Early Life Low-Calorie Sweetener Consumption Impacts Energy Balance during Adulthood
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Early Life Diets
2.3. Glucose Tolerance Test (GTT)
2.4. Cafeteria Diet Access in Adulthood
2.5. Open Field
2.6. Quantitative Polymerase Chain Reaction (qPCR)
2.7. Statistical Analysis
3. Results
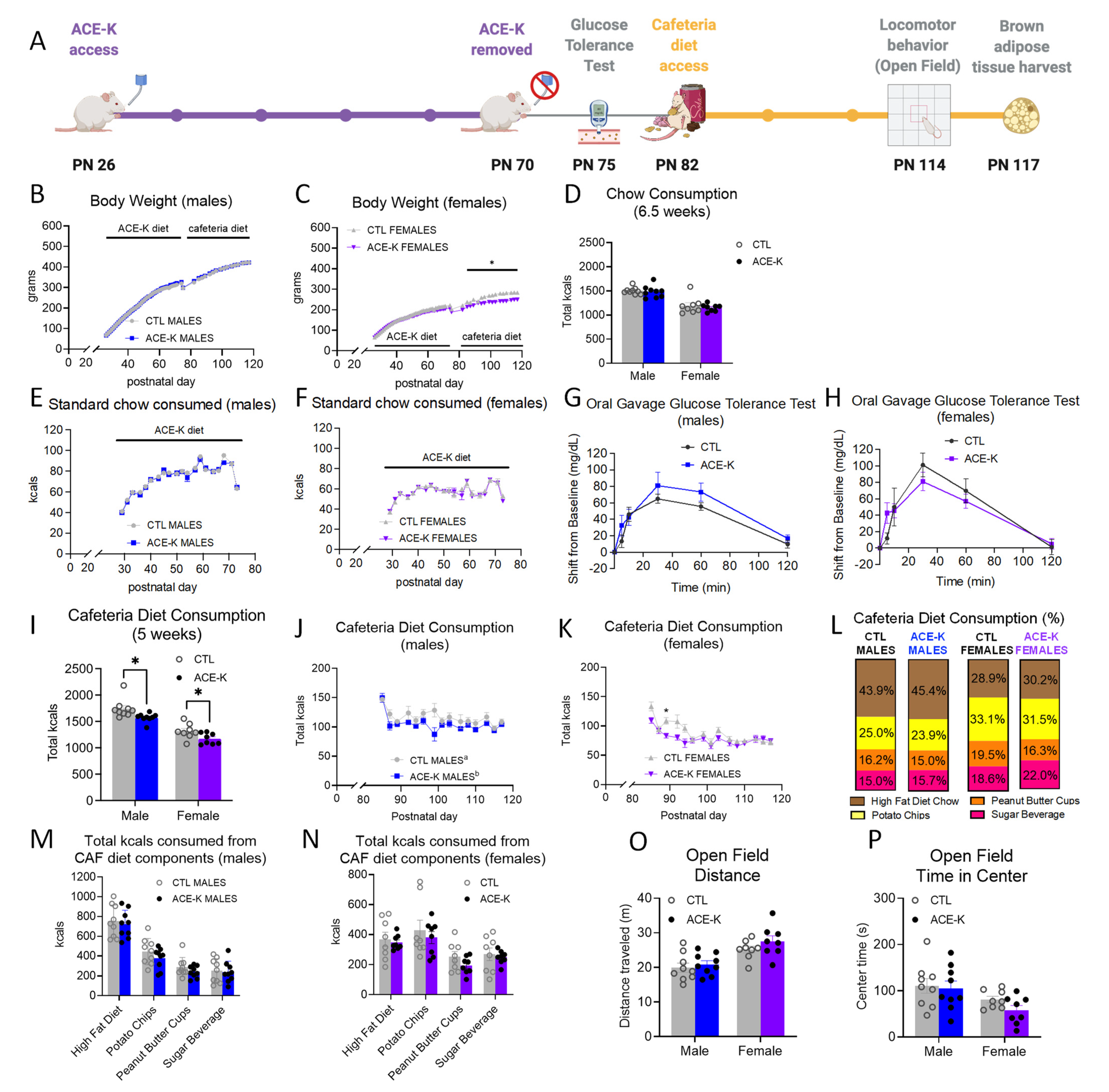
3.1. Early Life Habitual ACE-K Consumption Reduces the Total Caloric Intake of a Cafeteria Diet during Adulthood
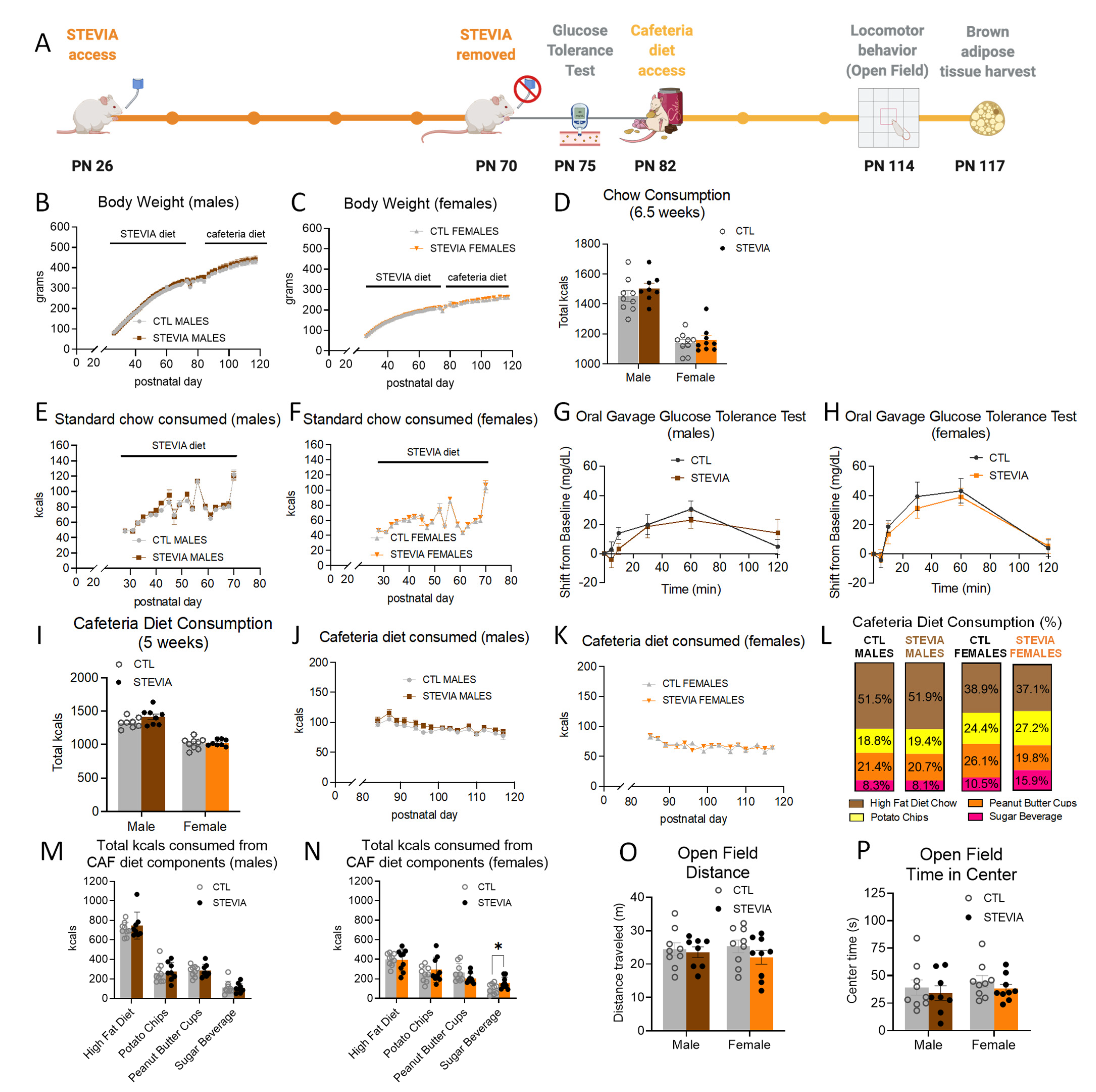
3.2. Early Life Habitual Sugar Consumption Does Not Impact Cafeteria Diet Consumption in Adulthood
3.3. Early Life Stevia Consumption Increases Sugar-Sweetened Beverage Consumption during Adult Cafeteria Diet Exposure
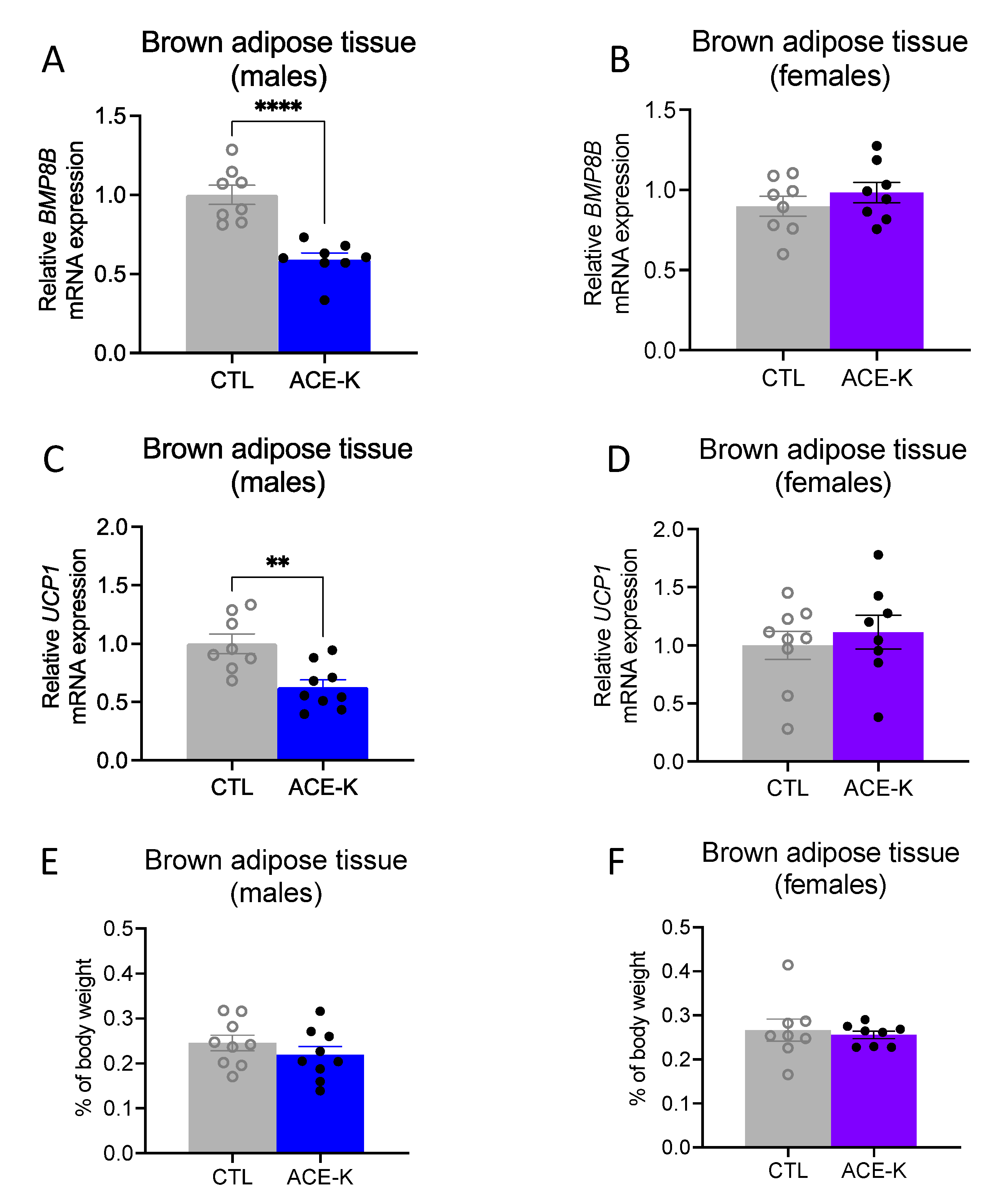
3.4. ACE-K Consumption during Early Life Reduces Brown Adipose Tissue Gene Expression of the Thermogenic Markers, BMP8B and UCP1, in Male Rats on a Cafeteria Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholas, L.M.; Ozanne, S.E. Early life programming in mice by maternal overnutrition: Mechanistic insights and interventional approaches. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2019, 374, 20180116. [Google Scholar] [CrossRef] [Green Version]
- Parlee, S.D.; MacDougald, O.A. Maternal nutrition and risk of obesity in offspring: The Trojan horse of developmental plasticity. Biochim. Biophys. Acta 2014, 1842, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Reyes, T.M. High-fat diet alters the dopamine and opioid systems: Effects across development. Int. J. Obes. Suppl. 2012, 2, S25–S28. [Google Scholar] [CrossRef] [Green Version]
- Magriplis, E.; Michas, G.; Petridi, E.; Chrousos, G.P.; Roma, E.; Benetou, V.; Cholopoulos, N.; Micha, R.; Panagiotakos, D.; Zampelas, A. Dietary Sugar Intake and Its Association with Obesity in Children and Adolescents. Children 2021, 8, 676. [Google Scholar] [CrossRef]
- Walton, J.; Bell, H.; Re, R.; Nugent, A.P. Current perspectives on global sugar consumption: Definitions, recommendations, population intakes, challenges and future direction. Nutr. Res. Rev. 2021, 1–22. [Google Scholar] [CrossRef]
- Belsky, D.W.; Moffitt, T.E.; Houts, R.; Bennett, G.G.; Biddle, A.K.; Blumenthal, J.A.; Evans, J.P.; Harrington, H.; Sugden, K.; Williams, B.; et al. Polygenic Risk, Rapid Childhood Growth, and the Development of Obesity. Arch. Pediatr. 2012, 166, 515–521. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 27 June 2022).
- Hall, K.D.; Butte, N.F.; Swinburn, B.A.; Chow, C.C. Dynamics of childhood growth and obesity: Development and validation of a quantitative mathematical model. Lancet Diabetes Endocrinol. 2013, 1, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Arisaka, O.; Ichikawa, G.; Koyama, S.; Sairenchi, T. Childhood obesity: Rapid weight gain in early childhood and subsequent cardiometabolic risk. Clin. Pediatr. Endocrinol. 2020, 29, 135–142. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Figueroa, J.; Zimmerman, T.; Swithers, S.E.; Welsh, J.A. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011–2016. Pediatr. Obes. 2019, 14, e12535. [Google Scholar] [CrossRef]
- Swithers, S.E.; Martin, A.A.; Davidson, T.L. High-intensity sweeteners and energy balance. Physiol. Behav. 2010, 100, 55–62. [Google Scholar] [CrossRef]
- Laska, M.N.; Murray, D.M.; Lytle, L.A.; Harnack, L.J. Longitudinal Associations Between Key Dietary Behaviors and Weight Gain Over Time: Transitions Through the Adolescent Years. Obesity 2012, 20, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Blum, J.W.; Jacobsen, D.J.; Donnelly, J.E. Beverage Consumption Patterns in Elementary School Aged Children across a Two-Year Period. J. Am. Coll. Nutr. 2005, 24, 93–98. [Google Scholar] [CrossRef]
- Chia, C.W.; Shardell, M.; Gravenstein, K.S.; Carlson, O.D.; Simonsick, E.M.; Ferrucci, L.; Egan, J.M. Regular low-calorie sweetener consumption is associated with increased secretion of glucose-dependent insulinotropic polypeptide. Diabetes Obes. Metab. 2018, 20, 2282–2285. [Google Scholar] [CrossRef]
- Shum, B.; Georgia, S. The Effects of Non-Nutritive Sweetener Consumption in the Pediatric Populations: What We Know, What We Don’t, and What We Need to Learn. Front. Endocrinol. 2021, 12, 625415. [Google Scholar] [CrossRef]
- Swithers, S.E.; Laboy, A.F.; Clark, K.; Cooper, S.; Davidson, T.L. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav. Brain Res. 2012, 233, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sylvetsky, A.C.; Jin, Y.; Clark, E.J.; Welsh, J.A.; Rother, K.I.; Talegawkar, S.A. Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. J. Acad. Nutr. Diet. 2017, 117, 441–448.e2. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Munger, S.D.; Sclafani, A.; De Araujo, I.E.; Roberts, A.; Molinary, S. Mechanisms for Sweetness. J. Nutr. 2012, 142, 1134S–1141S. [Google Scholar] [CrossRef] [Green Version]
- Mora, M.R.; Dando, R. The sensory properties and metabolic impact of natural and synthetic sweeteners. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1554–1583. [Google Scholar] [CrossRef]
- Russell, C.; Baker, P.; Grimes, C.; Lindberg, R.; Lawrence, M.A. Global trends in added sugars and non-nutritive sweetener use in the packaged food supply: Drivers and implications for public health. Public Health Nutr. 2022, 1–39. [Google Scholar] [CrossRef]
- Klug, C.; Rymon Lipinski, G.-W.; Bottger, D. Baking stability of acesulfame K. Z. Für Lebensm.-Unters. Und Forsch. 1992, 194, 476–478. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Jones, R.B.; Fodor, A.A.; Goran, M.I.; Kanoski, S.E. Early-Life Sugar Consumption Affects the Rat Microbiome Independently of Obesity. J. Nutr. 2017, 147, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Ackroff, K.; Sclafani, A. Flavor preferences conditioned by intragastric infusions of dilute polycose solutions. Physiol. Behav. 1994, 55, 957–962. [Google Scholar] [CrossRef]
- Harris, J.A.; Gorissen, M.C.; Bailey, G.K.; Westbrook, R.F. Motivational state regulates the content of learned flavor preferences. J. Exp. Psychol. Anim. Behav. Process. 2000, 26, 15–30. [Google Scholar] [CrossRef]
- Zhang, M.; Kelley, A.E. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology 2002, 159, 415–423. [Google Scholar] [CrossRef]
- Tsan, L.; Decarie-Spain, L.; Noble, E.E.; Kanoski, S.E. Western Diet Consumption During Development: Setting the Stage for Neurocognitive Dysfunction. Front. Neurosci. 2021, 15, 632312. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture and U.S. Department of Health and Human Services: Washington, DC, USA, 2020.
- Sclafani, A.; Bahrani, M.; Zukerman, S.; Ackroff, K. Stevia and Saccharin Preferences in Rats and Mice. Chem. Senses 2010, 35, 433–443. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K. Advantame Sweetener Preference in C57BL/6J Mice and Sprague-Dawley Rats. Chem. Senses 2015, 40, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Hsu, T.M.; Konanur, V.R.; Taing, L.; Usui, R.; Kayser, B.D.; Goran, M.I.; Kanoski, S.E. Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 2015, 25, 227–239. [Google Scholar] [CrossRef]
- Noble, E.E.; Hsu, T.M.; Liang, J.; Kanoski, S.E. Early-life sugar consumption has long-term negative effects on memory function in male rats. Nutr. Neurosci. 2019, 22, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Olson, C.A.; Davis, E.; Tsan, L.; Chen, Y.W.; Schade, R.; Liu, C.; Suarez, A.; Jones, R.B.; de La Serre, C.; et al. Gut microbial taxa elevated by dietary sugar disrupt memory function. Transl. Psychiatry 2021, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Tsan, L.; Sun, S.; Hayes, A.M.R.; Bridi, L.; Chirala, L.S.; Noble, E.E.; Fodor, A.A.; Kanoski, S.E. Early life Western diet-induced memory impairments and gut microbiome changes in female rats are long-lasting despite healthy dietary intervention. Nutr. Neurosci. 2021, 25, 2490–2506. [Google Scholar] [CrossRef] [PubMed]
- Belzung, C.; Griebel, G. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav. Brain Res. 2001, 125, 141–149. [Google Scholar] [CrossRef]
- Suarez, A.N.; Hsu, T.M.; Liu, C.M.; Noble, E.E.; Cortella, A.M.; Nakamoto, E.M.; Hahn, J.D.; de Lartigue, G.; Kanoski, S.E. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 2018, 9, 2181. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.A.; Wald, H.S.; Suarez, A.N.; Zubcevic, J.; Liu, C.M.; Cortella, A.M.; Kamitakahara, A.K.; Polson, J.W.; Arnold, M.; Grill, H.J.; et al. Ghrelin Signaling Affects Feeding Behavior, Metabolism, and Memory through the Vagus Nerve. Curr. Biol. 2020, 30, 4510–4518.e6. [Google Scholar] [CrossRef]
- Whittle, A.J.; Carobbio, S.; Martins, L.; Slawik, M.; Hondares, E.; Vazquez, M.J.; Morgan, D.; Csikasz, R.I.; Gallego, R.; Rodriguez-Cuenca, S.; et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012, 149, 871–885. [Google Scholar] [CrossRef] [Green Version]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.; Gray, C.; Li, M.; Segovia, S.; Vickers, M. Early Life Nutrition and Energy Balance Disorders in Offspring in Later Life. Nutrients 2015, 7, 8090–8111. [Google Scholar] [CrossRef]
- Bian, X.; Chi, L.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice. PLoS ONE 2017, 12, e0178426. [Google Scholar] [CrossRef]
- Tsan, L.; Chometton, S.; Hayes, A.M.R.; Klug, M.E.; Zuo, Y.; Sun, S.; Bridi, L.; Lan, R.; Fodor, A.A.; Noble, E.E.; et al. Early life low-calorie sweetener consumption disrupts glucose regulation, sugar-motivated behavior, and memory function in rats. JCI Insight 2022, 7, e157714. [Google Scholar] [CrossRef] [PubMed]
- Abo Elnaga, N.I.E.; Massoud, M.I.; Yousef, M.I.; Mohamed, H.H.A. Effect of stevia sweetener consumption as non-caloric sweetening on body weight gain and biochemical’s parameters in overweight female rats. Ann. Agric. Sci. 2016, 61, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, U.; Ahmad, R.S. Anti diabetic property of aqueous extract of Stevia rebaudiana Bertoni leaves in Streptozotocin-induced diabetes in albino rats. BMC Complement. Altern. Med. 2018, 18, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, M.; Rehfeld, A.; Frizzell, C.; Livingstone, C.; McGonagle, C.; Skakkebaek, N.E.; Wielogórska, E.; Connolly, L. In vitro bioassay investigations of the endocrine disrupting potential of steviol glycosides and their metabolite steviol, components of the natural sweetener Stevia. Mol. Cell. Endocrinol. 2016, 427, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.P.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef] [Green Version]
- Renwick, A.G. The metabolism of intense sweeteners. Xenobiotica 1986, 16, 1057–1071. [Google Scholar] [CrossRef]
- Volz, M.; Christ, O.; Eckert, H.; Herok, J.; Kellner, H.; Rupp, W. Kinetics and Biotransformation of Acesulfame-K. In Acesulfame-K; Marcel Dekker: New York, NY, USA, 1991; pp. 7–26. [Google Scholar]
- Udensi, U.K.T.; Paul, B. Potassium homeostasis, oxidative stress, and human disease. Int. J. Clin. Exp. Physiol. 2017, 4, 111. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, A.; Boileau, A.C.; Winkler, P.C.; Compton, J.C.; Prakash, I.; Jiang, X.; Mandarino, D.A. Pharmacokinetics of rebaudioside A and stevioside after single oral doses in healthy men. Food Chem. Toxicol. 2008, 46, S54–S60. [Google Scholar] [CrossRef]
- Roberts, A.; Renwick, A.G. Comparative toxicokinetics and metabolism of rebaudioside A, stevioside, and steviol in rats. Food Chem. Toxicol. 2008, 46, S31–S39. [Google Scholar] [CrossRef]
- Luukkaa, V.; Savontaus, E.; Rouru, J.; Virtanen, K.A.; Boss, O.; Huhtaniemi, I.; Koulu, M.; Pesonen, U.; Huupponen, R. Effects of estrous cycle and steroid replacement on the expression of leptin and uncoupling proteins in adipose tissue in the rat. Gynecol. Endocrinol. 2001, 15, 103–112. [Google Scholar] [CrossRef]
- Cong, W.-N.; Wang, R.; Cai, H.; Daimon, C.M.; Scheibye-Knudsen, M.; Bohr, V.A.; Turkin, R.; Wood, W.H.; Becker, K.G.; Moaddel, R.; et al. Long-Term Artificial Sweetener Acesulfame Potassium Treatment Alters Neurometabolic Functions in C57BL/6J Mice. PLoS ONE 2013, 8, e70257. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Clegg, D.J. Sex differences in the regulation of body weight. Physiol. Amp; Behav. 2009, 97, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaño, D.; Masoodi, H.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P. Stevia, sucralose and sucrose added to a maqui-Citrus beverage and their effects on glycemic response in overweight subjects: A randomized clinical trial. LWT 2021, 144, 111173. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayes, A.M.R.; Tsan, L.; Kao, A.E.; Schwartz, G.M.; Décarie-Spain, L.; Tierno Lauer, L.; Klug, M.E.; Schier, L.A.; Kanoski, S.E. Early Life Low-Calorie Sweetener Consumption Impacts Energy Balance during Adulthood. Nutrients 2022, 14, 4709. https://doi.org/10.3390/nu14224709
Hayes AMR, Tsan L, Kao AE, Schwartz GM, Décarie-Spain L, Tierno Lauer L, Klug ME, Schier LA, Kanoski SE. Early Life Low-Calorie Sweetener Consumption Impacts Energy Balance during Adulthood. Nutrients. 2022; 14(22):4709. https://doi.org/10.3390/nu14224709
Chicago/Turabian StyleHayes, Anna M. R., Linda Tsan, Alicia E. Kao, Grace M. Schwartz, Léa Décarie-Spain, Logan Tierno Lauer, Molly E. Klug, Lindsey A. Schier, and Scott E. Kanoski. 2022. "Early Life Low-Calorie Sweetener Consumption Impacts Energy Balance during Adulthood" Nutrients 14, no. 22: 4709. https://doi.org/10.3390/nu14224709
APA StyleHayes, A. M. R., Tsan, L., Kao, A. E., Schwartz, G. M., Décarie-Spain, L., Tierno Lauer, L., Klug, M. E., Schier, L. A., & Kanoski, S. E. (2022). Early Life Low-Calorie Sweetener Consumption Impacts Energy Balance during Adulthood. Nutrients, 14(22), 4709. https://doi.org/10.3390/nu14224709





