Aging Increases Susceptibility to Develop Cardiac Hypertrophy following High Sugar Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care and Treatment Groups
2.2. In Vivo Plantarflexor Function
2.3. Echocardiography
2.4. Serum Analyses
2.5. Mitochondrial Isolation and Respirometry
2.6. Histology
2.7. Gene Expression
2.8. Western Blotting
2.9. Statistical Analyses
3. Results
3.1. Old Mice Consuming a HS Diet for More Than 1 Week Became Frail and Developed Exacerbated Cardiac Hypertrophy
3.2. HS Diet for 1 Week Does Not Lead to Obesity or Hyperinsulinemia in Either Young or Old Mice
3.3. HS Diet for 1 Week Leads to Cardiac Hypertrophy with Preserved Function Specifically in Old Mice, an Effect Prevented by Elamepretide
3.4. Isolated Mitochondria from HS-Fed Mice Did Not Exhibit Significant Differences in Function or Protein Content
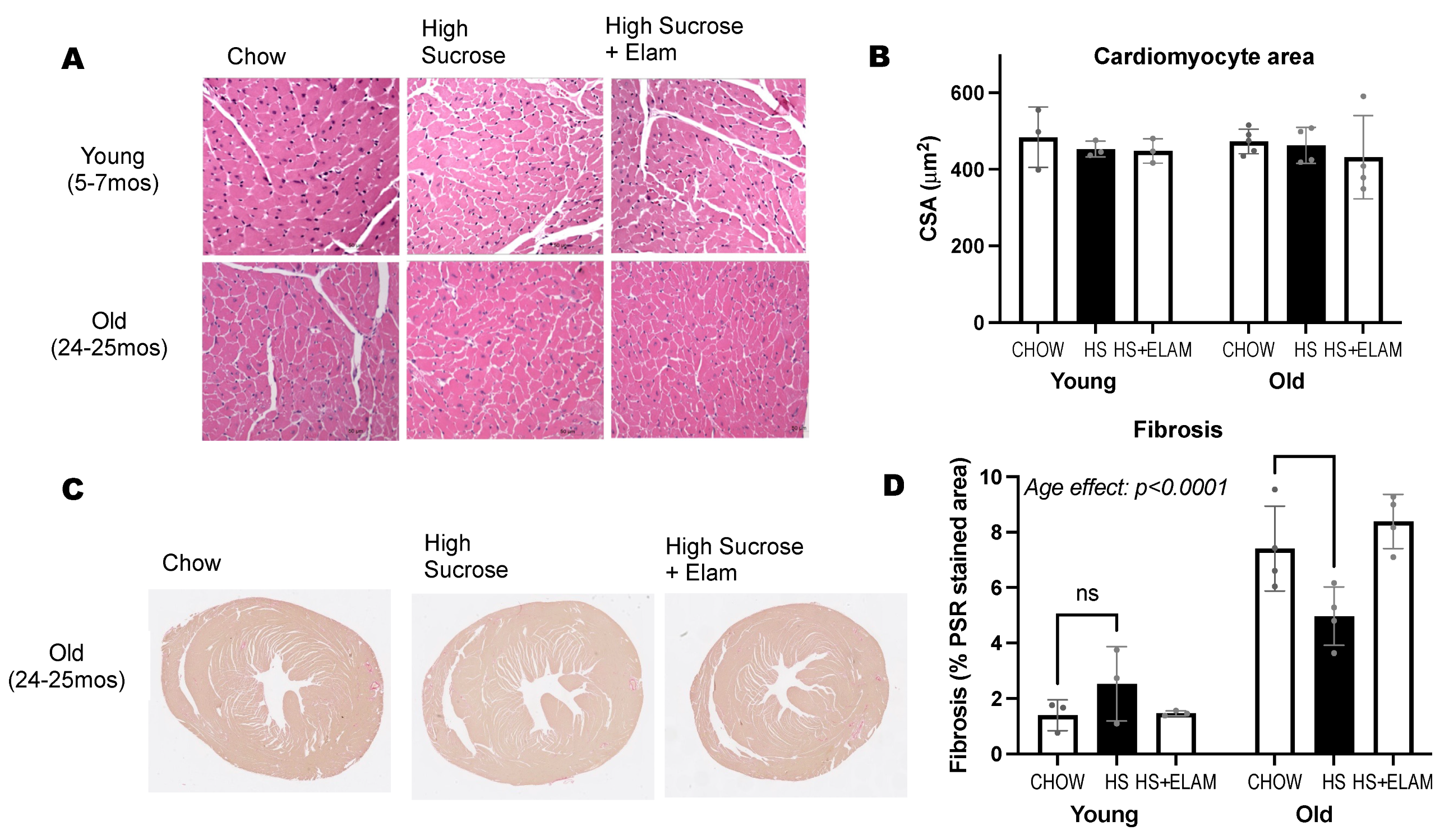
3.5. Old Hearts Have Increased Inflammation and Fibrosis, as Evidenced by Gene Expression and Histology, That Was Not Significantly Affected by HS Diet with or without ELAM
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern. Med. 2014, 174, 516–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeChristopher, L.R.; Auerbach, B.J.; Tucker, K.L. High fructose corn syrup, excess-free-fructose, and risk of coronary heart disease among African Americans—The Jackson Heart Study. BMC Nutr. 2020, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Andres-Hernando, A.; Orlicky, D.J.; Kuwabara, M.; Ishimoto, T.; Nakagawa, T.; Johnson, R.J.; Lanaspa, M.A. Deletion of Fructokinase in the Liver or in the Intestine Reveals Differential Effects on Sugar-Induced Metabolic Dysfunction. Cell Metab. 2020, 32, 117–127.e3. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Ishimoto, T.; Li, N.; Cicerchi, C.; Orlicky, D.J.; Ruzycki, P.; Ruzicky, P.; Rivard, C.; Inaba, S.; Roncal-Jimenez, C.A.; et al. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 2013, 4, 2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiao, Y.A.; Rabinovitch, P.S. The Aging Heart. Cold Spring Harb. Perspect. Med. 2015, 5, a025148. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Zhang, H.; Sweetwyne, M.; Whitson, J.; Ting, Y.S.; Basisty, N.; Pino, L.K.; Quarles, E.; Nguyen, N.H.; Campbell, M.D.; et al. Late-life restoration of mitochondrial function reverses cardiac dysfunction in old mice. eLife 2020, 9, e55513. [Google Scholar] [CrossRef]
- Whitson, J.A.; Bitto, A.; Zhang, H.; Sweetwyne, M.T.; Coig, R.; Bhayana, S.; Shankland, E.G.; Wang, L.; Bammler, T.K.; Mills, K.F.; et al. SS-31 and NMN: Two paths to improve metabolism and function in aged hearts. Aging Cell 2020, 19, e13213. [Google Scholar] [CrossRef]
- Birk, A.V.; Chao, W.M.; Bracken, C.; Warren, J.D.; Szeto, H.H. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br. J. Pharmacol. 2014, 171, 2017–2028. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.B.; Meng, Y.H.; Chang, S.; Zhang, R.Y.; Shi, C. High fructose causes cardiac hypertrophy via mitochondrial signaling pathway. Am. J. Transl. Res. 2016, 8, 4869–4880. [Google Scholar]
- Park, J.H.; Ku, H.J.; Kim, J.K.; Park, J.W.; Lee, J.H. Amelioration of High Fructose-Induced Cardiac Hypertrophy by Naringin. Sci. Rep. 2018, 8, 9464. [Google Scholar] [CrossRef] [Green Version]
- Bouchard-Thomassin, A.A.; Lachance, D.; Drolet, M.C.; Couet, J.; Arsenault, M. A high-fructose diet worsens eccentric left ventricular hypertrophy in experimental volume overload. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H125–H134. [Google Scholar] [CrossRef]
- Chess, D.J.; Lei, B.; Hoit, B.D.; Azimzadeh, A.M.; Stanley, W.C. Deleterious effects of sugar and protective effects of starch on cardiac remodeling, contractile dysfunction, and mortality in response to pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1853–H1860. [Google Scholar] [CrossRef]
- Valencia, A.P.; Samuelson, A.T.; Stuppard, R.; Marcinek, D.J. Functional recovery from eccentric injury is maintained in sarcopenic mouse muscle. JCSM Rapid Commun. 2021, 4, 222–231. [Google Scholar] [CrossRef]
- Chavez, J.D.; Tang, X.; Campbell, M.D.; Reyes, G.; Kramer, P.A.; Stuppard, R.; Keller, A.; Zhang, H.; Rabinovitch, P.S.; Marcinek, D.J.; et al. Mitochondrial protein interaction landscape of SS-31. Proc. Natl. Acad. Sci. USA 2020, 117, 15363–15373. [Google Scholar] [CrossRef]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef]
- Koren, M.J.; Devereux, R.B.; Casale, P.N.; Savage, D.D.; Laragh, J.H. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann. Intern. Med. 1991, 114, 345–352. [Google Scholar] [CrossRef]
- Pavillard, L.E.; Cañadas-Lozano, D.; Alcocer-Gómez, E.; Marín-Aguilar, F.; Pereira, S.; Robertson, A.A.B.; Muntané, J.; Ryffel, B.; Cooper, M.A.; Quiles, J.L.; et al. NLRP3-inflammasome inhibition prevents high fat and high sugar diets-induced heart damage through autophagy induction. Oncotarget 2017, 8, 99740–99756. [Google Scholar] [CrossRef] [Green Version]
- Cigliano, L.; Spagnuolo, M.S.; Crescenzo, R.; Cancelliere, R.; Iannotta, L.; Mazzoli, A.; Liverini, G.; Iossa, S. Short-Term Fructose Feeding Induces Inflammation and Oxidative Stress in the Hippocampus of Young and Adult Rats. Mol. Neurobiol. 2018, 55, 2869–2883. [Google Scholar] [CrossRef]
- Rizvi, F.; Preston, C.C.; Emelyanova, L.; Yousufuddin, M.; Viqar, M.; Dakwar, O.; Ross, G.R.; Faustino, R.S.; Holmuhamedov, E.L.; Jahangir, A. Effects of Aging on Cardiac Oxidative Stress and Transcriptional Changes in Pathways of Reactive Oxygen Species Generation and Clearance. J. Am. Heart Assoc. 2021, 10, e019948. [Google Scholar] [CrossRef]
- Sweetwyne, M.T.; Pippin, J.W.; Eng, D.G.; Hudkins, K.L.; Chiao, Y.A.; Campbell, M.D.; Marcinek, D.J.; Alpers, C.E.; Szeto, H.H.; Rabinovitch, P.S.; et al. The mitochondrial-targeted peptide, SS-31, improves glomerular architecture in mice of advanced age. Kidney Int. 2017, 91, 1126–1145. [Google Scholar] [CrossRef] [Green Version]
- Siegel, M.P.; Kruse, S.E.; Percival, J.M.; Goh, J.; White, C.C.; Hopkins, H.C.; Kavanagh, T.J.; Szeto, H.H.; Rabinovitch, P.S.; Marcinek, D.J. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 2013, 12, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, X.; Li, M.; Li, J.; Xiao, W.; Ma, W.; Chen, X.; Liang, X.; Tang, S.; Luo, Y. Mitochondria-targeted antioxidant peptide SS31 protects the retinas of diabetic rats. Curr. Mol. Med. 2013, 13, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.; Boros, L.G.; Nolen, G.T.; Chang, C.W.; Wabitsch, M.; Beger, R.D.; Kaput, J. Metabolic fate of fructose in human adipocytes: A targeted. Metabolomics 2015, 11, 529–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eissing, L.; Scherer, T.; Tödter, K.; Knippschild, U.; Greve, J.W.; Buurman, W.A.; Pinnschmidt, H.O.; Rensen, S.S.; Wolf, A.M.; Bartelt, A.; et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 2013, 4, 1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [Green Version]
- Debray, F.G.; Seyssel, K.; Fadeur, M.; Tappy, L.; Paquot, N.; Tran, C. Effect of a high fructose diet on metabolic parameters in carriers for hereditary fructose intolerance. Clin. Nutr. 2021, 40, 4246–4254. [Google Scholar] [CrossRef]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef]
- Softic, S.; Meyer, J.G.; Wang, G.X.; Gupta, M.K.; Batista, T.M.; Lauritzen, H.P.M.M.; Fujisaka, S.; Serra, D.; Herrero, L.; Willoughby, J.; et al. Dietary Sugars Alter Hepatic Fatty Acid Oxidation via Transcriptional and Post-translational Modifications of Mitochondrial Proteins. Cell Metab. 2019, 30, 735–753.e734. [Google Scholar] [CrossRef]
- Bode, C.; Schumacher, H.; Goebell, H.; Zelder, O.; Pelzel, H. Fructose induced depletion of liver adenine nucleotides in man. Horm. Metab. Res. 1971, 3, 289–290. [Google Scholar] [CrossRef]
- Kim, I.Y.; Ye, B.M.; Kim, M.J.; Kim, S.R.; Lee, D.W.; Kim, H.J.; Rhee, H.; Song, S.H.; Seong, E.Y.; Lee, S.B. Association between serum uric acid and left ventricular hypertrophy/left ventricular diastolic dysfunction in patients with chronic kidney disease. PLoS ONE 2021, 16, e0251333. [Google Scholar] [CrossRef]
- Høieggen, A.; Alderman, M.H.; Kjeldsen, S.E.; Julius, S.; Devereux, R.B.; De Faire, U.; Fyhrquist, F.; Ibsen, H.; Kristianson, K.; Lederballe-Pedersen, O.; et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004, 65, 1041–1049. [Google Scholar] [CrossRef]
- Loader, J.; Meziat, C.; Watts, R.; Lorenzen, C.; Sigaudo-Roussel, D.; Stewart, S.; Reboul, C.; Meyer, G.; Walther, G. Effects of Sugar-Sweetened Beverage Consumption on Microvascular and Macrovascular Function in a Healthy Population. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, K.; Wylie, A.T.; Tucker, K.L.; Hamp, T.J.; Gharaibeh, R.Z.; Fodor, A.A.; Cullen, J.M. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am. J. Clin. Nutr. 2013, 98, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Hertle, S.; Spanagel, R.; Bilbao, A. Female mice are more prone to develop an addictive-like phenotype for sugar consumption. Sci. Rep. 2021, 11, 7364. [Google Scholar] [CrossRef]
- Merz, A.A.; Cheng, S. Sex differences in cardiovascular ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef]
- Valencia, A.P.; Schappal, A.E.; Morris, E.M.; Thyfault, J.P.; Lowe, D.A.; Spangenburg, E.E. The presence of the ovary prevents hepatic mitochondrial oxidative stress in young and aged female mice through glutathione peroxidase 1. Exp. Gerontol. 2016, 73, 14–22. [Google Scholar] [CrossRef]





| Diet | Chow (Picolab 5008C33) | High Sucrose (HS) (Bioserv Custom) | |
|---|---|---|---|
| Composition by weight | Protein (%) | ~23.6 | 20.5 |
| Fat (%) | ~6.7–8.1 | 7.0 | |
| Carbohydrate (%) | ~50.3 | 65.8 | |
| Sucrose (%) | ~3.23 | 65.8 | |
| Composition by calories | Protein (%kcal) | 27 | 20 |
| Fat (%kcal) | 17 | 15 | |
| Carbohydrate (%kcal) | 57 | 64 | |
| Sucrose (%kcal) | ~3.6 | 64 | |
| Physiological fuel value (kcal/g) | 3.56 | 4.08 |
| Variable | Young Chow | Young HS | Young HS + ELAM | Old Chow | Old HS | Old HS + ELAM | Age-Effect p-Value | Treatment Effect p-Value |
|---|---|---|---|---|---|---|---|---|
| Sample size (n) | 8 | 10 | 10 | 9 | 9 | 8 | ||
| Body weight pre-diet (g) | 37.06 ± 2.39 | 36.98 ± 2.36 | 35.96 ± 4.32 | 41.14 ± 3.96 | 43.98 ± 5.09 | 40.64 ± 2.97 | <0.0001 | - |
| Body weight post-diet (g) | 38.34 ± 2.32 | 39.32 ± 2.29 $ | 38.0 ±4.11 $ | 41.56 ± 4.96 | 42.69 ± 3.66 | 40.13 ± 2.39 | <0.01 | 0.25 |
| Heart (mg) | 174.03 ± 12.55 | 184.32 ±14.34 | 179.1 ± 18.52 | 216.03 ± 18.52 | 237.76 ± 21.9 * | 218.91 ± 18.71 | <0.0001 | <0.05 |
| Liver (g) | 1.67 ± 0.09 | 1.77 ± 0.25 | 1.85 ± 0.25 | 2.14 ± 0.34 | 2.41 ± 0.38 | 2.18 ± 0.26 | <0.0001 | 0.16 |
| Gastrocnemius (mg) | 188.23 ± 9.76 | 192.89 ± 13.13 | 185.83 ± 20.09 | 176.97 ± 19.9 | 179.89 ±13.06 | 174.63 ± 17.28 | <0.0001 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia, A.P.; Whitson, J.A.; Wang, S.; Nguyen, L.; den Hartigh, L.J.; Rabinovitch, P.S.; Marcinek, D.J. Aging Increases Susceptibility to Develop Cardiac Hypertrophy following High Sugar Consumption. Nutrients 2022, 14, 4645. https://doi.org/10.3390/nu14214645
Valencia AP, Whitson JA, Wang S, Nguyen L, den Hartigh LJ, Rabinovitch PS, Marcinek DJ. Aging Increases Susceptibility to Develop Cardiac Hypertrophy following High Sugar Consumption. Nutrients. 2022; 14(21):4645. https://doi.org/10.3390/nu14214645
Chicago/Turabian StyleValencia, Ana P., Jeremy A. Whitson, Shari Wang, Leon Nguyen, Laura J. den Hartigh, Peter S. Rabinovitch, and David J. Marcinek. 2022. "Aging Increases Susceptibility to Develop Cardiac Hypertrophy following High Sugar Consumption" Nutrients 14, no. 21: 4645. https://doi.org/10.3390/nu14214645
APA StyleValencia, A. P., Whitson, J. A., Wang, S., Nguyen, L., den Hartigh, L. J., Rabinovitch, P. S., & Marcinek, D. J. (2022). Aging Increases Susceptibility to Develop Cardiac Hypertrophy following High Sugar Consumption. Nutrients, 14(21), 4645. https://doi.org/10.3390/nu14214645







