Sensitivity and Specificity of Body Mass Index for Sarcopenic Dysphagia Diagnosis among Patients with Dysphagia: A Multi-Center Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Source
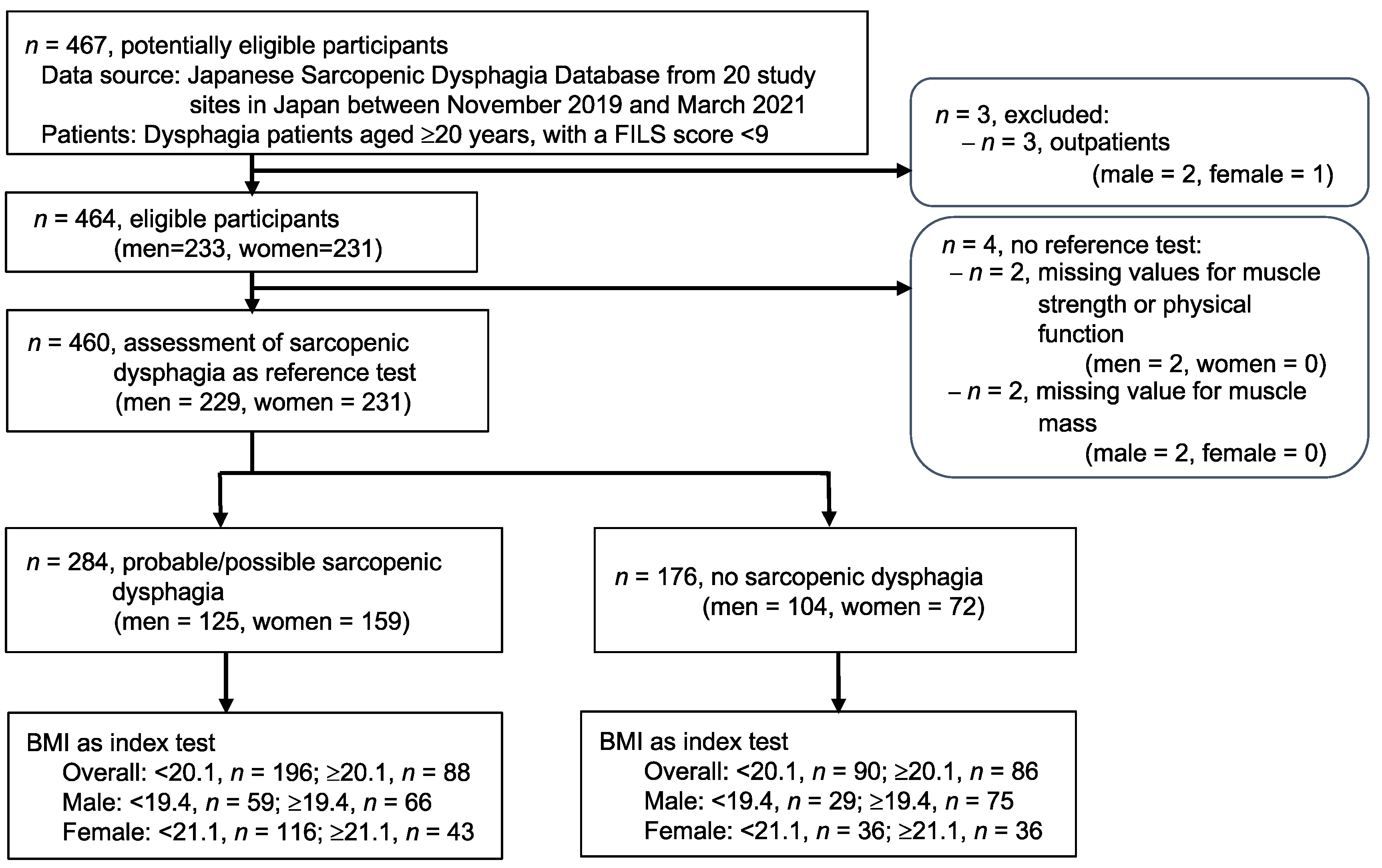
2.3. Participants
2.4. Index Test
2.5. Reference Test
2.6. Other Variables
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clavé, P.; Shaker, R. Dysphagia: Current Reality and Scope of the Problem. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, I.; Fujiu-Kurachi, M.; Arai, H.; Hyodo, M.; Kagaya, H.; Maeda, K.; Mori, T.; Nishioka, S.; Oshima, F.; Ogawa, S.; et al. Sarcopenia and Dysphagia: Position Paper by Four Professional Organizations. Geriatr. Gerontol. Int. 2019, 19, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, H.; Kishima, M.; Itoda, M.; Fujishima, I.; Kunieda, K.; Ohno, T.; Shigematsu, T.; Oshima, F.; Mori, T.; Ogawa, N.; et al. Diagnosis and Treatment of Sarcopenic Dysphagia: A Scoping Review. Dysphagia 2021, 36, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Takahashi, R.; Murakami, T. The Prevalence and Prognosis of Sarcopenic Dysphagia in Patients Who Require Dysphagia Rehabilitation. J. Nutr. Health Aging 2019, 23, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Nakayama, E.; Tohara, H.; Takahashi, O.; Ohnishi, S.; Tsuzuki, H.; Hayata, M.; Takehisa, T.; Takehisa, Y.; Ueda, K. Diagnostic Accuracy of Lip Force and Tongue Strength for Sarcopenic Dysphagia in Older Inpatients: A Cross-Sectional Observational Study. Clin. Nutr. 2019, 38, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Takaki, M.; Akagi, J. Decreased Skeletal Muscle Mass and Risk Factors of Sarcopenic Dysphagia: A Prospective Observational Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Sakuma, K. Sarcopenic Dysphagia as a New Concept. In Sarcopenic Dysphagia as a New Concept; Dionyssiotis, Y., Ed.; IntechOpen: Rijeka, Croatia; London, UK, 2017. [Google Scholar]
- Nagano, A.; Nishioka, S.; Wakabayashi, H. Rehabilitation Nutrition for Iatrogenic Sarcopenia and Sarcopenic Dysphagia. J. Nutr. Health Aging 2019, 23, 256–265. [Google Scholar] [CrossRef]
- Bonilha, H.S.; Simpson, A.N.; Ellis, C.; Mauldin, P.; Martin-Harris, B.; Simpson, K. The One-Year Attributable Cost of Post-Stroke Dysphagia. Dysphagia 2014, 29, 545–552. [Google Scholar] [CrossRef]
- WHO. Expert Consultation. Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Global BMI Mortality Collaboration; Di Angelantonio, E.; Bhupathiraju, S.; Wormser, D.; Gao, P.; Kaptoge, S.; Berrington de Gonzalez, A.; Cairns, B.; Huxley, R.; Jackson, C.; et al. Body-Mass Index and All-Cause Mortality: Individual-Participant-Data Meta-Analysis of 239 Prospective Studies in Four Continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakadate, A.; Otaka, Y.; Kondo, K.; Yamamoto, R.; Matsuura, D.; Honaga, K.; Muraoka, K.; Akaboshi, K.; Liu, M. Age, Body Mass Index, and White Blood Cell Count Predict the Resumption of Oral Intake in Subacute Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, Y.; Nakayama, S.; Taniguchi, H.; Ohori, I.; Komatsu, N.; Nishimura, H.; Katsuki, Y. Factors Predicting Recovery of Oral Intake in Stroke Survivors with Dysphagia in a Convalescent Rehabilitation Ward. J. Stroke Cerebrovasc. Dis. 2017, 26, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ishida, Y.; Nonogaki, T.; Shimizu, A.; Yamanaka, Y.; Matsuyama, R.; Kato, R.; Mori, N. Development and Predictors of Sarcopenic Dysphagia during Hospitalization of Older Adults. Nutrients 2019, 12, 70. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.; Naganuma, A.; Ogawa, Y.; Inagawa, M.; Nishioka, S.; Momosaki, R.; Wakabayashi, H. Calf Circumference and Stroke Are Independent Predictors for an Improvement in the Food Intake Level Scale in the Japanese Sarcopenic Dysphagia Database. Eur. Geriatr. Med. 2022, 13, 1211–1220. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, S.; Wakabayashi, H.; Fujishima, I.; Kishima, M.; Itoda, M.; Yamakawa, M.; Wada, F.; Kato, R.; Furiya, Y.; Nishioka, S.; et al. Construction and Quality Evaluation of the Japanese Sarcopenic Dysphagia Database. J. Nutr. Health Aging 2021, 25, 926–932. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Kishima, M.; Itoda, M.; Fujishima, I.; Kunieda, K.; Ohno, T.; Shigematsu, T.; Oshima, F.; Mori, T.; Ogawa, N.; et al. Prevalence of Hoarseness and Its Association with Severity of Dysphagia in Patients with Sarcopenic Dysphagia. J. Nutr. Health Aging 2022, 26, 266–271. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Kunieda, K.; Ohno, T.; Fujishima, I.; Hojo, K.; Morita, T. Reliability and Validity of a Tool to Measure the Severity of Dysphagia: The Food Intake LEVEL Scale. J. Pain Symptom Manag. 2013, 46, 201–206. [Google Scholar] [CrossRef]
- Nagai, T.; Wakabayashi, H.; Nishioka, S.; Momosaki, R. Functional Prognosis in Patients with Sarcopenic Dysphagia: An Observational Cohort Study from the Japanese Sarcopenic Dysphagia Database. Geriatr. Gerontol. Int. 2022, 22, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Hasegawa, W.; Yasunaga, H.; Sunohara, M.; Jo, T.; Takami, K.; Matsui, H.; Fushimi, K.; Nagase, T. Paradoxical Association between Body Mass Index and In-Hospital Mortality in Elderly Patients with Chronic Obstructive Pulmonary Disease in Japan. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1337–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, H.; Maeda, K.; Nishioka, S.; Shamoto, H.; Momosaki, R. Impact of Body Mass Index on Activities of Daily Living in Inpatients with Acute Heart Failure. J. Nutr. Health Aging 2019, 23, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, G.; Morita, K.; Matsui, H.; Yasunaga, H.; Fushimi, K.; Sanada, H. Association between Pressure Injury Status and Hospital Discharge to Home: A Retrospective Observational Cohort Study Using a National Inpatient Database. Ann. Clin. Epidemiol. 2020, 2, 38–50. [Google Scholar] [CrossRef]
- Suzuki, R.; Sakata, N.; Fushimi, K. Association of Body Mass Index with Clostridioides Difficile Infection among Older Patients with Pneumonia in Japan. Geriatr. Gerontol. Int. 2022, 22, 63–67. [Google Scholar] [CrossRef]
- Mori, T.; Fujishima, I.; Wakabayashi, H.; Oshima, F.; Itoda, M.; Kunieda, K.; Kayashita, J.; Nishioka, S.; Sonoda, A.; Kuroda, Y.; et al. Development, Reliability, and Validity of a Diagnostic Algorithm for Sarcopenic Dysphagia. JCSM Clin. Rep. 2017, 2, 1–10. [Google Scholar]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Fischer, J.E.; Bachmann, L.M.; Jaeschke, R. A Readers’ Guide to the Interpretation of Diagnostic Test Properties: Clinical Example of Sepsis. Intensive Care Med. 2003, 29, 1043–1051. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 2 June 2022).
- Kaiser, M.J.; Bauer, J.M.; Rämsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.S.; Charlton, K.E.; Maggio, M.; et al. Frequency of Malnutrition in Older Adults: A Multinational Perspective Using the Mini Nutritional Assessment. J. Am. Geriatr. Soc. 2010, 58, 1734–1738. [Google Scholar] [CrossRef]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarui, I.; Okada, E.; Okada, C.; Saito, A.; Takimoto, H. Trends in BMI among Elderly Japanese Population: Findings from 1973 to 2016 Japan National Health and Nutrition Survey. Public Health Nutr. 2020, 23, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Klugar, M.; Ding, S.; Carmody, D.P.; Hakonsen, S.J.; Jadotte, Y.T.; White, S.; Munn, Z. Diagnostic Test Accuracy: Methods for Systematic Review and Meta-Analysis. Int. J. Evid. Based Healthc. 2015, 13, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Abu-Ghanem, S.; Graf, A.; Govind, J. Diagnosis of Sarcopenic Dysphagia in the Elderly: Critical Review and Future Perspectives. Dysphagia 2022, 37, 1093–1102. [Google Scholar] [CrossRef]
- Kunieda, K.; Fujishima, I.; Wakabayashi, H.; Ohno, T.; Shigematsu, T.; Itoda, M.; Oshima, F.; Mori, T.; Ogawa, N.; Ogawa, S. Relationship between Tongue Pressure and Pharyngeal Function Assessed Using High-Resolution Manometry in Older Dysphagia Patients with Sarcopenia: A Pilot Study. Dysphagia 2021, 36, 33–40. [Google Scholar] [CrossRef]

| Variable | Level | Overall | Sarcopenic Dysphagia | ||
|---|---|---|---|---|---|
| Probable/Possible | No | p Value | |||
| n | 460 | 284 | 176 | ||
| Age, years (median (IQR)) | 83.0 (76.0, 88.0) | 85.0 (79.0, 89.0) | 78.0 (70.0, 85.0) | <0.001 | |
| Age category (%) | <65 years | 36 (7.8) | 12 (4.2) | 24 (13.6) | <0.001 |
| 65–74 years | 64 (13.9) | 27 (9.5) | 37 (21.0) | ||
| 75–84 years | 159 (34.6) | 92 (32.4) | 67 (38.1) | ||
| ≥85 years | 201 (43.7) | 153 (53.9) | 48 (27.3) | ||
| Gender (%) | Male | 229 (49.8) | 125 (44.0) | 104 (59.1) | 0.002 |
| Female | 231 (50.2) | 159 (56.0) | 72 (40.9) | ||
| Hospital type (%) | Acute hospital | 202 (43.9) | 124 (43.7) | 78 (44.3) | 0.94 |
| Rehabilitation hospital | 208 (45.2) | 130 (45.8) | 78 (44.3) | ||
| Long-term hospital | 50 (10.9) | 30 (10.6) | 20 (11.4) | ||
| FILS at baseline (%) | 1 | 80 (17.4) | 28 (9.9) | 52 (29.5) | <0.001 |
| 2 | 34 (7.4) | 24 (8.5) | 10 (5.7) | ||
| 3 | 15 (3.3) | 11 (3.9) | 4 (2.3) | ||
| 4 | 7 (1.5) | 3 (1.1) | 4 (2.3) | ||
| 5 | 9 (2.0) | 8 (2.8) | 1 (0.6) | ||
| 6 | 30 (6.5) | 22 (7.7) | 8 (4.5) | ||
| 7 | 167 (36.3) | 100 (35.2) | 67 (38.1) | ||
| 8 | 118 (25.7) | 88 (31.0) | 30 (17.0) | ||
| Primary diagnosis that led to hospitalization (%) | Diseases of the circulatory system | 165 (35.9) | 68 (23.9) | 97 (55.1) | <0.001 |
| Cerebrovascular disease | 130 (28.3) | 41 (14.4) | 89 (50.6) | ||
| Injury, poisoning, and certain other consequences of external causes | 135 (29.3) | 105 (37.0) | 30 (17.0) | ||
| Diseases of the respiratory system | 57 (12.4) | 50 (17.6) | 7 (4.0) | ||
| Diseases of the nervous system | 26 (5.7) | 10 (3.5) | 16 (9.1) | ||
| Cancer | 25 (5.4) | 10 (3.5) | 15 (8.5) | ||
| Diseases of the musculoskeletal system and connective tissue | 18 (3.9) | 16 (5.6) | 2 (1.1) | ||
| Diseases of the digestive system | 13 (2.8) | 11 (3.9) | 2 (1.1) | ||
| Diseases of the genitourinary system | 13 (2.8) | 9 (3.2) | 4 (2.3) | ||
| Endocrine, nutritional, and metabolic diseases | 4 (0.9) | 3 (1.1) | 1 (0.6) | ||
| Diseases of the skin and subcutaneous tissue | 2 (0.4) | 1 (0.4) | 1 (0.6) | ||
| Infectious disease | 1 (0.2) | 1 (0.4) | 0 (0.0) | ||
| Missing | 1 (0.2) | 0 (0.0) | 1 (0.6) | ||
| BMI, kg/m2 (median (IQR)) | 19.9 (17.3, 22.6) | 19.4 (17.0, 21.9) | 20.9 (18.3, 24.1) | <0.001 | |
| BMI category (%) | <18.5 kg/m2 | 171 (37.2) | 123 (43.3) | 48 (27.3) | 0.001 |
| 18.5–22.9 kg/m2 | 184 (40.0) | 110 (38.7) | 74 (42.0) | ||
| 23.0–24.9 kg/m2 | 50 (10.9) | 27 (9.5) | 23 (13.1) | ||
| 25.0–29.9 kg/m2 | 50 (10.9) | 23 (8.1) | 27 (15.3) | ||
| ≥30.0 kg/m2 | 5 (1.1) | 1 (0.4) | 4 (2.3) | ||
| Barthel index score (median (IQR)) | 20.0 (5.0, 50.0) | 20.0 (0.0, 50.0) | 22.50 (10.0, 50.0) | 0.320 | |
| Barthel index (%) | Independent (100) | 5 (1.1) | 1 (0.4) | 4 (2.3) | 0.155 |
| Partial assistance (21–99) | 222 (48.3) | 138 (48.6) | 84 (47.7) | ||
| Total assistance (0–20) | 233 (50.7) | 145 (51.1) | 88 (50.0) | ||
| Calf circumference, cm (median (IQR)) | 28.0 (25.1, 31.0) | 27.0 (24.5, 29.5) | 30.5 (27.1, 32.5) | <0.001 | |
| Hand grip strength, kg (median (IQR)) | 12.0 (6.3, 18.7) | 10.8 (6.0, 15.0) | 16.7 (7.1, 25.0) | <0.001 | |
| GLIM malnutrition (%) | 301 (65.4) | 208 (73.2) | 93 (52.8) | <0.001 | |
| Duration between admission and evaluation, days (median (IQR)) | 3.0 (2.0, 6.0) | 3.0 (2.0, 7.0) | 3.0 (2.0, 6.0) | 0.509 | |
| Group | n | BMI < 20 kg/m2 (%) | Min (kg/m2) | Q25 (kg/m2) | Median (kg/m2) | Q75 (kg/m2) | Max (kg/m2) | |
|---|---|---|---|---|---|---|---|---|
| Overall | 460 | 50.2 | 9.9 | 17.3 | 19.9 | 22.6 | 32.3 | |
| Probable/possible sarcopenic dysphagia | 284 | 56.7 | 9.9 | 17.0 | 19.4 | 21.9 | 32.3 | |
| -Men | 125 | 49.6 | 13.8 | 16.9 | 20.0 | 22.4 | 32.3 | |
| -Women | 159 | 62.3 | 9.9 | 17.0 | 19.2 | 21.2 | 29.9 | |
| No sarcopenic dysphagia | 176 | 39.8 | 12.2 | 18.3 | 20.9 | 24.1 | 30.8 | |
| Subgroup of no sarcopenic dysphagia according to gender and cause of dysphagia | ||||||||
| Gender | Cause of dysphagia | n | BMI < 20 kg/m2 (%) | Min (kg/m2) | Q25 (kg/m2) | Median (kg/m2) | Q75 (kg/m2) | Max (kg/m2) |
| Men | 104 | 35.6 | 12.2 | 18.8 | 20.9 | 23.8 | 30.8 | |
| -Subgroup | Cerebral infarction | 60 | 26.7 | 12.2 | 19.6 | 21.6 | 24.3 | 30.5 |
| Cerebral hemorrhage | 10 | 40.0 | 16.2 | 18.4 | 22.2 | 26.1 | 30.8 | |
| Subarachnoid hemorrhage | 3 | 33.3 | 14.9 | 17.7 | 20.5 | 22.4 | 24.2 | |
| Parkinson’s disease | 9 | 55.6 | 15.4 | 18.0 | 19.9 | 21.1 | 26.4 | |
| Dementia | 1 | 0 | 20.1 | 20.1 | 20.1 | 20.1 | 20.1 | |
| Cancer | 7 | 71.4 | 14.3 | 15.8 | 17.0 | 19.8 | 20.1 | |
| Unspecified reasons | 13 | 46.2 | 15.7 | 18.9 | 20.3 | 22.3 | 24.7 | |
| Missing | 1 | 0 | 24.2 | 24.2 | 24.2 | 24.2 | 24.2 | |
| Gender | Cause of dysphagia | n | BMI < 20 kg/m2 (%) | Min (kg/m2) | Q25 (kg/m2) | Median (kg/m2) | Q75 (kg/m2) | Max (kg/m2) |
| Women | 72 | 45.8 | 13.5 | 17.4 | 20.8 | 24.5 | 30.2 | |
| -Subgroup | Cerebral infarction | 18 | 44.4 | 14.2 | 16.1 | 21.6 | 25.9 | 30.2 |
| Cerebral hemorrhage | 10 | 40.0 | 13.5 | 18.0 | 20.6 | 21.8 | 24.7 | |
| Subarachnoid hemorrhage | 6 | 33.3 | 14.8 | 20.6 | 24.0 | 26.1 | 27.2 | |
| Parkinson’s disease | 6 | 83.3 | 15.6 | 15.8 | 16.6 | 19.2 | 23.9 | |
| Dementia | 11 | 54.5 | 13.8 | 17.1 | 19.5 | 22.1 | 25.8 | |
| Cancer | 1 | 0 | 22.9 | 22.9 | 22.9 | 22.9 | 22.9 | |
| Unspecified reasons | 20 | 40.0 | 14.8 | 18.8 | 21.9 | 25.1 | 28.4 | |
| Variable | AUC (95% CI) | BMI Cut-Off, | n | Tp (n) | Fp (n) | Fn (n) | Tn (n) | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| kg/m2 | (95% CI) % | (95% CI) % | (95% CI) % | (95% CI) % | |||||||
| Cut-off as per ROC | |||||||||||
| Overall | 0.61 (0.56–0.66) | 20.1 | 460 | 165 | 70 | 119 | 106 | 58.1 (52.1–63.9) | 60.2 (52.6–67.5) | 70.2 (63.9–76.0) | 47.1 (40.4–53.9) |
| Subgroup | |||||||||||
| Men | 0.60 (0.52–0.67) | 19.4 | 229 | 59 | 29 | 66 | 75 | 47.2 (38.2–56.3) | 72.1 (62.5–80.5) | 67.0 (56.2–76.7) | 53.2 (44.6–61.6) |
| Women | 0.60 (0.52–0.69) | 21.1 | 231 | 116 | 36 | 43 | 36 | 73.0 (65.3–79.7) | 50.0 (38.0–62.0) | 76.3 (68.7–82.8) | 45.6 (34.3–57.2) |
| Patients aged ≥ 65 years | 0.62 (0.57–0.68) | 20.1 | 424 | 163 | 58 | 109 | 94 | 59.8 (53.8–65.8) | 61.8 (53.6–69.6) | 73.8 (67.4–79.4) | 46.3 (39.3–53.4) |
| Acute hospital | 0.62 (0.54–0.70) | 19.4 | 202 | 61 | 24 | 63 | 54 | 49.2 (40.1–58.3) | 69.2 (57.8–79.2) | 71.8 (61.0–81.0) | 46.2 (36.9–55.6) |
| Rehabilitation hospital | 0.59 (0.51–0.68) | 19.6 | 208 | 71 | 28 | 59 | 50 | 54.6 (45.7–63.4) | 64.1 (52.4–74.7) | 71.7 (61.8–80.3) | 45.9 (36.3–55.7) |
| Long-term care hospital | 0.60 (0.44–0.76) | 19.9 | 50 | 16 | 6 | 14 | 14 | 53.3 (34.3–71.7) | 70.0 (45.7–88.1) | 57.1 (37.2–75.5) | 63.6 (40.6–82.8) |
| Cut-off based on the WHO BMI classification | |||||||||||
| Overall | 18.5 | 460 | 123 | 48 | 161 | 128 | 43.3 (37.5–49.3) | 72.7 (65.5–79.2) | 71.9 (65.6–78.5) | 44.3 (49.8–61.5) | |
| 23 | 460 | 233 | 122 | 51 | 54 | 82.0 (77.1–86.3) | 30.7 (24.0–38.1) | 65.6 (60.4–70.6) | 51.4 (41.5–61.3) | ||
| 25 | 460 | 260 | 145 | 24 | 31 | 91.5 (87.7–94.5) | 17.6 (12.3–24.1) | 64.2 (59.3–68.9) | 56.4 (42.3–69.7) | ||
| 30 | 460 | 283 | 172 | 1 | 4 | 99.6 (98.1–100) | 2.3 (0.6–5.7) | 62.2 (57.6–66.7) | 80.0 (28.4–99.5) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togashi, S.; Wakabayashi, H.; Ohinata, H.; Nishioka, S.; Kokura, Y.; Momosaki, R. Sensitivity and Specificity of Body Mass Index for Sarcopenic Dysphagia Diagnosis among Patients with Dysphagia: A Multi-Center Cross-Sectional Study. Nutrients 2022, 14, 4494. https://doi.org/10.3390/nu14214494
Togashi S, Wakabayashi H, Ohinata H, Nishioka S, Kokura Y, Momosaki R. Sensitivity and Specificity of Body Mass Index for Sarcopenic Dysphagia Diagnosis among Patients with Dysphagia: A Multi-Center Cross-Sectional Study. Nutrients. 2022; 14(21):4494. https://doi.org/10.3390/nu14214494
Chicago/Turabian StyleTogashi, Shintaro, Hidetaka Wakabayashi, Hironori Ohinata, Shinta Nishioka, Yoji Kokura, and Ryo Momosaki. 2022. "Sensitivity and Specificity of Body Mass Index for Sarcopenic Dysphagia Diagnosis among Patients with Dysphagia: A Multi-Center Cross-Sectional Study" Nutrients 14, no. 21: 4494. https://doi.org/10.3390/nu14214494
APA StyleTogashi, S., Wakabayashi, H., Ohinata, H., Nishioka, S., Kokura, Y., & Momosaki, R. (2022). Sensitivity and Specificity of Body Mass Index for Sarcopenic Dysphagia Diagnosis among Patients with Dysphagia: A Multi-Center Cross-Sectional Study. Nutrients, 14(21), 4494. https://doi.org/10.3390/nu14214494









