effects of rosmarinic acid through mitogen-activated protein kinase phosphatase-1 and brain-derived neurotrophic factor modulation. J. Funct. Foods 2015, 14, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Akbarian, S.; Davis, R. Keep the ‘phospho’ on MAPK, be happy. Nat. Med. 2010, 16, 1187–1188. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Qiao, M. Mechanisms of extracellular signal-regulated kinase/cAMP response element-binding protein/brain-derived neurotrophic factor signal transduction pathway in depressive disorder. Neural Regen. Res. 2013, 8, 843–852. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Wu, X.H.; Feng, Y.; Xie, X.F.; Fan, Y.H.; Yan, S.; Zhao, Q.Y.; Peng, C.; You, Z.L. Salvianolic acid B ameliorates depressive-like behaviors in chronic mild stress-treated mice: Involvement of the neuroinflammatory pathway. Acta Pharmacol. Sin. 2016, 37, 1141–1153. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic Acids: Potential Source of Natural Drugs for the Treatment of Fibrosis Disease and Cancer. Front. Pharmacol. 2019, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Guo, Y.; Dang, R.; Yang, M.; Liao, D.; Li, H.; Sun, Z.; Feng, Q.; Xu, P. Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: Involvement of autophagy and NLRP3 inflammasome. J. Neuroinflammation 2017, 14, 239. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Casas-Barquero, N.; Williams, M.R.; Romero-Guillena, S.L.; Cañadas-Lozano, D.; Bullón, P.; Sánchez-Alcazar, J.A.; Navarro-Pando, J.M.; Cordero, M.D. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017, 121, 114–121. [Google Scholar] [CrossRef]
- Gassen, N.C.; Rein, T. Is There a Role of Autophagy in Depression and Antidepressant Action? Front. Psychiatry 2019, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivatives-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]
- Shakeri, A.Z.; Mohammad, R.; Sahebkar, A. Ellagic Acid: A Logical Lead for Drug Development? Curr. Pharm. Des. 2018, 24, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Girish, C.; Raj, V.; Arya, J.; Balakrishnan, S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur. J. Pharmacol. 2012, 682, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Bedel, H.A.; Kencebay, M.C.; Özbey, G.; Usta, C. The antidepressant like activity of ellagic acid andits effect on hippocampal brain derived neurotrophic factor levels in mouse depression models. Nat. Prod. Res. 2017, 32, 2932–2935. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Salimi, N.; Soltani, A.; Amini-Khoei, H. Implication of NMDA-NO pathway in the antidepressant-like effect of ellagic acid in male mice. Neuropeptides 2019, 76, 101928. [Google Scholar] [CrossRef]
- Dhir, A.; Kulkarni, S.K. Nitric oxide and major depression. Nitric. Oxide 2011, 24, 125–131. [Google Scholar] [CrossRef]
- Akkol, K.E.; Dereli, F.T.G.; Ilhan, M. Assessment of Antidepressant Effect of the Aerial Parts of Micromeria myrtifolia Boiss. & Hohen on Mice. Molecules 2019, 24, 1869. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Chou, M.L.; Chen, W.C.; Lai, Y.S.; Lu, K.H.; Hao, C.W.; Sheen, L.Y. A medicinal herb, Melissa officinalis L. ameliorates depressive-like behavior of rats in the forced swimming test via regulating the serotonergic neurotransmitter. J. Ethnopharmacol. 2015, 175, 266–272. [Google Scholar] [CrossRef]
- Li, Y.C.; Shen, J.D.; Li, Y.Y.; Huang, Q. Antidepressant effects of the water extract from Taraxacum officinale leaves and roots in mice. Pharm. Biol. 2014, 52, 1028–1032. [Google Scholar] [CrossRef]
- Gao, C.; Kong, S.; Guo, B.; Liang, X.; Duan, H.; Li, D. Antidepressive Effects of Taraxacum Officinale in a Mouse Model of Depression Are Due to Inhibition of Corticosterone Levels and Modulation of Mitogen-Activated Protein Kinase Phosphatase-1 (Mkp-1) and Brain-Derived Neurotrophic Factor (Bdnf) Expression. Med. Sci. Monit. 2019, 25, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Sobolev, A.P.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Di Giovanni, C.; Xiao, J.; Shirooie, S.; Sokeng, A.J.T.; et al. Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (molina) stuntz) in a mouse model of Post-stroke depression. Food Chem. Toxicol. 2019, 129, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Caprioli, G.; Iannarelli, R.; Sokeng, A.J.T.; Braidy, N.; Khanjani, S.; Moghaddam, A.H.; Atanasov, A.G.; et al. The water extract of tutsan (Hypericum androsaemum L.) red berries exerts antidepressive-like effects and in vivo antioxidant activity in a mouse model of post-stroke depression. Biomed. Pharmacother. 2018, 99, 290–298. [Google Scholar] [CrossRef]
- Barauna, S.C.; Magro, D.D.; Brueckheimer, M.B.; Maia, T.P.; Sala, G.A.B.N.; Döhler, A.W.; Harger, M.C.; de Melo, D.F.M.; de Gasper, A.L.; Alberton, M.D. Antioxidant and antidepressant-like effects of Eugenia catharinensis D. Legrand in an animal model of depression induced by corticosterone. Metab. Brain Dis. 2018, 33, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, B.; Kiasalari, Z.; Roghani, M.; Khalili, M.; Ansari, F. Antidepressant and anxiolytic activity of Lavandula officinalis aerial parts hydroalcoholic extract in scopolamine-treated rats. Pharm. Biol. 2017, 55, 958–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daodee, S.; Monthakantirat, O.; Ruengwinitwong, K.; Gatenakorn, K.; Maneenet, J.; Khamphukdee, C.; Sekeroglu, N.; Chulikhit, Y.; Kijjoa, A. Effects of the Ethanol Extract of Dipterocarpus alatus Leaf on the Unpredictable Chronic Mild Stress-Induced Depression in ICR Mice and Its Possible Mechanism of Action. Molecules 2019, 24, 3396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dattilo, V.; Amato, R.; Perrotti, N.; Gennarelli, M. The Emerging Role of SGK1 (Serum- and Glucocorticoid-Regulated Kinase 1) in Major Depressive Disorder: Hypothesis and Mechanisms. Front. Genet. 2020, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 3, 265–280. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Ma, H.; Yin, H.; Wang, P.; Bai, B.; Guo, L.; Geng, Q. Associations between depression, nutrition, and outcomes among individuals with coronary artery disease. Nutrition 2021, 86, 111157. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Gutiérrez-Rojas, L.; Molina, R.; Rodríguez-Jimenez, R.; et al. Biological Role of Nutrients, Food and Dietary Patterns in the Prevention and Clinical Management of Major Depressive Disorder. Nutrients 2022, 15, 3099. [Google Scholar] [CrossRef]
- LaChance, L.R.; Ramsey, D. Antidepressant foods: An evidence-based nutrient profiling system for depression. World J. Psychiatry 2018, 8, 97–104. [Google Scholar] [CrossRef]
- Khosravi, M.; Sotoudeh, G.; Amini, M.; Raisi, F.; Mansoori, A.; Hosseinzadeh, M. The relationship between dietary patterns and depression mediated by serum levels of Folate and vitamin B12. BMC Psychiatry 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 3, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, R.; Horton, S.; Hereford, C.; Ridpath, L.; Foster, R.; Boothe, E. Pro-inflammatory diet and depressive symptoms in the healthcare setting. BMC Psychiatry 2022, 22, 125. [Google Scholar] [CrossRef] [PubMed]
- Mechlinska, A.; Włodarczyk, A.; Gruchała-Niedoszytko, M.; Małgorzewicz, S.; Cubała, W.J. Dietary Patterns of Treatment–Resistant Depression Patients. Nutrients 2022, 14, 3766. [Google Scholar] [CrossRef]
- Sarris, J.; Thomson, R.; Hargraves, F.; Eaton, M.; De Manincor, M.; Veronese, N.; Solmi, M.; Stubbs, B.; Yung, A.R.; Firth, J. Multiple lifestyle factors and depressed mood: A cross-sectional and longitudinal analysis of the UK Biobank (N = 84,860). BMC Med. 2020, 1, 354. [Google Scholar] [CrossRef]
- Khosravi, M.; Sotoudeh, G.; Majdzadeh, R.; Nejati, S.; Darabi, S.; Raisi, F.; Esmaillzadeh, A.; Sorayani, M. Healthy and Unhealthy Dietary Patterns Are Related to Depression: A Case-Control Study. Psychiatry Investig. 2015, 4, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef] [Green Version]
- Pano, O.; Martínez-Lapiscina, E.H.; Sayón-Orea, C.; Martinez-Gonzalez, M.A.; Martinez, J.A.; Sanchez-Villegas, A. Healthy diet, depression and quality of life: A narrative review of biological mechanisms and primary prevention opportunities. World J. Psychiatry 2021, 11, 997–1016. [Google Scholar] [CrossRef]
- Głąbska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef] [Green Version]
- Lordan, R. Dietary supplements and nutraceuticals market growth during the coronavirus pandemic—Implications for consumers and regulatory oversight. Pharma Nutr. 2021, 18, 100282. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Miller, J.W. Challenges in conducting clinical nutrition research. Nutr. Rev. 2017, 7, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacka, F.N. Nutritional Psychiatry: Where to Next? EBioMedicine 2017, 17, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Huang, X.; Wang, Y.; Xie, Y.; Qiu, X.; Ren, P.; Gao, L.; Zhou, H.; Zhang, H.; Qiao, M. Ferulic acid-induced anti-depression and prokinetics similar to Chaihu-Shugan-San via polypharmacology. Brain Res. Bull. 2011, 86, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, J.; Rodrigues, A.F.; Rós, A.S.; De Castro, A.B.; De Lima, D.D.; Magro, D.D.; Zeni, A.L. Ferulic acid chronic treatment exerts antidepressant-like effect: Role of antioxidant defense system. Metab. Brain Dis. 2015, 30, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, M.L.d.S.; Martins, V.G.d.Q.A.; Silva, A.P.d.; Rocha, H.A.O.; Rachetti, V.d.P.S.; Scortecci, K.C. Phenolic Acids as Antidepressant Agents. Nutrients 2022, 14, 4309. https://doi.org/10.3390/nu14204309
Cordeiro MLdS, Martins VGdQA, Silva APd, Rocha HAO, Rachetti VdPS, Scortecci KC. Phenolic Acids as Antidepressant Agents. Nutrients. 2022; 14(20):4309. https://doi.org/10.3390/nu14204309
Chicago/Turabian StyleCordeiro, Maria Lúcia da Silva, Verônica Giuliani de Queiroz Aquino Martins, Ariana Pereira da Silva, Hugo Alexandre Oliveira Rocha, Vanessa de Paula Soares Rachetti, and Katia Castanho Scortecci. 2022. "Phenolic Acids as Antidepressant Agents" Nutrients 14, no. 20: 4309. https://doi.org/10.3390/nu14204309
APA StyleCordeiro, M. L. d. S., Martins, V. G. d. Q. A., Silva, A. P. d., Rocha, H. A. O., Rachetti, V. d. P. S., & Scortecci, K. C. (2022). Phenolic Acids as Antidepressant Agents. Nutrients, 14(20), 4309. https://doi.org/10.3390/nu14204309







