Rehabilitation Nutrition in Patients with Chronic Kidney Disease and Cachexia
Abstract
:1. Introduction
2. Overview of Chronic Kidney Disease
2.1. Definition, Prevalence, and Prognosis of Chronic Kidney Disease
2.2. Physical Function, Muscle Strength, and Skeletal Muscle Mass in Patients with Chronic Kidney Disease
3. Overview of Cachexia
3.1. Definition, Prevalence, and Prognosis of Cachexia
3.2. Interventions of Cachexia
4. Chronic Kidney Disease and Cachexia/Protein-Energy Wasting
4.1. Previous Studies of Chronic Kidney Disease and Cachexia
4.2. Definition, Prevalence, and Prognosis of Protein-Energy Wasting
5. Rehabilitation Nutrition in Chronic Kidney Disease and Cachexia/Protein-Energy Wasting
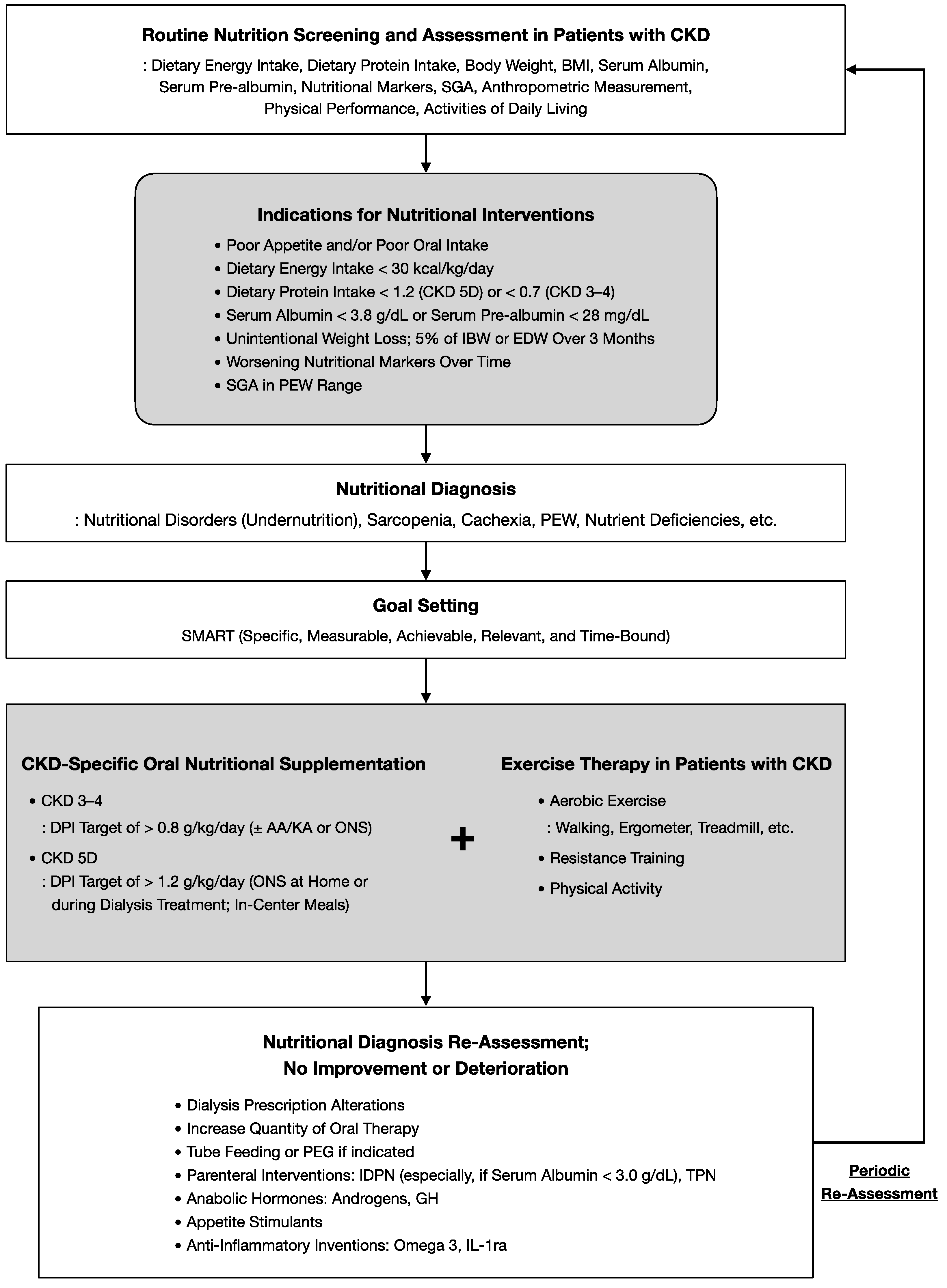
5.1. Rehabilitation Nutrition
5.2. Nutritional Management
5.3. Exercise Therapy
5.4. Rehabilitation Nutrition for Chronic Kidney Disease
6. Other Interventions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [CrossRef]
- von Haehling, S.; Anker, M.S.; Anker, S.D. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: Facts and numbers update 2016. J. Cachexia Sarcopenia Muscle 2016, 7, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mutsert, R.; Grootendorst, D.C.; Boeschoten, E.W.; Brandts, H.; Van Manen, J.G.; Krediet, R.T.; Dekker, F.W. Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients. Am. J. Clin. Nutr. 2009, 89, 787–793. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, H. Rehabilitation nutrition in general and family medicine. J. Gen. Fam. Med. 2017, 18, 153–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, A.; Nishioka, S.; Wakabayashi, H. Rehabilitation Nutrition for Iatrogenic Sarcopenia and Sarcopenic Dysphagia. J. Nutr. Health Aging 2019, 23, 256–265. [Google Scholar] [CrossRef]
- Kakehi, S.; Wakabayashi, H.; Inuma, H.; Inose, T.; Shioya, M.; Aoyama, Y.; Hara, T.; Uchimura, K.; Tomita, K.; Okamoto, M.; et al. Rehabilitation Nutrition and Exercise Therapy for Sarcopenia. World J. Men’s Health 2022, 40, 1–10. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Zheng, R.; Carter, C.E.; Mak, R.H. Cachexia/Protein energy wasting syndrome in CKD: Causation and treatment. Semin. Dial. 2019, 32, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Heiwe, S.; Jacobson, S.H. Exercise training for adults with chronic kidney disease. Cochrane Database Syst. Rev. 2011, 10, CD003236. [Google Scholar] [CrossRef]
- Ammirati, A.L. Chronic Kidney Disease. Rev. Assoc. Méd. Bras. 2020, 66, s03–s09. [Google Scholar] [CrossRef]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Chan, W. Chronic Kidney Disease and Nutrition Support. Nutr. Clin. Pract. 2021, 36, 312–330. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronco, C.; Ronco, F.; McCullough, P.A. A Call to Action to Develop Integrated Curricula in Cardiorenal Medicine. Blood Purif. 2017, 44, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Matsushita, K.; Abate, K.H.; Al-Aly, Z.; Arnlov, J.; Asayama, K.; Atkins, R.; Badawi, A.; Ballew, S.H.; Banerjee, A.; et al. Global Cardiovascular and Renal Outcomes of Reduced GFR. J. Am. Soc. Nephrol. 2017, 28, 2167–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roshanravan, B.; Robinson-Cohen, C.; Patel, K.V.; Ayers, E.; Littman, A.J.; de Boer, I.H.; Ikizler, T.A.; Himmelfarb, J.; Katzel, L.I.; Kestenbaum, B.; et al. Association between physical performance and all-cause mortality in CKD. J. Am. Soc. Nephrol. 2013, 24, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Roshanravan, B.; Khatri, M.; Robinson-Cohen, C.; Levin, G.; Patel, K.V.; de Boer, I.H.; Seliger, S.; Ruzinski, J.; Himmelfarb, J.; Kestenbaum, B. A prospective study of frailty in nephrology-referred patients with CKD. Am. J. Kidney Dis. 2012, 60, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Bàràny, P.; Heimbürger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative Associations of Muscle Mass and Muscle Strength with Mortality in Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. Control of food intake and muscle wasting in cachexia. Int. J. Biochem. Cell Biol. 2013, 45, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Marinho, R.; Alcantara, P.S.M.; Ottoch, J.P.; Seelaender, M. Role of Exosomal MicroRNAs and myomiRs in the Development of Cancer Cachexia-Associated Muscle Wasting. Front. Nutr. 2017, 4, 69. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Ding, H.; Zhou, Y.; Doan, H.A.; Sin, K.W.T.; Zhu, Z.J.; Flores, R.; Wen, Y.; Gong, X.; et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 2017, 8, 589. [Google Scholar] [CrossRef] [Green Version]
- Kir, S.; Komaba, H.; Garcia, A.P.; Economopoulos, K.P.; Liu, W.; Lanske, B.; Hodin, R.A.; Spiegelman, B.M. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab. 2016, 23, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Prim. 2018, 4, 17105. [Google Scholar] [CrossRef]
- Christensen, H.M.; Kistorp, C.; Schou, M.; Keller, N.; Zerahn, B.; Frystyk, J.; Schwarz, P.; Faber, J. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine 2013, 43, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Vest, A.R.; Chan, M.; Deswal, A.; Givertz, M.M.; Lekavich, C.; Lennie, T.; Litwin, S.E.; Parsly, L.; Rodgers, J.E.; Rich, M.W.; et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J. Card. Fail. 2019, 25, 380–400. [Google Scholar] [CrossRef]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.-J.; Zhao, J.-R.; Hao, J.; Li, B.; Huo, Y.; Han, Y.-L.; Wan, L.-L.; Li, J.; Huang, J.; Lu, J.; et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, M.; Keshavarz-Fathi, M.; Baracos, V.; Arends, J.; Mahmoudi, M.; Rezaei, N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit. Rev. Oncol. Hematol. 2018, 127, 91–104. [Google Scholar] [CrossRef]
- Morley, J.E. Pharmacologic Options for the Treatment of Sarcopenia. Calcif. Tissue Int. 2016, 98, 319–333. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, K.C.H.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional Recommendations for the Management of Sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Grande, A.J.; Silva, V.; Maddocks, M. Exercise for cancer cachexia in adults: Executive summary of a Cochrane Collaboration systematic review. J. Cachexia Sarcopenia Muscle 2015, 6, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Bye, A.; Stene, G.; Baracos, V.; Strasser, F.; Griffiths, G.; Maddocks, M.; Fallon, M.; et al. Cancer cachexia: Rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 2018, 8, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naito, T.; Mitsunaga, S.; Miura, S.; Tatematsu, N.; Inano, T.; Mouri, T.; Tsuji, T.; Higashiguchi, T.; Inui, A.; Okayama, T.; et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, H.; Arai, H.; Inui, A. The regulatory approval of anamorelin for treatment of cachexia in patients with non-small cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 14–16. [Google Scholar] [CrossRef] [PubMed]
- McKeaveney, C.; Slee, A.; Adamson, G.; Davenport, A.; Farrington, K.; Fouque, D.; Kalantar-Zadeh, K.; Mallett, J.; Maxwell, A.P.; Mullan, R.; et al. Using a generic definition of cachexia in patients with kidney disease receiving haemodialysis: A longitudinal (pilot) study. Nephrol. Dial. Transpl. 2021, 36, 1919–1926. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obi, Y.; Qader, H.; Kovesdy, C.P.; Kalantar-Zadeh, K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A Practical Approach to Nutrition, Protein-Energy Wasting, Sarcopenia, and Cachexia in Patients with Chronic Kidney Disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies From the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Shirai, N.; Inoue, T.; Ogawa, M.; Okamura, M.; Morishita, S.; Suguru, Y.; Tsubaki, A. Relationship between Nutrition-Related Problems and Falls in Hemodialysis Patients: A Narrative Review. Nutrients 2022, 14, 3225. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [Green Version]
- Van Der Veer, S.N.; Jager, K.J.; Nache, A.M.; Richardson, D.; Hegarty, J.; Couchoud, C.; De Keizer, N.F.; Tomson, C.R.V. Translating knowledge on best practice into improving quality of RRT care: A systematic review of implementation strategies. Kidney Int. 2011, 80, 1021–1034. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Sadu Singh, B.K.; Khor, B.H.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2017, 36, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Kistler, B.M.; Moore, L.W.; Benner, D.; Biruete, A.; Boaz, M.; Brunori, G.; Chen, J.; Drechsler, C.; Guebre-Egziabher, F.; Hensley, M.K.; et al. The International Society of Renal Nutrition and Metabolism Commentary on the National Kidney Foundation and Academy of Nutrition and Dietetics KDOQI Clinical Practice Guideline for Nutrition in Chronic Kidney Disease. J. Ren. Nutr. 2021, 31, 116–120.e111. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, T.; Kabayama, M.; Ryuno, H.; Tanaka, K.; Kiyoshige, E.; Akagi, Y.; Godai, K.; Sugimoto, K.; Akasaka, H.; Takami, Y.; et al. Association between protein intake and changes in renal function among Japanese community-dwelling older people: The SONIC study. Geriatr. Gerontol. Int. 2022, 22, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Machida, S.; Matsumoto, N.; Shibagaki, Y.; Sakurada, T. Age Modifies the Association of Dietary Protein Intake with All-Cause Mortality in Patients with Chronic Kidney Disease. Nutrients 2018, 10, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier-Jean, A.; Prince, R.L.; Lewis, J.R.; Craig, J.C.; Hodgson, J.M.; Lim, W.H.; Teixeira-Pinto, A.; Wong, G. Dietary plant and animal protein intake and decline in estimated glomerular filtration rate among elderly women: A 10-year longitudinal cohort study. Nephrol. Dial. Transpl. 2021, 36, 1640–1647. [Google Scholar] [CrossRef]
- Nishioka, S.; Nakahara, S.; Takasaki, M.; Shiohama, N.; Kokura, Y.; Suzuki, T.; Yokoi-Yoshimura, Y.; Nii, M.; Maeda, K.; Wakabayashi, H. The concept of aggressive nutrition therapy and clinical indication: A position paper. Clin. Nutr. ESPEN 2022. [Google Scholar] [CrossRef]
- Fitschen, P.J.; Biruete, A.; Jeong, J.; Wilund, K.R. Efficacy of beta-hydroxy-beta-methylbutyrate supplementation in maintenance hemodialysis patients. Hemodial. Int. 2017, 21, 107–116. [Google Scholar] [CrossRef]
- Baier, S.; Johannsen, D.; Abumrad, N.; Rathmacher, J.A.; Nissen, S.; Flakoll, P. Year-long Changes in Protein Metabolism in Elderly Men and Women Supplemented With a Nutrition Cocktail of β-Hydroxy-β-methylbutyrate (HMB), L-Arginine, and L-Lysine. J. Parenter. Enter. Nutr. 2009, 33, 71–82. [Google Scholar] [CrossRef]
- Evans, A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003, 41, S13–S26. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Koziol, B.J.; Shapiro, J.I.; Brass, E.P. Carnitine metabolism during exercise in patients on chronic hemodialysis. Kidney Int. 1992, 41, 1613–1619. [Google Scholar] [CrossRef] [Green Version]
- Steiber, A.L.; Davis, A.T.; Spry, L.; Strong, J.; Buss, M.L.; Ratkiewicz, M.M.; Weatherspoon, L.J. Carnitine Treatment Improved Quality-of-Life Measure in a Sample of Midwestern Hemodialysis Patients. J. Parenter. Enter. Nutr. 2006, 30, 10–15. [Google Scholar] [CrossRef]
- Yano, J.; Kaida, Y.; Maeda, T.; Hashida, R.; Tonan, T.; Nagata, S.; Hazama, T.; Nakayama, Y.; Ito, S.; Kurokawa, Y.; et al. L-carnitine supplementation vs cycle ergometer exercise for physical activity and muscle status in hemodialysis patients: A randomized clinical trial. Ther. Apher. Dial. 2021, 25, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Yamagishi, S.-I.; Sakai, K.; Kaida, Y.; Adachi, T.; Ando, R.; Okuda, S. Potential Inhibitory Effects of L-Carnitine Supplementation on Tissue Advanced Glycation End Products in Patients with Hemodialysis. Rejuvenation Res. 2013, 16, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, K.; Kaida, Y.; Yamagishi, S.I.; Tanaka, H.; Yokoro, M.; Yano, J.; Sakai, K.; Kurokawa, Y.; Taguchi, K.; Nakayama, Y.; et al. L-Carnitine Supplementation Improves Self-Rating Depression Scale Scores in Uremic Male Patients Undergoing Hemodialysis. Lett. Drug Des. Discov. 2017, 14, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef]
- Hendriks, F.K.; Smeets, J.S.J.; Janneau, M.X.; Broers, N.J.H.; Frank, M.; Verdijk, L.B.; Kooman, J.P.; Luc, J.C. Amino acid removal during hemodialysis can be compensated for by protein ingestion and is not compromised by intradialytic exercise: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2021, 114, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Deleaval, P.; Luaire, B.; Laffay, P.; Jambut-Cadon, D.; Stauss-Grabo, M.; Canaud, B.; Chazot, C. Short-Term Effects of Branched-Chain Amino Acids-Enriched Dialysis Fluid on Branched-Chain Amino Acids Plasma Level and Mass Balance: A Randomized Cross-Over Study. J. Ren. Nutr. 2020, 30, 61–68. [Google Scholar] [CrossRef]
- Yamagata, K.; Hoshino, J.; Sugiyama, H.; Hanafusa, N.; Shibagaki, Y.; Komatsu, Y.; Konta, T.; Fujii, N.; Kanda, E.; Sofue, T.; et al. Clinical practice guideline for renal rehabilitation: Systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren. Replace. Ther. 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, S.A.; Castle, E.; Lindup, H.; Mayes, J.; Waite, I.; Grant, D.; Mangahis, E.; Crabb, O.; Shevket, K.; Macdougall, I.C.; et al. Mortality and morbidity following exercise-based renal rehabilitation in patients with chronic kidney disease: The effect of programme completion and change in exercise capacity. Nephrol. Dial. Transpl. 2019, 34, 618–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiwe, S.; Jacobson, S.H. Exercise training in adults with CKD: A systematic review and meta-analysis. Am. J. Kidney Dis. 2014, 64, 383–393. [Google Scholar] [CrossRef]
- Cheema, B.S.; Chan, D.; Fahey, P.; Atlantis, E. Effect of Progressive Resistance Training on Measures of Skeletal Muscle Hypertrophy, Muscular Strength and Health-Related Quality of Life in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Sport. Med. 2014, 44, 1125–1138. [Google Scholar] [CrossRef]
- Kim, J.C.; Kalantar-Zadeh, K.; Kopple, J.D. Frailty and Protein-Energy Wasting in Elderly Patients with End Stage Kidney Disease. J. Am. Soc. Nephrol. 2013, 24, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Cheema, B.S.; Fiatarone Singh, M.A. Progressive resistance training and nutrition in renal failure. J. Ren. Nutr. 2007, 17, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.J.; Gore, E.F.; Baker, L.A.; Watson, E.L.; Smith, A.C. Muscle power and physical dysfunction: A model for tailoring rehabilitation in chronic kidney disease. Nephrology 2021, 26, 790–797. [Google Scholar] [CrossRef]
- Tentori, F.; Elder, S.J.; Thumma, J.; Pisoni, R.L.; Bommer, J.; Fissell, R.B.; Fukuhara, S.; Jadoul, M.; Keen, M.L.; Saran, R.; et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Correlates and associated outcomes. Nephrol. Dial. Transpl. 2010, 25, 3050–3062. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.M.; Tawney, K.; Bacchetti, P.; Johansen, K.L. Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am. J. Kidney Dis. 2003, 41, 447–454. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Sheshadri, A.; Hsu, F.-C.; Chen, S.-H.; Jotwani, V.; Tranah, G.; Fielding, R.A.; Liu, C.K.; Ix, J.; Coca, S.G.; et al. Effect of Structured, Moderate Exercise on Kidney Function Decline in Sedentary Older Adults. JAMA Intern. Med. 2022, 182, 650. [Google Scholar] [CrossRef]
- Chen, I.R.; Wang, S.-M.; Liang, C.-C.; Kuo, H.-L.; Chang, C.-T.; Liu, J.-H.; Lin, H.-H.; Wang, I.K.; Yang, Y.-F.; Chou, C.-Y.; et al. Association of Walking with Survival and RRT Among Patients with CKD Stages 3–5. Clin. J. Am. Soc. Nephrol. 2014, 9, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Matsuzawa, R.; Abe, Y.; Hoshi, K.; Yoneki, K.; Harada, M.; Watanabe, T.; Shimoda, T.; Suzuki, Y.; Matsunaga, Y.; et al. Utility of Regular Management of Physical Activity and Physical Function in Hemodialysis Patients. Kidney Blood Press. Res. 2018, 43, 1505–1515. [Google Scholar] [CrossRef]
- Saitoh, M.; Ogawa, M.; Dos Santos, M.R.; Kondo, H.; Suga, K.; Itoh, H.; Tabata, Y. Effects of Intradialytic Resistance Exercise on Protein Energy Wasting, Physical Performance and Physical Activity in Ambulatory Patients on Dialysis: A Single-Center Preliminary Study in a Japanese Dialysis Facility. Ther. Apher. Dial. 2016, 20, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, K.; Kamijo-Ikemori, A.; Yasuda, T.; Hotta, C.; Izawa, K.P.; Watanabe, S.; Sugaya, T.; Kimura, K. Moderate-intensity single exercise session does not induce renal damage. J. Clin. Lab. Anal. 2013, 27, 177–180. [Google Scholar] [CrossRef]
- Kotoku, K.; Yasuno, T.; Kawakami, S.; Fujimi, K.; Matsuda, T.; Nakashima, S.; Uehara, Y.; Tanaka, H.; Saito, T.; Higaki, Y. Effect of exercise intensity on renal blood flow in patients with chronic kidney disease stage 2. Clin. Exp. Nephrol. 2019, 23, 621–628. [Google Scholar] [CrossRef]
- Hiraki, K.; Shibagaki, Y.; Izawa, K.P.; Hotta, C.; Wakamiya, A.; Sakurada, T.; Yasuda, T.; Kimura, K. Effects of home-based exercise on pre-dialysis chronic kidney disease patients: A randomized pilot and feasibility trial. BMC Nephrol. 2017, 18, 198. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Takeuchi, I.; Iida, Y.; Takahashi, K.; Nagano, F.; Miyazaki, S.; Shirado, K.; Yoshimura, Y.; Momosaki, R.; Maeda, K.; et al. Disease-specific Nutritional Physical Therapy: A Position Paper by the Japanese Association of Rehabilitation Nutrition (Secondary Publication). JMA J. 2022, 5, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, K.; Yasuda, T.; Hotta, C.; Izawa, K.P.; Morio, Y.; Watanabe, S.; Sakurada, T.; Shibagaki, Y.; Kimura, K. Decreased physical function in pre-dialysis patients with chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, K.; Hotta, C.; Izawa, K.P.; Sakurada, T.; Shibagaki, Y. Dietary protein intake is strongly and positively related with muscle strength in patients with pre-dialysis chronic kidney disease. Clin. Exp. Nephrol. 2017, 21, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Ottaviani, E.; Guerriero, C.; Di Daniele, F.; Pietroboni Zaitseva, A.; Di Daniele, N. Uremic Sarcopenia and Its Possible Nutritional Approach. Nutrients 2021, 13, 147. [Google Scholar] [CrossRef]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyère, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef] [Green Version]
- Rondanelli, M.; Cereda, E.; Klersy, C.; Faliva, M.A.; Peroni, G.; Nichetti, M.; Gasparri, C.; Iannello, G.; Spadaccini, D.; Infantino, V.; et al. Improving rehabilitation in sarcopenia: A randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J. Cachexia Sarcopenia Muscle 2020, 11, 1535–1547. [Google Scholar] [CrossRef]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef] [Green Version]
- Gullett, N.P.; Hebbar, G.; Ziegler, T.R. Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting. Am. J. Clin. Nutr. 2010, 91, 1143S–1147S. [Google Scholar] [CrossRef] [Green Version]
- Johansen, K.L.; Painter, P.L.; Sakkas, G.K.; Gordon, P.; Doyle, J.; Shubert, T. Effects of Resistance Exercise Training and Nandrolone Decanoate on Body Composition and Muscle Function among Patients Who Receive Hemodialysis: A Randomized, Controlled Trial. J. Am. Soc. Nephrol. 2006, 17, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Brunori, G.; Leiserowitz, M.; Fouque, D. Growth hormone induces anabolism in malnourished maintenance haemodialysis patients. Nephrol. Dial. Transpl. 2005, 20, 952–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deboer, M.D.; Zhu, X.; Levasseur, P.R.; Inui, A.; Hu, Z.; Han, G.; Mitch, W.E.; Taylor, J.E.; Halem, H.A.; Dong, J.Z.; et al. Ghrelin treatment of chronic kidney disease: Improvements in lean body mass and cytokine profile. Endocrinology 2008, 149, 827–835. [Google Scholar] [CrossRef] [PubMed]

| Criteria | Cachexia (Cachexia Consensus Working Group) | Protein-Energy Wasting (International Society of Renal Nutrition and Metabolism) |
|---|---|---|
| Dietary intake (Anorexia) | Unintentional low dietary energy intake < 20 kcal/kg/day Unintentional low dietary energy intake < 70% of usual food intake Poor appetite | Unintentional low dietary protein intake < 0.80 g/kg/day for at least 2 months for dialysis patients or <0.6 g/kg/day for patients with CKD stages 2–5 Unintentional low dietary energy intake < 25 kcal/kg/day for at least 2 months |
| Serum chemistry | Serum albumin < 3.2 g/dL | Serum albumin < 3.8 g/dL |
| Anaemia: haemoglobin < 12 g/dL | Serum pre-albumin (transthyretin) < 30 mg/dL (for maintenance dialysis patients only; levels may vary according to GFR level for patients with CKD stages 2–5) | |
| Increased inflammatory markers: CRP > 0.5 mg/dL, IL-6 > 4.6 pg/mL | Serum cholesterol < 100 mg/dL | |
| Body mass | N/A | BMI < 23 kg/m2 (a lower BMI might be desirable for certain Asian population; weight must be oedema-free mass, for example, post-dialysis dry weight) Unintentional weight loss over time: 5% over 3 months or 10% over 6 months Total body fat percentage < 10% |
| Muscle mass | Reduced mid-upper arm muscle circumference < 10th percentile for age and sex Reduction in appendicle skeletal muscle index on DEXA (kg/m2) by <5.45 and <7.25 in women and men, respectively | Muscle wasting: reduced muscle mass 5% over 3 months or 10% over 6 months Reduced mid-arm muscle circumference (reduction > 10% in relation to 50th percentile of reference population) Low creatinine appearance |
| Muscle strength | Decreased muscle strength (lowest tertile, e.g., handgrip strength | N/A |
| Fatigue | Physical or mental weariness resulting from exertion; inability to continue exercise at the same intensity with a resultant deterioration in performance | N/A |
| Diagnosis of cachexia/PEW | Weight loss of at least 5% in 12 months or less in the presence of underlying illness, plus three of the other criteria | At least three of the four listed categories (and at least one test in each of the selected categories) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamura, M.; Inoue, T.; Ogawa, M.; Shirado, K.; Shirai, N.; Yagi, T.; Momosaki, R.; Kokura, Y. Rehabilitation Nutrition in Patients with Chronic Kidney Disease and Cachexia. Nutrients 2022, 14, 4722. https://doi.org/10.3390/nu14224722
Okamura M, Inoue T, Ogawa M, Shirado K, Shirai N, Yagi T, Momosaki R, Kokura Y. Rehabilitation Nutrition in Patients with Chronic Kidney Disease and Cachexia. Nutrients. 2022; 14(22):4722. https://doi.org/10.3390/nu14224722
Chicago/Turabian StyleOkamura, Masatsugu, Tatsuro Inoue, Masato Ogawa, Kengo Shirado, Nobuyuki Shirai, Takuma Yagi, Ryo Momosaki, and Yoji Kokura. 2022. "Rehabilitation Nutrition in Patients with Chronic Kidney Disease and Cachexia" Nutrients 14, no. 22: 4722. https://doi.org/10.3390/nu14224722
APA StyleOkamura, M., Inoue, T., Ogawa, M., Shirado, K., Shirai, N., Yagi, T., Momosaki, R., & Kokura, Y. (2022). Rehabilitation Nutrition in Patients with Chronic Kidney Disease and Cachexia. Nutrients, 14(22), 4722. https://doi.org/10.3390/nu14224722










