Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain and Children’s Cognitive Development: A Birth Cohort Study
Abstract
1. Introduction
2. Materials and Methods
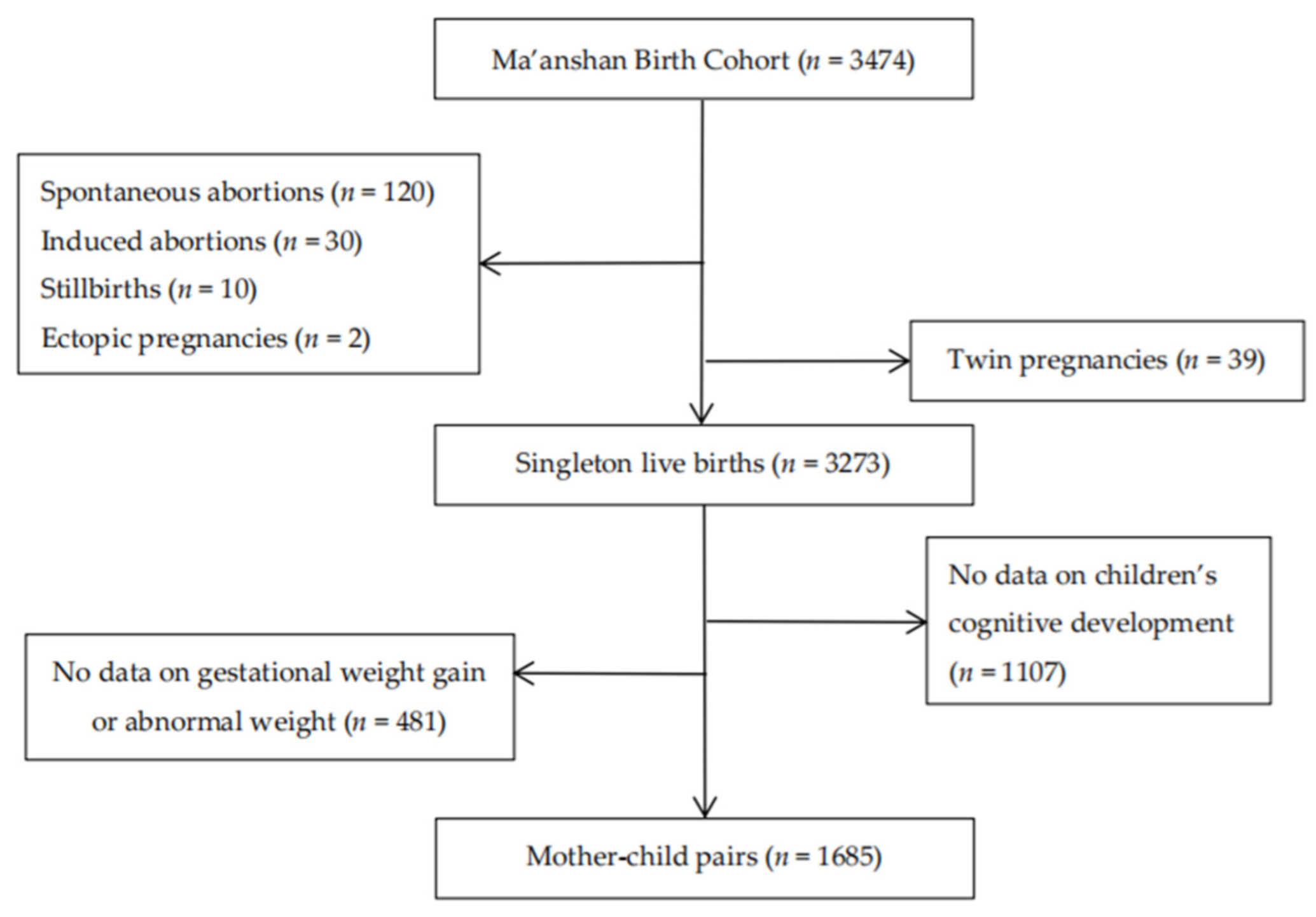
2.1. Study Population
2.2. Assessment of Maternal Pre-Pregnancy BMI and GWG
2.3. Children’s Cognitive Development Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Participants
3.2. Association between Pre-Pregnancy BMI and GWG
3.3. Distribution of Children’s Cognitive Ability under Different Pre-Pregnancy BMI and GWG Categories
3.4. The Effect of Maternal Pre-Pregnancy BMI on Children’s Cognitive Development under Different GWG Classifications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantor, A.G.; Jungbauer, R.M.; McDonagh, M.; Blazina, I.; Marshall, N.E.; Weeks, C.; Fu, R.; LeBlanc, E.S.; Chou, R. Counseling and Behavioral Interventions for Healthy Weight and Weight Gain in Pregnancy: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 2094–2109. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.; Yaktine, A. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. In Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar]
- Windham, G.C.; Anderson, M.; Lyall, K.; Daniels, J.L.; Kral, T.V.E.; Croen, L.A.; Levy, S.E.; Bradley, C.B.; Cordero, C.; Young, L.; et al. Maternal Pre-pregnancy Body Mass Index and Gestational Weight Gain in Relation to Autism Spectrum Disorder and other Developmental Disorders in Offspring. Autism. Res. 2019, 12, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Matias, S.L.; Pearl, M.; Lyall, K.; Croen, L.A.; Kral, T.V.E.; Fallin, D.; Lee, L.C.; Bradley, C.B.; Schieve, L.A.; Windham, G.C. Maternal prepregnancy weight and gestational weight gain in association with autism and developmental disorders in offspring. Obesity 2021, 29, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Deputy, N.P.; Dub, B.; Sharma, A.J. Prevalence and Trends in Prepregnancy Normal Weight–48 States, New York City, and District of Columbia, 2011-2015. MMWR. Morb. Mortal. Wkly. Rep. 2018, 66, 1402–1407. [Google Scholar] [CrossRef]
- Fayed, A.; Wahabi, H.A.; Esmaeil, S.; Elkouny, R.; Elmorshedy, H.; Bakhsh, H. Independent effect of gestational weight gain and prepregnancy obesity on pregnancy outcomes among Saudi women: A sub-cohort analysis from Riyadh mother and baby cohort study (RAHMA). PLoS ONE 2022, 17, e0262437. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019, 126, 984–995. [Google Scholar] [CrossRef]
- Perichart-Perera, O.; Muñoz-Manrique, C.; Reyes-López, A.; Tolentino-Dolores, M.; Espino, Y.S.S.; Ramírez-González, M.C. Metabolic markers during pregnancy and their association with maternal and newborn weight status. PLoS ONE 2017, 12, e0180874. [Google Scholar] [CrossRef]
- Widen, E.M.; Kahn, L.G.; Cirillo, P.; Cohn, B.; Kezios, K.L.; Factor-Litvak, P. Prepregnancy overweight and obesity are associated with impaired child neurodevelopment. Matern. Child. Nutr. 2018, 14, e12481. [Google Scholar] [CrossRef]
- Tong, L.; Kalish, B.T. The impact of maternal obesity on childhood neurodevelopment. J. Perinatol. 2021, 41, 928–939. [Google Scholar] [CrossRef]
- Keim, S.A.; Pruitt, N.T. Gestational weight gain and child cognitive development. Int. J. Epidemiol. 2012, 41, 414–422. [Google Scholar] [CrossRef]
- Martínez-Hortelano, J.A.; Álvarez-Bueno, C.; Cavero-Redondo, I.; Herráiz-Adillo, Á.; Berlanga-Macías, C.; Martínez-Vizcaíno, V. Gestational weight gain and offspring’s cognitive skills: A systematic review and meta-analysis. BMC Pediatr. 2020, 20, 533. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S.; et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021, 33, 923–938.e926. [Google Scholar] [CrossRef] [PubMed]
- Coo, H.; Fabrigar, L.; Davies, G.; Fitzpatrick, R.; Flavin, M. Are observed associations between a high maternal prepregnancy body mass index and offspring IQ likely to be causal? J. Epidemiol. Community Health 2019, 73, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, E.; Gardiner, J.; Williams, C.; Melhuish, E.; Barnes, J.; Sutcliffe, A. Maternal prepregnancy BMI and child cognition: A longitudinal cohort study. Pediatrics 2013, 131, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.J.; Richardson, G.A.; Hutcheon, J.A.; Himes, K.P.; Brooks, M.M.; Day, N.L.; Bodnar, L.M. Maternal Obesity and Excessive Gestational Weight Gain Are Associated with Components of Child Cognition. J. Nutr. 2015, 145, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Pugh, S.J.; Hutcheon, J.A.; Richardson, G.A.; Brooks, M.M.; Himes, K.P.; Day, N.L.; Bodnar, L.M. Child academic achievement in association with pre-pregnancy obesity and gestational weight gain. J. Epidemiol. Community Health 2016, 70, 534–540. [Google Scholar] [CrossRef]
- Widen, E.M.; Nichols, A.R.; Kahn, L.G.; Factor-Litvak, P.; Insel, B.J.; Hoepner, L.; Dube, S.M.; Rauh, V.; Perera, F.; Rundle, A. Prepregnancy obesity is associated with cognitive outcomes in boys in a low-income, multiethnic birth cohort. BMC Pediatr. 2019, 19, 507. [Google Scholar] [CrossRef]
- Hinkle, S.N.; Albert, P.S.; Sjaarda, L.A.; Grewal, J.; Grantz, K.L. Trajectories of maternal gestational weight gain and child cognition assessed at 5 years of age in a prospective cohort study. J. Epidemiol. Community Health 2016, 70, 696–703. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and managing the global epidemic. In Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; Volume 894, pp. i–xii, 1–253. [Google Scholar]
- Ji, H.; Fan, H.; Ai, J.; Shi, C.; Bi, J.; Chen, Y.H.; Lu, X.P.; Chen, Q.H.; Tian, J.M.; Bao, C.J.; et al. Neurocognitive deficits and sequelae following severe hand, foot, and mouth disease from 2009 to 2017, in JiangSu Province, China: A long-term follow-up study. Int. J. Infect. Dis. 2022, 115, 245–255. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Wu, X.Y.; Yan, S.Q.; Huang, K.; Tong, J.; Gao, H.; Xie, Y.; Tao, S.M.; Ding, P.; Zhu, P.; et al. Domain- and trimester-specific effect of prenatal phthalate exposure on preschooler cognitive development in the Ma’anshan Birth Cohort (MABC) study. Environ. Int. 2020, 142, 105882. [Google Scholar] [CrossRef]
- Diemer, E.W.; Hudson, J.I.; Javaras, K.N. More (Adjustment) Is Not Always Better: How Directed Acyclic Graphs Can Help Researchers Decide Which Covariates to Include in Models for the Causal Relationship between an Exposure and an Outcome in Observational Research. Psychother. Psychosom. 2021, 90, 289–298. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Infant and Young Child Feeding: Model Chapter for Textbooks for Medical Students and Allied Health Professionals; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Adane, A.A.; Mishra, G.D.; Tooth, L.R. Diabetes in Pregnancy and Childhood Cognitive Development: A Systematic Review. Pediatrics 2016, 137, e20154234. [Google Scholar] [CrossRef] [PubMed]
- Gumusoglu, S.B.; Chilukuri, A.S.S.; Santillan, D.A.; Santillan, M.K.; Stevens, H.E. Neurodevelopmental Outcomes of Prenatal Preeclampsia Exposure. Trends Neurosci. 2020, 43, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Perumal, N.; Manji, K.P.; Darling, A.M.; Kisenge, R.R.; Kvestad, I.; Hysing, M.; Belinger, D.C.; Urassa, W.; Strand, T.A.; Duggan, C.P.; et al. Gestational Age, Birth Weight, and Neurocognitive Development in Adolescents in Tanzania. J. Pediatr. 2021, 236, 194–203. [Google Scholar] [CrossRef]
- Koh, K. Maternal breastfeeding and children’s cognitive development. Soc. Sci. Med. 2017, 187, 101–108. [Google Scholar] [CrossRef]
- Sadruddin, A.F.A.; Ponguta, L.A.; Zonderman, A.L.; Wiley, K.S.; Grimshaw, A.; Panter-Brick, C. How do grandparents influence child health and development? A systematic review. Soc. Sci. Med. 2019, 239, 112476. [Google Scholar] [CrossRef]
- Guilloty, N.I.; Soto, R.; Anzalota, L.; Rosario, Z.; Cordero, J.F.; Palacios, C. Diet, Pre-pregnancy BMI, and Gestational Weight Gain in Puerto Rican Women. Matern. Child. Health J. 2015, 19, 2453–2461. [Google Scholar] [CrossRef]
- Neves, P.A.R.; Gatica-Domínguez, G.; Santos, I.S.; Bertoldi, A.D.; Domingues, M.; Murray, J.; Silveira, M.F. Poor maternal nutritional status before and during pregnancy is associated with suspected child developmental delay in 2-year old Brazilian children. Sci. Rep. 2020, 10, 1851. [Google Scholar] [CrossRef]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal prepregnancy obesity and child neurodevelopment in the Collaborative Perinatal Project. Int. J. Epidemiol. 2014, 43, 783–792. [Google Scholar] [CrossRef]
- Lee, P.; Tse, L.A.; László, K.D.; Wei, D.; Yu, Y.; Li, J. Association of maternal gestational weight gain with intellectual developmental disorder in the offspring: A nationwide follow-up study in Sweden. BJOG 2022, 129, 540–549. [Google Scholar] [CrossRef]
- Monthé-Drèze, C.; Rifas-Shiman, S.L.; Gold, D.R.; Oken, E.; Sen, S. Maternal obesity and offspring cognition: The role of inflammation. Pediatr. Res. 2019, 85, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Girchenko, P.; Lahti-Pulkkinen, M.; Heinonen, K.; Reynolds, R.M.; Laivuori, H.; Lipsanen, J.; Villa, P.M.; Hämäläinen, E.; Kajantie, E.; Lahti, J.; et al. Persistently High Levels of Maternal Antenatal Inflammation Are Associated with and Mediate the Effect of Prenatal Environmental Adversities on Neurodevelopmental Delay in the Offspring. Biol. Psychiatry 2020, 87, 898–907. [Google Scholar] [CrossRef]
- Sureshchandra, S.; Marshall, N.E.; Wilson, R.M.; Barr, T.; Rais, M.; Purnell, J.Q.; Thornburg, K.L.; Messaoudi, I. Inflammatory Determinants of Pregravid Obesity in Placenta and Peripheral Blood. Front. Physiol. 2018, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Eastman, A.J.; Moore, R.E.; Townsend, S.D.; Gaddy, J.A.; Aronoff, D.M. The Influence of Obesity and Associated Fatty Acids on Placental Inflammation. Clin. Ther. 2021, 43, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Khambadkone, S.G.; Cordner, Z.A.; Tamashiro, K.L.K. Maternal stressors and the developmental origins of neuropsychiatric risk. Front. Neuroendocrinol. 2020, 57, 100834. [Google Scholar] [CrossRef] [PubMed]
- Cordner, Z.A.; Khambadkone, S.G.; Boersma, G.J.; Song, L.; Summers, T.N.; Moran, T.H.; Tamashiro, K.L.K. Maternal high-fat diet results in cognitive impairment and hippocampal gene expression changes in rat offspring. Exp. Neurol. 2019, 318, 92–100. [Google Scholar] [CrossRef]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Nutritional and developmental programming effects of insulin. J. Neuroendocrinol. 2021, 33, e12933. [Google Scholar] [CrossRef]
- Gage, S.H.; Lawlor, D.A.; Tilling, K.; Fraser, A. Associations of maternal weight gain in pregnancy with offspring cognition in childhood and adolescence: Findings from the Avon Longitudinal Study of Parents and Children. Am. J. Epidemiol. 2013, 177, 402–410. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Magnano San Lio, R.; Agodi, A. Maternal Dietary Patterns Are Associated with Pre-Pregnancy Body Mass Index and Gestational Weight Gain: Results from the “Mamma & Bambino” Cohort. Nutrients 2019, 11, 1308. [Google Scholar] [CrossRef]
- Freitas-Vilela, A.A.; Pearson, R.M.; Emmett, P.; Heron, J.; Smith, A.; Emond, A.; Hibbeln, J.R.; Castro, M.B.T.; Kac, G. Maternal dietary patterns during pregnancy and intelligence quotients in the offspring at 8 years of age: Findings from the ALSPAC cohort. Matern. Child. Nutr. 2018, 14, e12431. [Google Scholar] [CrossRef]
- Tzeng, Y.L.; Chen, S.L.; Chen, C.F.; Wang, F.C.; Kuo, S.Y. Sleep Trajectories of Women Undergoing Elective Cesarean Section: Effects on Body Weight and Psychological Well-Being. PLoS ONE 2015, 10, e0129094. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, W.; Tan, T.; He, W.; Dong, Z.; Wang, Y.T.; Han, H. Maternal sleep deprivation at different stages of pregnancy impairs the emotional and cognitive functions, and suppresses hippocampal long-term potentiation in the offspring rats. Mol. Brain 2016, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.M.; Dalman, C.; Rai, D.; Lee, B.K.; Karlsson, H. The Association of Paternal IQ With Autism Spectrum Disorders and Its Comorbidities: A Population-Based Cohort Study. J. Am. Acad. Child. Adolesc. Psychiatry 2020, 59, 410–421. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Pre-Pregnancy BMI (kg/m2) | p Values | |||
|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25.0–29.9 | ≥30 | ||
| Demographic characteristics | |||||
| Maternal educational level [n(%)] | 0.08 | ||||
| Junior high school or below | 40(15.3) | 245(19.8) | 40(25.2) | 6(20.7) | |
| Senior middle school | 72(27.6) | 257(20.8) | 37(23.3) | 6(20.7) | |
| Junior college or above | 149(57.1) | 734(59.4) | 82(51.6) | 17(58.6) | |
| Paternal educational level [n(%)] | <0.001 | ||||
| Junior high school or below | 28(10.7) | 160(12.9) | 41(25.8) | 7(24.1) | |
| Senior middle school | 84(32.2) | 355(28.7) | 43(27.0) | 8(27.6) | |
| Junior college or above | 149(57.1) | 721(58.3) | 75(47.2) | 14(48.3) | |
| Household monthly income per capita (yuan) [n(%)] | 0.46 | ||||
| ≤2500 | 78(29.9) | 340(27.5) | 52(32.7) | 11(37.9) | |
| 2500~4000 | 94(36.0) | 511(41.3) | 62(39.0) | 11(37.9) | |
| >4000 | 89(34.1) | 385(31.1) | 45(28.3) | 7(24.1) | |
| Maternal characteristics | |||||
| Age (years) [Mean(SD)] | 25.6(2.9) | 26.6(3.4) | 28.3(4.4) | 28.0(3.8) | <0.001 |
| IQ [Mean(SD)] | 96.7(10.4) | 96.3(10.8) | 95.0(10.7) | 99.6(9.0) | 0.13 |
| Occupation [n(%)] | 0.50 | ||||
| No job | 116(44.4) | 485(39.2) | 69(43.4) | 14(48.3) | |
| Mental job | 121(46.4) | 621(50.2) | 71(44.7) | 11(37.9) | |
| Manual job | 24(9.2) | 130(10.5) | 19(11.9) | 4(13.8) | |
| Parity [n(%)] | <0.001 | ||||
| Nulliparous | 249(95.4) | 1120(90.6) | 130(81.8) | 22(75.9) | |
| Multiparous | 12(4.6) | 116(9.4) | 29(18.2) | 7(24.1) | |
| Previous adverse pregnancy outcomes [n(%)] | 0.001 | ||||
| Did not have | 150(57.5) | 769(62.2) | 73(45.9) | 15(51.7) | |
| Had | 111(42.5) | 467(37.8) | 86(54.1) | 14(48.3) | |
| Pregnancy complications [n(%)] | <0.001 | ||||
| Did not have | 245(93.9) | 1044(84.5) | 94(59.1) | 16(55.2) | |
| Had | 16(6.1) | 192(15.5) | 65(40.9) | 13(44.8) | |
| Smoking during pregnancy [n(%)] | 0.20 | ||||
| No | 251(96.2) | 1188(96.1) | 148(93.1) | 29(100.0) | |
| Yes | 10(3.8) | 48(3.9) | 11(6.9) | 0 | |
| Drinking during pregnancy [n(%)] | 0.81 | ||||
| No | 243(93.1) | 1149(93.0) | 150(94.3) | 28(96.6) | |
| Yes | 18(6.9) | 87(7.0) | 9(5.7) | 1(3.4) | |
| Children’s characteristics | |||||
| Gestational age (week) [Mean(SD)] | 39.1(1.3) | 39.2(1.3) | 38.9(1.5) | 38.0(2.7) | <0.001 |
| Birth weight (g)a [Mean(SD)] | 3248.3(444.9) | 3385.8(410.5) | 3502.7(495.2) | 3280.0(682.7) | <0.001 |
| Children’s sex [n(%)] | 0.97 | ||||
| Boy | 137(52.5) | 653(52.8) | 84(52.8) | 14(48.3) | |
| Girl | 124(47.5) | 583(47.2) | 75(47.2) | 15(51.7) | |
| Exclusive breastfeeding for 6 months a [n(%)] | 0.98 | ||||
| No | 228(87.4) | 1093(88.4) | 144(90.6) | 25(86.2) | |
| Yes | 25(9.6) | 112(9.1) | 14(8.8) | 3(10.3) | |
| Main caregivers before 3 years of age a [n(%)] | 0.85 | ||||
| Parents | 126(48.3) | 634(51.3) | 80(50.3) | 14(48.3) | |
| Grandparents | 133(51.0) | 594(48.1) | 79(49.7) | 14(48.3) | |
| GWG | Pre-Pregnancy BMI (kg/m2) | p Value | |||
|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25.0–29.9 | ≥30 | ||
| Inadequate GWG | 27(10.3) | 104(8.4) | 4(2.5) | 3(10.3) | <0.001 |
| Adequate GWG | 119(45.6) | 419(33.9) | 21(13.2) | 2(6.9) | |
| Excessive GWG | 115(44.1) | 713(57.7) | 134(84.3) | 24(82.8) | |
| GWG Classifications | Inadequate GWG | Adequate GWG | Excessive GWG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Pregnancy BMI | <18.5 | 18.5~24.9 | 25.0~29.9 | ≥30 | <18.5 | 18.5~24.9 | 25.0~29.9 | ≥30 | <18.5 | 18.5~24.9 | 25.0~29.9 | ≥30 | |
| VCI | 1 | 26(96.3) | 95(91.3) | 4(100.0) | 2(66.7) | 114(95.8) | 394(94.0) | 18(85.7) | 2(100.0) | 102(88.7) | 671(94.1) | 124(92.5) | 18(75.0) |
| 2 | 1(3.7) | 9(8.7) | 0 | 1(33.3) | 5(4.2) | 25(6.0) | 3(14.3) | 0 | 13(11.3) | 42(5.9) | 10(7.5) | 6(25.0) | |
| VSI | 1 | 21(77.8) | 96(92.3) | 3(75.0) | 3(100.0) | 117(98.3) | 386(92.1) | 20(95.2) | 1(50.0) | 99(86.1) | 644(90.3) | 116(86.6) | 21(87.5) |
| 2 | 6(22.2) | 8(7.7) | 1(25.0) | 0 | 2(1.7) | 33(7.9) | 1(4.8) | 1(50.0) | 16(13.9) | 69(9.7) | 18(13.4) | 3(12.5) | |
| FRI | 1 | 23(100.0) | 84(87.5) | 3(75.0) | 3(100.0) | 98(91.6) | 334(90.0) | 15(93.8) | 1(50.0) | 90(89.1) | 572(92.4) | 110(91.7) | 20(95.2) |
| 2 | 0 | 12(12.5) | 1(25.0) | 0 | 9(8.4) | 37(10.0) | 1(6.3) | 1(50.0) | 11(10.9) | 47(7.6) | 10(8.3) | 1(4.8) | |
| WMI | 1 | 25(92.6) | 95(91.3) | 4(100.0) | 2(66.7) | 110(92.4) | 379(90.5) | 20(95.2) | 2(100.0) | 100(87.0) | 658(92.3) | 119(88.8) | 22(91.7) |
| 2 | 2(7.4) | 9(8.7) | 0 | 1(33.3) | 9(7.6) | 40(9.5) | 1(4.8) | 0 | 15(13.0) | 55(7.7) | 15(11.2) | 2(8.3) | |
| PSI | 1 | 19(82.6) | 85(88.5) | 4(100.0) | 3(100.0) | 96(89.7) | 328(88.4) | 14(87.5) | 2(100.0) | 88(87.1) | 554(89.5) | 98(81.7) | 18(85.7) |
| 2 | 4(17.4) | 11(11.5) | 0 | 0 | 11(10.3) | 43(11.6) | 2(12.5) | 0 | 13(12.9) | 65(10.5) | 22(18.3) | 3(14.3) | |
| FSIQ | 1 | 25(92.6) | 98(94.2) | 4(100.0) | 2(66.7) | 115(96.6) | 395(94.3) | 19(90.5) | 2(100.0) | 101(87.8) | 680(95.4) | 123(91.8) | 20(83.3) |
| 2 | 2(7.4) | 6(5.8) | 0 | 1(33.3) | 4(3.4) | 24(5.7) | 2(9.5) | 0 | 14(12.2) | 33(4.6) | 11(8.2) | 4(16.7) | |
| GWG Classifications | Inadequate GWG | Adequate GWG | Excessive GWG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Pregnancy BMI | <18.5 | 18.5~24.9 | ≥25.0 | <18.5 | 18.5~24.9 | ≥25.0 | <18.5 | 18.5~24.9 | 25.0~29.9 | ≥30 | |
| VCI | Model 1 | 0.43 (0.05–3.38) | Ref | 1.65 (0.21–13.03) | 0.70 (0.27–1.84) | Ref | 2.19 (0.66–7.24) | 1.92 (1.03–3.58) | Ref | 1.27 (0.64–2.53) | 4.24 (1.80–9.98) |
| Model 2 | 0.59 (0.05–6.99) | Ref | 1.56 (0.13–19.42) | 0.80 (0.30–2.15) | Ref | 2.12 (0.60–7.54) | 1.78 (0.94–3.35) | Ref | 1.05 (0.51–2.18) | 3.71 (1.49–9.22) | |
| VSI | Model 1 | 2.89 (1.00–8.33) | Ref | 1.86 (0.23–14.85) | 0.21 (0.05–0.89) | Ref | 1.10 (0.27–4.60) | 1.44 (0.84–2.48) | Ref | 1.39 (0.83–2.33) | 1.29 (0.41–4.10) |
| Model 2 | 2.35 (0.67–8.29) | Ref | 2.34 (0.19–28.96) | 0.22 (0.05–0.92) | Ref | 1.42 (0.32–6.26) | 1.27 (0.73–2.20) | Ref | 1.18 (0.69–2.02) | 1.08 (0.33–3.51) | |
| FRI | Model 1 | - | Ref | 1.14 (0.15–8.79) | 0.84 (0.41–1.75) | Ref | 1.11 (0.27–4.62) | 1.43 (0.74–2.77) | Ref | 1.10 (0.56–2.17) | 0.63 (0.09–4.55) |
| Model 2 | - | Ref | 1.32 (0.13–13.59) | 0.86 (0.41–1.82) | Ref | 1.14 (0.26–5.06) | 1.34 (0.69–2.64) | Ref | 0.87 (0.42–1.82) | 0.37 (0.05–2.71) | |
| WMI | Model 1 | 0.86 (0.19–3.96) | Ref | 1.65 (0.21–13.03) | 0.79 (0.38–1.63) | Ref | 0.46 (0.06–3.31) | 1.69 (0.96–2.99) | Ref | 1.45 (0.82–2.57) | 1.08 (0.26–4.43) |
| Model 2 | 1.77 (0.29–10.72) | Ref | 1.44 (0.13–16.00) | 0.83 (0.39–1.76) | Ref | 0.51 (0.07–3.81) | 1.64 (0.92–2.92) | Ref | 1.49 (0.82–2.70) | 1.03 (0.25–4.30) | |
| PSI | Model 1 | 1.52 (0.48–4.77) | Ref | - | 0.89 (0.46–1.72) | Ref | 0.96 (0.23–3.96) | 1.23 (0.68–2.22) | Ref | 1.75 (1.08–2.83) | 1.36 (0.43–4.33) |
| Model 2 | 1.95 (0.55–6.97) | Ref | - | 0.83 (0.42–1.62) | Ref | 1.55 (0.35–6.78) | 1.19 (0.65–2.18) | Ref | 1.69 (1.02–2.81) | 1.19 (0.37–3.85) | |
| FSIQ | Model 1 | 1.28 (0.26–6.36) | Ref | 2.48 (0.30–20.57) | 0.59 (0.20–1.69) | Ref | 1.52 (0.36–6.42) | 2.63 (1.41–4.92) | Ref | 1.77 (0.90–3.51) | 3.60 (1.28–10.16) |
| Model 2 | 1.23 (0.18–8.30) | Ref | 1.57 (0.11–21.85) | 0.60 (0.21–1.76) | Ref | 2.30 (0.52–10.22) | 2.53 (1.34–4.76) | Ref | 1.40 (0.68–2.89) | 2.81 (0.95–8.25) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.; Lu, J.; Yan, S.; Tao, F.; Huang, K. Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain and Children’s Cognitive Development: A Birth Cohort Study. Nutrients 2022, 14, 4613. https://doi.org/10.3390/nu14214613
Hao X, Lu J, Yan S, Tao F, Huang K. Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain and Children’s Cognitive Development: A Birth Cohort Study. Nutrients. 2022; 14(21):4613. https://doi.org/10.3390/nu14214613
Chicago/Turabian StyleHao, Xuemei, Jingru Lu, Shuangqin Yan, Fangbiao Tao, and Kun Huang. 2022. "Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain and Children’s Cognitive Development: A Birth Cohort Study" Nutrients 14, no. 21: 4613. https://doi.org/10.3390/nu14214613
APA StyleHao, X., Lu, J., Yan, S., Tao, F., & Huang, K. (2022). Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain and Children’s Cognitive Development: A Birth Cohort Study. Nutrients, 14(21), 4613. https://doi.org/10.3390/nu14214613





