Association of CYP2R1 and VDR Polymorphisms with Metabolic Syndrome Components in Non-Diabetic Brazilian Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Collection of Data and Blood Samples
2.3. Genetics Analyses
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Nehus, E.; Mitsnefes, M. Childhood Obesity and the Metabolic Syndrome. Pediatr. Clin. N. Am. 2019, 66, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Flemming, G.M.C.; Bussler, S.; Körner, A.; Kiess, W. Definition and early diagnosis of metabolic syndrome in children. J. Pediatr. Endocrinol. Metab. 2020, 33, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalidi, B.; Kimball, S.M.; Kuk, J.L.; Ardern, C.I. Metabolically healthy obesity, vitamin D, and all-cause and cardiometabolic mortality risk in NHANES III. Clin. Nutr. 2019, 38, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Massaro, J.M.; Fox, C.S.; Larson, M.G.; Keyes, M.J.; McCabe, E.L.; Robins, S.J.; O’Donnell, C.J.; Hoffmann, U.; Jacques, P.F.; et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes 2010, 59, 242–248. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat Weight Disord. 2017, 22, 27–41. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Lubberts, E.; Bener, A. Editorial: Immune-Modulatory Effects of Vitamin D. Front. Immunol. 2020, 11, 596611. [Google Scholar] [CrossRef]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.-F. Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies. Nutrients 2022, 14, 2049. [Google Scholar] [CrossRef]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina 2019, 55, 541. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of Vitamin D in the Metabolic Syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Kosmopoulos, M.; Nikas, I.P.; Spartalis, M.; Kassi, E.; Goulis, D.G.; Lambrinoudaki, I.; Siasos, G. The Impact of Obesity on the Association between Vitamin D Deficiency and Cardiovascular Disease. Nutrients 2019, 14, 2458. [Google Scholar]
- Al-Daghri, N.M.; Amer, O.E.; Khattak, M.N.K.; Sabico, S.; Ghouse, A.A.M.; Al-Saleh, Y.; Aljohani, N.; Alfawaz, H.; Alokail, M.S. Effects of different vitamin D supplementation strategies in reversing metabolic syndrome and its component risk factors in adolescents. J. Steroid Biochem. Mol. Biol. 2019, 191, 105378. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Walton, R.T.; Griffiths, C.J.; Martineau, A.R. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: Review of genetic association studies. J. Steroid Biochem. Mol. Biol. 2016, 164, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Totonchi, H.; Rezaei, R.; Noori, S.; Azarpira, N.; Mokarram, P.; Imani, D. Vitamin D Receptor Gene Polymorphisms and the Risk of Metabolic Syndrome (MetS): A Meta-Analysis. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 943–955. [Google Scholar] [CrossRef]
- Thacher, T.D.; Levine, M.A. CYP2R1 mutations causing vitamin D-deficiency rickets. J. Steroid Biochem. Mol. Biol. 2017, 173, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Xue, Z.; Ji, H.; Zhang, D.; Wang, Y. Effects of CYP2R1 gene variants on vitamin D levels and status: A systematic review and meta-analysis. Gene 2018, 15, 361–369. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, D.; Lin, J.; Li, X.; Liu, Y.; Gao, J.; Xue, Y.; Zhang, Y.; Ding, R.; Huang, G.; et al. The influence of CYP2R1 polymorphisms and gene-obesity interaction with hypertension risk in a Chinese rural population. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 241–248. [Google Scholar] [CrossRef]
- Bakos, B.; Szili, B.; Szabó, B.; Horváth, P.; Kirschner, G.; Kósa, J.P.; Toldy, E.; Lakatos, P.; Tabák, A.G.; Takács, I. Genetic variants of VDR and CYP2R1 affect BMI independently of serum vitamin D concentrations. BMC Med. Genet. 2020, 21, 129. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Cavadino, A.; Berry, D.J.; Mangino, M.; Andrews, P.; Moore, J.H.; Spector, T.D.; Power, C.; Järvelin, M.R.; Hyppönen, E. Interaction between allelic variations in vitamin D receptor and retinoid X receptor genes on metabolic traits. BMC Genet. 2014, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Karonova, T.; Grineva, E.; Belyaeva, O.; Bystrova, A.; Jude, E.B.; Andreeva, A.; Kostareva, A.; Pludowski, P. Relationship Between Vitamin D Status and Vitamin D Receptor Gene Polymorphisms With Markers of Metabolic Syndrome Among Adults. Front. Endocrinol. 2018, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lu, W.; Gong, X.; Zhou, J.; Wu, F. Association of vitamin D receptor polymorphisms with metabolic syndrome-related components: A cross-sectional study. J. Clin. Lab. Anal. 2021, 35, e23829. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Su, K.; Ding, Z.; Zhang, Z.; Wang, C. Association of Vitamin D Receptor Gene Polymorphisms with Metabolic Syndrome in Chinese Children. Int. J. Gen. Med. 2021, 14, 57–66. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneve, Switzerland, 2006. [Google Scholar]
- Zimmet, P.; Alberti, G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children and adolescents. Lancet 2007, 369, 2059–2061. [Google Scholar] [CrossRef]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Júnior, D.M.; et al. Brazilian Guidelines of Hypertension—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava, B.A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, R.; Guinó, E.; Moreno, V. Análisis estadístico de polimorfismos genéticos en estudios epidemiológicos. Gac. Sanit 2005, 19, 333–341. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef]
- Qin, Z.S.; Niu, T.; Liu, J.S. Partition-ligation-expectation-maximization algorithm for haplotype inference with single-nucleotide polymorphisms. Am. J. Hum. Genet. 2002, 71, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, G.; Li, Y.; Liu, X.; Liu, L.; Yang, K.; Wang, C.; Wei, S. Evaluation of the Associations of GC and CYP2R1 Genes and Gene-Obesity Interactions with Type 2 Diabetes Risk in a Chinese Rural Population. Ann. Nutr. Metab. 2020, 76, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, F.; Yu, S.; Zhang, D.; Wang, J.; Han, H.; Sun, H.; Xue, Y.; Ba, Y.; Wang, C.; et al. Triangular relationship between CYP2R1 gene polymorphism, serum 25(OH)D3 levels and T2DM in a Chinese rural population. Gene 2018, 678, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, L.; Li, C.; Gai, Z.; Li, Y. Vitamin D and Vitamin D Receptor: New Insights in the Treatment of Hypertension. Curr. Protein Pept. Sci. 2019, 20, 984–995. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.; Manson, J.E.; Buring, J.E.; Gaziano, J.M.; Sesso, H.D. A prospective study of plasma vitamin D metabolites, vitamin D receptor gene polymorphisms, and risk of hypertension in men. Eur. J. Nutr. 2013, 52, 1771–1779. [Google Scholar] [CrossRef]
- Legarth, C.; Grimm, D.; Wehland, M.; Bauer, J.; Krüger, M. The Impact of Vitamin D in the Treatment of Essential Hypertension. Int. J. Mol. Sci. 2018, 19, 455. [Google Scholar] [CrossRef]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin. Hypertens. 2018, 24, 9. [Google Scholar] [CrossRef]
- Simpson, R.U.; Hershey, S.H.; Nibbelink, K.A. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J. Steroid. Biochem. Mol. Biol. 2007, 103, 521–524. [Google Scholar] [CrossRef]
- Hajj, A.; Chedid, R.; Chouery, E.; Megarbané, A.; Gannagé-Yared, M.H. Relationship between vitamin D receptor gene polymorphisms, cardiovascular risk factors and adiponectin in a healthy young population. Pharmacogenomics 2016, 17, 1675–1686. [Google Scholar] [CrossRef]
- Wang, L.; Chu, A.; Buring, J.E.; Ridker, P.M.; Chasman, D.I.; Sesso, H.D. Common genetic variations in the vitamin D pathway in relation to blood pressure. Am. J. Hypertens. 2014, 27, 1387–1395. [Google Scholar] [CrossRef]
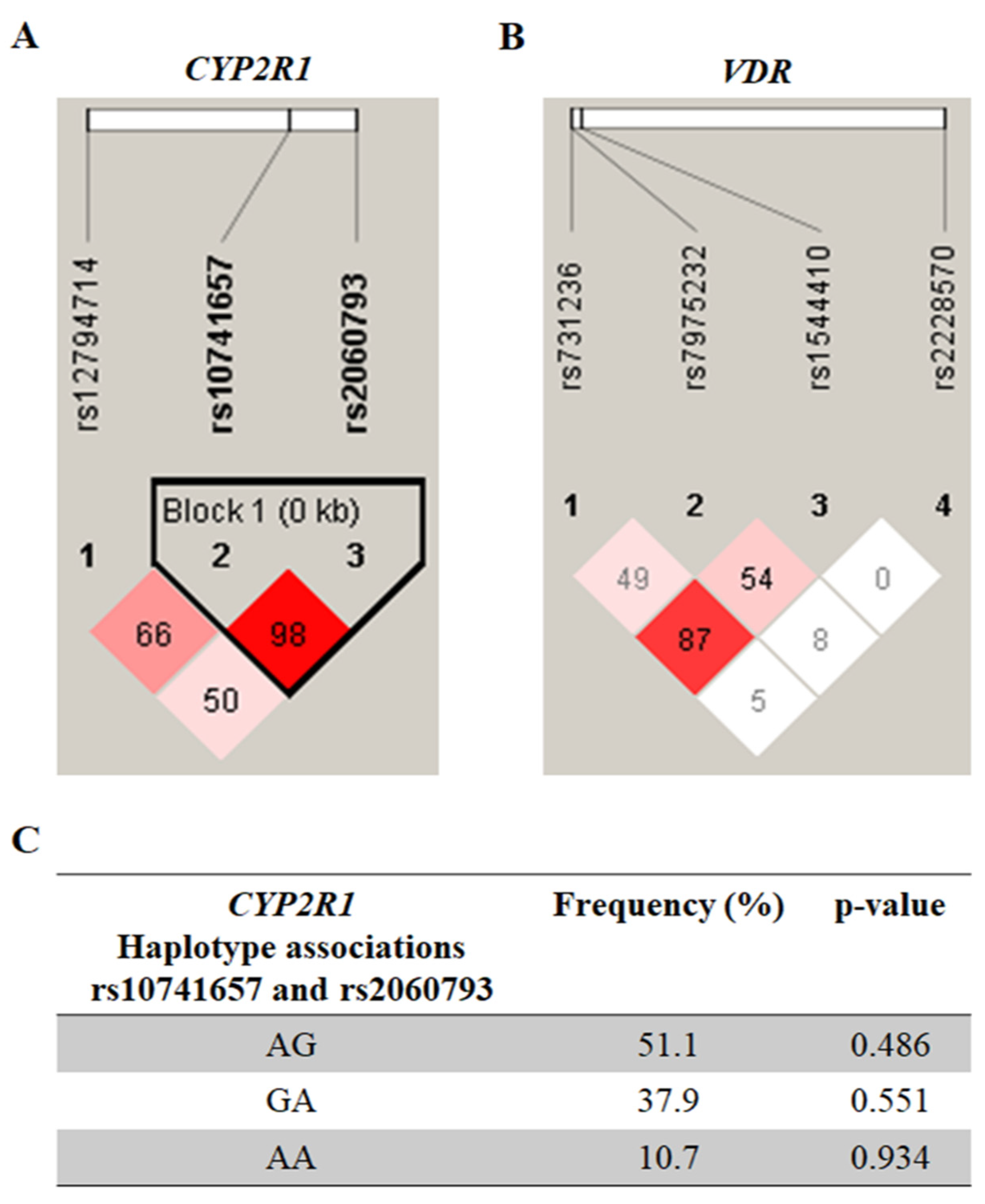
| Variables | Non-MS (n = 126) | MS (n = 48) | p-Value a |
|---|---|---|---|
| Age, mean (SD), years | 11 (10.1) | 11 (10.1) | 0.530 |
| Sex (female n (%)/male n (%) | 61 (48.4)/65 (51.6) | 22 (45.8)/26 (54.2) | 0.761 |
| BMI, mean (SD), kg/m2 | 26.5 (3.7) | 28.4 (4) | 0.003 |
| WC, mean (SD), cm | 86 (10) | 91 (17) | 0.017 |
| SBP, median (IQR), mmHg | 110 (109–120) | 120 (110–130) | 0.072 |
| DBP, median (IQR), mmHg | 70 (65–80) | 70 (60–80) | 0.302 |
| Fasting glucose, mean (SD), mg/dL | 92 (7) | 98 (8) | <0.001 |
| Total cholesterol, mean (SD), mg/dL | 172 (31) | 171 (36) | 0.980 |
| HDL-c, mean (SD), mg/dL | 42 (7) | 33 (5) | <0.001 |
| LDL-c, mean (SD), mg/dL | 110 (26) | 106 (27) | 0.381 |
| Triglycerides, median (IQR), mg/dL | 93 (71–119) | 158 (108–209) | <0.001 |
| 25-hydroxyvitamin D, mean (SD), ng/dL | 31.9 (10.3) | 29.9 (8.2) | 0.114 |
| 25-hydroxyvitamin D deficiency, n (%) | 9 (5.2) | 8 (4.7) | 0.082 |
| SNPs | Genotypes /Alleles | Non-MS (n = 126) | MS (n = 48) | ap-Value | OR | 95% CI | bp-Value |
|---|---|---|---|---|---|---|---|
| CYP2R1 | |||||||
| rs10741657 (A > G) | AA | 43 (34.1) | 20 (41.7) | 0.859 | 1.00 | - | - |
| AG | 67 (53.2) | 22 (45.8) | 0.71 | 0.34–1.45 | 0.362 | ||
| GG | 16 (12.7) | 6 (12.5) | 0.81 | 0.27–2.37 | 0.792 | ||
| A | 153 (60.7) | 62 (64.6) | 1.00 | - | - | ||
| G | 99 (39.3) | 34 (35.4) | 0.84 | 0.52–1.37 | 0.538 | ||
| rs2060793 (A > G) | AA | 17 (13.5) | 11 (22.9) | 0.999 | 1.00 | - | - |
| AG | 93 (73.8) | 30 (62.5) | 0.50 | 0.21–1.18 | 0.156 | ||
| GG | 16 (12.7) | 7 (14.6) | 0.68 | 0.21–2.17 | 0.567 | ||
| A | 127 (50.4) | 52 (54.2) | 1.00 | - | - | ||
| G | 125 (49.6) | 44 (45.8) | 0.86 | 0.54–1.36 | 0.550 | ||
| rs12794714 (A > G) | AA | 31 (24.6) | 7 (14.6) | 0.149 | 1.00 | - | - |
| AG | 75 (59.5) | 25 (52.1) | 1.48 | 0.58–3.77 | 0.502 | ||
| GG | 20 (15.9) | 16 (33.3) | 3.54 | 1.24–10.14 | 0.023 | ||
| A | 137 (54.4) | 39 (40.6) | 1.00 | - | - | ||
| G | 115 (45.6) | 57 (59.4) | 1.74 | 1.09–2.84 | 0.023 | ||
| VDR | |||||||
| rs2228570 (A > G) | AA | 57 (45.2) | 20 (41.7) | 0.587 | 1.00 | - | - |
| AG | 51 (40.5) | 20 (41.7) | 1.12 | 0.54–2.31 | 0.853 | ||
| GG | 18 (14.3) | 8 (16.7) | 1.27 | 0.48–3.36 | 0.620 | ||
| A | 165 (65.5) | 60 (62.5) | 1.00 | - | - | ||
| G | 87 (34.5) | 36 (37.5) | 1.14 | 0.70–1.85 | 0.617 | ||
| rs731236 (A > G) | AA | 28 (22.2) | 15 (31.2) | 0.435 | 1.00 | - | - |
| AG | 77 (61.1) | 27 (56.2) | 0.65 | 0.3–1.41 | 0.317 | ||
| GG | 21 (16.7) | 6 (12.5) | 0.53 | 0.18–1.61 | 0.296 | ||
| A | 133 (52.8) | 57 (59.4) | 1.00 | - | - | ||
| G | 119 (47.2) | 39 (40.6) | 0.76 | 0.47–1.22 | 0.280 | ||
| rs1544410 (T > C) | TT | 30 (23.8) | 12 (25.0) | 0.061 | 1.00 | - | - |
| TC | 82 (65.1) | 31 (64.6) | 0.95 | 0.43–2.08 | 0.999 | ||
| CC | 14 (11.1) | 5 (10.4) | 0.89 | 0.26–3.03 | 0.998 | ||
| T | 142 (56.3) | 55 (57.3) | 1.00 | - | - | ||
| C | 110 (43.7) | 41 (42.7) | 0.96 | 0.59–1.53 | 0.617 | ||
| rs7975232 (A > C) | AA | 45 (35.7) | 15 (31.2) | 0.217 | 1.00 | - | - |
| AC | 51 (40.5) | 19 (39.6) | 1.12 | 0.51–2.45 | 0.842 | ||
| CC | 30 (23.8) | 14 (29.2) | 1.40 | 0.59–3.32 | 0.509 | ||
| A | 141 (56) | 49 (51) | 1.00 | - | - | ||
| C | 111 (44) | 47 (49) | 1.22 | 0.76–1.94 | 0.470 |
| Variables | OR (95% CI) | p-Value |
|---|---|---|
| CYP2R1 genotypes (SNP) | ||
| GG (rs10741657) | 1.52 (0.52–4.43) | 0.443 |
| GG (rs2060793) | 1.45 (0.57–3.68) | 0.438 |
| GG (rs12794714) | 2.74 (1.14–6.58) | 0.024 |
| VDR genotypes (SNP) | ||
| GG (rs2228570) | 0.74 (0.27–2.04) | 0.561 |
| GG (rs731236) | 0.85 (0.23–3.18) | 0.807 |
| CC (rs1544410) | 1.25 (0.29–5.43) | 0.770 |
| CC (rs7975232) | 1.67 (0.74–3.76) | 0.218 |
| 25-hydroxyvitamin D | 0.97 (0.93–1.01) | 0.134 |
| SNPs/ Models | Genotypes | Abdominal Obesity | Hyperglycemia | Hypertension | Low HDL-c | High TG | VitD Deficiency | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| rs10741657 | |||||||||||||
| Dominant | AA | 1 | 0.099 | 1 | 0.187 | 1 | 0.060 | 1 | 0.236 | 1 | 0.488 | 1 | 0.499 |
| AG + GG | 0.97 (0.93–1.01) | 1.97 (0.72–5.38) | 2.89 (0.96–8.69) | 0.64 (0.30–1.34) | 1.35 (0.58–3.17) | 0.78 (0.38–1.60) | |||||||
| Recessive | AA + AG | 1 | 0.726 | 1 | 0.026 | 1 | 0.974 | 1 | 0.149 | 1 | 0.394 | 1 | 0.343 |
| GG | 1.01 (0.95–1.07) | 3.90 (1.18–12.92) | 1.03 (0.23–4.61) | 0.43 (0.14–1.35) | 1.67 (0.52–5.38) | 0.59 (0.20–1.74) | |||||||
| rs2060793 | |||||||||||||
| Dominant | AA | 1 | 0.738 | 1 | 0.037 | 1 | 0.970 | 1 | 0.101 | 1 | 0.490 | 1 | 0.226 |
| AG + GG | 0.99 (0.93–1.05) | 0.28 (0.08–0.92) | 1.03 (0.23–4.58) | 2.57 (0.83–7.95) | 0.67 (0.21–2.12) | 1.92 (0.67–5.53) | |||||||
| Recessive | AA + AG | 1 | 0.830 | 1 | 0.076 | 1 | 0.430 | 1 | 0.593 | 1 | 0.358 | 1 | 0.162 |
| GG | 1.04 (0.99–1.10) | 0.15 (0.02–1.22) | 0.59 (0.16–2.17) | 1.29 (0.50–3.31) | 1.60 (0.59–4.31) | 1.97 (0.76–5.11) | |||||||
| rs12794714 | |||||||||||||
| Dominant | AA | 1 | 0.406 | 1 | 0.415 | 1 | 0.409 | 1 | 0.573 | 1 | 0.739 | 1 | 0.079 |
| AG + GG | 1.02 (0.97–1.07) | 0.65 (0.23–1.88) | 1.80 (0.45–7.21) | 1.28 (0.54–3.02) | 0.85 (0.33–2.19) | 2.11 (0.32–4.83) | |||||||
| Recessive | AA + AG | 1 | 0.542 | 1 | 0.992 | 1 | 0.994 | 1 | 0.288 | 1 | 0.259 | 1 | 0.308 |
| GG | 1.01 (0.97–1.06) | 1.01 (0.33–3.09) | 1.01 (0.31–3.21) | 1.62 (0.66–3.97) | 1.70 (0.68–4.30) | 1.57 (0.66–3.72) | |||||||
| SNPs/ Models | Genotypes | Abdominal Obesity | Hyperglycemia | Hypertension | Low HDL-c | High TG | VitD Deficiency | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| rs2228570 | |||||||||||||
| Dominant | AA | 1 | 0.412 | 1 | 0.167 | 1 | 0.597 | 1 | 0.945 | 1 | 0.621 | 1 | 0.064 |
| AG + GG | 1.02 (0.98–1.06) | 0.52 (0.21–1.31) | 1.31 (0.48–3.60) | 1.03 (0.50–2.11) | 1.23 (0.54–2.78) | 0.51 (0.25–1.04) | |||||||
| Recessive | AA + AG | 1 | 0.074 | 1 | 0.634 | 1 | 0.598 | 1 | 0.532 | 1 | 0.161 | 1 | 0.485 |
| GG | 1.05 (0.10–1.11) | 0.72 (0.18–2.83) | 0.69 (0.17–2.77) | 0.72 (0.26–2.00) | 0.33 (0.07–1.55) | 0.70 (0.25–1.92) | |||||||
| rs731236 | |||||||||||||
| Dominant | AA | 1 | 0.366 | 1 | 0.333 | 1 | 0.506 | 1 | 0.435 | 1 | 0.814 | 1 | 0.363 |
| AG + GG | 1.02 (0.98–1.07) | 0.69 (0.55–5.82) | 1.79 (0.23–2.07) | 0.72 (0.32–1.64) | 0.90 (0.37–2.20) | 0.69 (0.31–1.53) | |||||||
| Recessive | AA + AG | 1 | 0.326 | 1 | 0.675 | 1 | 0.300 | 1 | 0.874 | 1 | 0.862 | 1 | 0.903 |
| GG | 0.98 (0.93–1.03) | 0.75 (0.20–2.88) | 1.96 (0.55–6.97) | 1.08 (0.41–2.85) | 0.91 (0.30–2.71) | 1.06 (0.42–2.69) | |||||||
| rs1544410 | |||||||||||||
| Dominant | TT | 1 | 0.577 | 1 | 0.221 | 1 | 0.385 | 1 | 0.898 | 1 | 0.757 | 1 | 0.121 |
| TC + CC | 1.01 (0.97–1.06) | 2.26 (0.61–8.33) | 1.76 (0.49–6.24) | 1.06 (0.46–2.42) | 0.87 (0.35–2.15) | 0.52 (0.23–1.19) | |||||||
| Recessive | TT + TC | 1 | 0.249 | 1 | 0.901 | 1 | 0.536 | 1 | 0.518 | 1 | 0.909 | 1 | 0.881 |
| CC | 0.97 (0.91–1.02) | 1.09 (0.27–4.37) | 1.61 (0.35–7.31) | 1.45 (0.47–4.44) | 1.07 (0.32–3.62) | 0.92 (0.32–2.67) | |||||||
| rs7975232 | |||||||||||||
| Dominant | AA | 1 | 0.521 | 1 | 0.820 | 1 | 0.281 | 1 | 0.670 | 1 | 0.606 | 1 | 0.978 |
| AC + CC | 1.01 (0.97–1.05) | 0.894 (0.34–2.35) | 1.88 (0.60–5.89) | 1.18 (0.55–2.50) | 0.80 (0.35–1.84) | 0.99 (0.48–2.04) | |||||||
| Recessive | AA + AC | 1 | 0.230 | 1 | 0.183 | 1 | 0.002 | 1 | 0.299 | 1 | 0.126 | 1 | 0.509 |
| CC | 0.97 (0.93–1.02) | 2.00 (0.72–5.53) | 5.91 (1.91–18.32) | 1.59 (0.67–3.78) | 0.42 (0.14–1.28) | 0.75 (0.33–1.74) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, E.P.d.S.; Lima, S.C.V.d.C.; Galdino, O.A.; Arrais, R.F.; de Souza, K.S.C.; de Rezende, A.A. Association of CYP2R1 and VDR Polymorphisms with Metabolic Syndrome Components in Non-Diabetic Brazilian Adolescents. Nutrients 2022, 14, 4612. https://doi.org/10.3390/nu14214612
Araújo EPdS, Lima SCVdC, Galdino OA, Arrais RF, de Souza KSC, de Rezende AA. Association of CYP2R1 and VDR Polymorphisms with Metabolic Syndrome Components in Non-Diabetic Brazilian Adolescents. Nutrients. 2022; 14(21):4612. https://doi.org/10.3390/nu14214612
Chicago/Turabian StyleAraújo, Eduarda Pontes dos Santos, Severina Carla Vieira da Cunha Lima, Ony Araújo Galdino, Ricardo Fernando Arrais, Karla Simone Costa de Souza, and Adriana Augusto de Rezende. 2022. "Association of CYP2R1 and VDR Polymorphisms with Metabolic Syndrome Components in Non-Diabetic Brazilian Adolescents" Nutrients 14, no. 21: 4612. https://doi.org/10.3390/nu14214612
APA StyleAraújo, E. P. d. S., Lima, S. C. V. d. C., Galdino, O. A., Arrais, R. F., de Souza, K. S. C., & de Rezende, A. A. (2022). Association of CYP2R1 and VDR Polymorphisms with Metabolic Syndrome Components in Non-Diabetic Brazilian Adolescents. Nutrients, 14(21), 4612. https://doi.org/10.3390/nu14214612






