Heat-Treated Meat Origin Tracing and Authenticity through a Practical Multiplex Polymerase Chain Reaction Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection and DNA Extraction
2.2. Design of Species-Specific Primers
2.3. Simplex and Multiplex PCR Assays
2.4. Sequencing of PCR Products
2.5. Tests of Specificity, Sensitivity and Reproducibility of Primers
3. Results
3.1. Specificity Assays of Designed Primers
3.2. Sensitivity of Multiplex PCR Assay
3.3. Reproducibility of Multiplex PCR Assay in Heat Processing Meat
3.4. Application of Multiplex PCR Assay on Commercial Meat Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galal-Khallaf, A. Multiplex PCR and 12S rRNA gene sequencing for detection of meat adulteration: A case study in the Egyptian markets. Gene 2021, 764, 145062. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.; Abd El-Hack, M.E.; Tiwari, R.; Dhama, K. Identification of different animal species in meat and meat products: Trends and advances. Adv. Anim. Vet. Sci. 2015, 3, 334–346. [Google Scholar] [CrossRef]
- Zhou, S.; Zhong, G.; Zhou, H.; Zhang, X.; Zeng, X.; Wu, Z.; Pan, D.; Cai, Z.; He, J.; Liu, Q. A heptaplex PCR assay for molecular traceability of species origin with high efficiency and practicality in both raw and heat processing meat materials. Front. Nutr. 2022, 9, 890537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Liu, S.Y.; Meng, F.B.; Liu, D.Y.; Zhang, Y.; Wang, W.; Zhang, J.M. Comparative review and the recent progress in detection technologies of meat product adulteration. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2256–2296. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Jalbani, Y.M.; Zhang, G.L.; Zhao, Z.Y.; Wang, Z.Y.; Zhao, X.Y.; Chen, A.L. Detection of goat meat adulteration by real-time PCR based on a reference primer. Food Chem. 2019, 277, 554–557. [Google Scholar] [CrossRef]
- Li, J.C.; Li, J.P.; Xu, S.G.; Xiong, S.Y.; Yang, J.N.; Chen, X.; Wang, S.W.; Qiao, X.L.; Zhou, T. A rapid and reliable multiplex PCR assay for simultaneous detection of fourteen animal species in two tubes. Food Chem. 2019, 295, 395–402. [Google Scholar] [CrossRef]
- Singh, A.; Bhargava, P. PCR based strategies to identify and differentiate species specific meat. J. Pharm. Chem. Biol. Sci. 2019, 7, 36–43. [Google Scholar]
- Premanandh, J. Horse meat scandal—A wake-up call for regulatory authorities. Food Control 2013, 34, 568–569. [Google Scholar] [CrossRef]
- Uddin, S.M.K.; Hossain, M.A.M.; Chowdhury, Z.Z.; Johan, M.R. Detection and discrimination of seven highly consumed meat species simultaneously in food products using heptaplex PCR-RFLP assay. J. Food Compos. Anal. 2021, 100, 103938. [Google Scholar] [CrossRef]
- Wang, W.J.; Liu, J.J.; Zhang, Q.D.; Zhou, X.; Liu, B. Multiplex PCR assay for identification and quantification of bovine and equine in minced meats using novel specific nuclear DNA sequences. Food Control 2019, 105, 29–37. [Google Scholar] [CrossRef]
- Li, J.C.; Li, J.P.; Liu, R.X.; Wei, Y.X.; Wang, S.W. Identification of eleven meat species in foodstuff by a hexaplex real-time PCR with melting curve analysis. Food Control 2021, 121, 107599. [Google Scholar] [CrossRef]
- Chung, S.M.; Hellberg, R.S. Effects of poor sanitation procedures on cross-contamination of animal species in ground meat products. Food Control 2020, 109, 106927. [Google Scholar] [CrossRef]
- da Costa, P.A.; Cobuccio, L.; Mainali, D.; Rault, M.; Cavin, C. Rapid analysis of food raw materials adulteration using laser direct infrared spectroscopy and imaging. Food Control 2020, 113, 107114. [Google Scholar] [CrossRef]
- Mansouri, M.; Khalilzadeh, B.; Barzegari, A.; Shoeibi, S.; Isildak, S.; Bargahi, N.; Omidi, Y.; Dastmalchi, S.; Rashidi, M.R. Design a highly specific sequence for electrochemical evaluation of meat adulteration in cooked sausages. Biosens. Bioelectron. 2020, 150, 111916. [Google Scholar] [CrossRef] [PubMed]
- Barbin, D.F.; Badaro, A.T.; Honorato, D.C.B.; Ida, E.Y.; Shimokomaki, M. Identification of turkey meat and processed products using near infrared spectroscopy. Food Control 2020, 107, 106816. [Google Scholar] [CrossRef]
- Leng, T.; Li, F.; Xiong, L.A.; Xiong, Q.; Zhu, M.T.; Chen, Y. Quantitative detection of binary and ternary adulteration of minced beef meat with pork and duck meat by NIR combined with chemometrics. Food Control 2020, 113, 107203. [Google Scholar] [CrossRef]
- Cai, Z.D.; Zhou, S.; Liu, Q.Q.; Ma, H.; Yuan, X.Y.; Gao, J.Q.; Cao, J.X.; Pan, D.D. A simple and reliable single tube septuple PCR assay for simultaneous identification of seven meat species. Foods 2021, 10, 1083. [Google Scholar] [CrossRef]
- Poser, R.; Detsch, R.; Fischer, K.; Muller, W.D.; Behrschmidt, M.; Schwagele, F. Animal- and plant species in meat product treated under different temperature regimes—Identification by means of polymerase chain reaction, hybridisation applying specific DNA-probes and ELISA. Adv. Anim. Vet. Sci. 2000, 80, 87–89. [Google Scholar]
- Ali, M.E.; Razzak, M.A.; Abd Hamid, S.B.; Rahman, M.M.; Al Amin, M.; Abd Rashid, N.R. Asing. Multiplex PCR assay for the detection of five meat species forbidden in Islamic foods. Food Chem. 2015, 177, 214–224. [Google Scholar] [CrossRef]
- Vaithiyanathan, S.; Vishnuraj, M.R.; Reddy, G.N.; Kulkarni, V.V. Application of DNA technology to check misrepresentation of animal species in illegally sold meat. Biocatal. Agric. Biotechol. 2018, 16, 564–568. [Google Scholar] [CrossRef]
- Liu, W.W.; Tao, J.; Xue, M.; Ji, J.G.; Zhang, Y.H.; Zhang, L.J.; Sun, W.P. A multiplex PCR method mediated by universal primers for the identification of eight meat ingredients in food products. Eur. Food Res. Technol. 2019, 245, 2385–2392. [Google Scholar] [CrossRef]
- Martin, I.; Garcia, T.; Fajardo, V.; Rojas, M.; Pegels, N.; Hernandez, P.E.; Gonzalez, I.; Martin, R. SYBR-Green real-time PCR approach for the detection and quantification of pig DNA in feedstuffs. Meat Sci. 2009, 82, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Yaman, B.N.; Celik, P.A.; Mutlu, M.B.; Cabuk, A. A Combinational Analysis of Acidophilic Bacterial Diversity of an Iron-Rich Environment. Geomicrobiol. J. 2020, 37, 877–889. [Google Scholar] [CrossRef]
- Prusakova, O.V.; Glukhova, X.A.; Afanas’eva, G.V.; Trizna, Y.A.; Nazarova, L.F.; Beletsky, I.P. A simple and sensitive two-tube multiplex PCR assay for simultaneous detection of ten meat species. Meat Sci. 2018, 137, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Saleem, M.S.; Imran, M.; Khan, W.A.; Ashraf, K.; Zahoor, M.Y.; Rashid, I.; Rehman, H.U.; Nadeem, A.; Ali, S.; et al. Single tube multiplex PCR assay for the identification of banned meat species. Food Addit. Contam. Part B 2020, 13, 284–291. [Google Scholar] [CrossRef]
- Wang, W.J.; Wang, X.K.; Zhang, Q.D.; Liu, Z.H.; Zhou, X.; Liu, B. A multiplex PCR method for detection of five animal species in processed meat products using novel species-specific nuclear DNA sequences. Eur. Food Res. Technol. 2020, 246, 1351–1360. [Google Scholar] [CrossRef]
- Mafra, I.; Ferreira, I.M.P.L.V.O.; Oliveira, M.B.P.P. Food authentication by PCR-based methods. Eur. Food Res. Technol. 2008, 227, 649–665. [Google Scholar] [CrossRef]
- Cai, Z.D.; Zhong, G.W.; Liu, Q.Q.; Yang, X.Q.; Zhang, X.X.; Zhou, S.; Zeng, X.Q.; Wu, Z.; Pan, D.D. Molecular authentication of twelve meat species through a promising two-tube hexaplex polymerase chain reaction technique. Front. Nutr. 2022, 9, 813962. [Google Scholar] [CrossRef]
- Kim, M.J.; Yoo, I.; Yang, S.M.; Suh, S.M.; Kim, H.Y. Development and validation of a multiplex PCR assay for simultaneous detection of chicken, turkey and duck in processed meat products. Int. J. Food Sci. Technol. 2018, 53, 2673–2679. [Google Scholar] [CrossRef]
- Sultana, S.; Hossain, M.A.M.; Zaidul, I.S.M.; Ali, M.E. Multiplex PCR to discriminate bovine, porcine, and fish DNA in gelatin and confectionery products. LWT-Food Sci. Technol. 2018, 92, 169–176. [Google Scholar] [CrossRef]
- Zhao, G.; Shen, X.; Liu, Y.L.; Xie, P.C.; Yao, C.Y.; Li, X.M.; Sun, Y.M.; Lei, Y.; Lei, H.T. Direct lysis-multiplex polymerase chain reaction assay for beef fraud substitution with chicken, pork and duck. Food Control 2021, 129, 108252. [Google Scholar] [CrossRef]
- Safdar, M.; Junejo, Y.; Arman, K.; Abasiyanik, M.F. A highly sensitive and specific tetraplex PCR assay for soybean, poultry, horse and pork species identification in sausages: Development and validation. Meat Sci. 2014, 98, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.Z.; Qu, W.; Xu, J.G.; Qiao, D.Q.; Yao, L.; Xue, F.; Chen, W. A sensitive multiplex PCR protocol for simultaneous detection of chicken, duck, and pork in beef samples. J. Food Sci. Technol. 2019, 56, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Hang, X.R.; Geng, R.Q. Molecular detection of adulteration in commercial buffalo meat products by multiplex PCR assay. Food Sci. Technol. 2019, 39, 344–348. [Google Scholar] [CrossRef]
- Balakrishna, K.; Sreerohini, S.; Parida, M. Ready-to-use single tube quadruplex PCR for differential identification of mutton, chicken, pork and beef in processed meat samples. Food Addit. Contam. Part A 2019, 36, 1435–1444. [Google Scholar] [CrossRef]
- Liu, W.W.; Wang, X.N.; Tao, J.; Xi, B.S.; Xue, M.; Sun, W.P. A Multiplex PCR Assay Mediated by Universal Primers for the Detection of Adulterated Meat in Mutton. J. Food Prot. 2019, 82, 325–330. [Google Scholar] [CrossRef]
- Safdar, M.; Junejo, Y. The development of a hexaplex-conventional PCR for identification of six animal and plant species in foodstuffs. Food Chem. 2016, 192, 745–749. [Google Scholar] [CrossRef]
- Matsunaga, T.; Chikuni, K.; Tanabe, R.; Muroya, S.; Shibata, K.; Yamada, J.; Shinmura, Y. A quick and simple method for the identification of meat species and meat products by PCR assay. Meat Sci. 1999, 51, 143–148. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, W.; Zhu, M.R.; Wen, Y.J.; Xie, T.; He, X.Q.; Zhang, Y.F.; Cao, S.Z.; Niu, L.L.; Zhang, H.P.; et al. Molecular identification of adulteration in mutton based on mitochondrial 16S rRNA gene. Mitochondrial DNA 2016, 27, 628–632. [Google Scholar] [CrossRef]
- Doosti, A.; Dehkordi, P.G.; Rahimi, E. Molecular assay to fraud identification of meat products. J. Food Sci. Technol. 2014, 51, 148–152. [Google Scholar] [CrossRef]
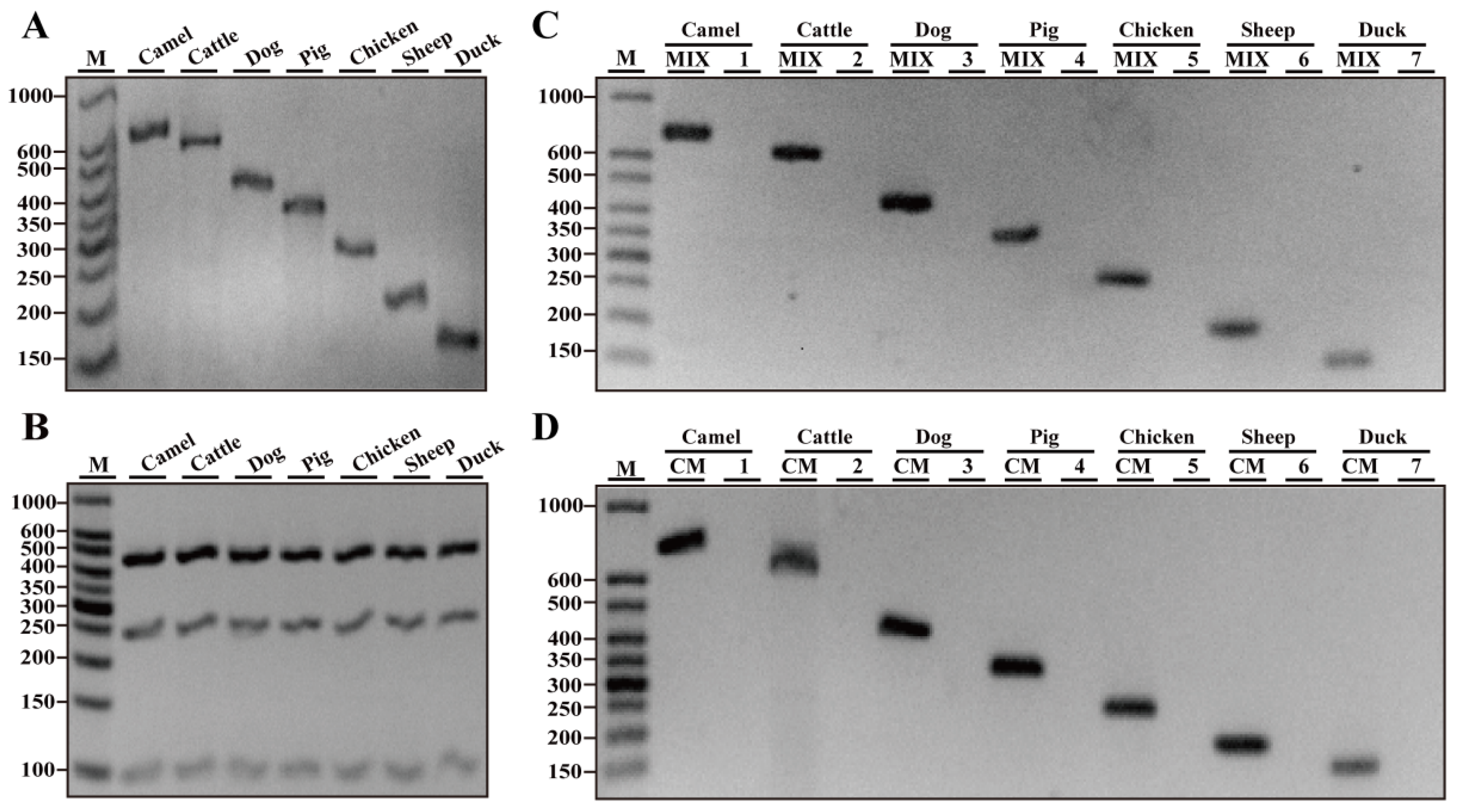
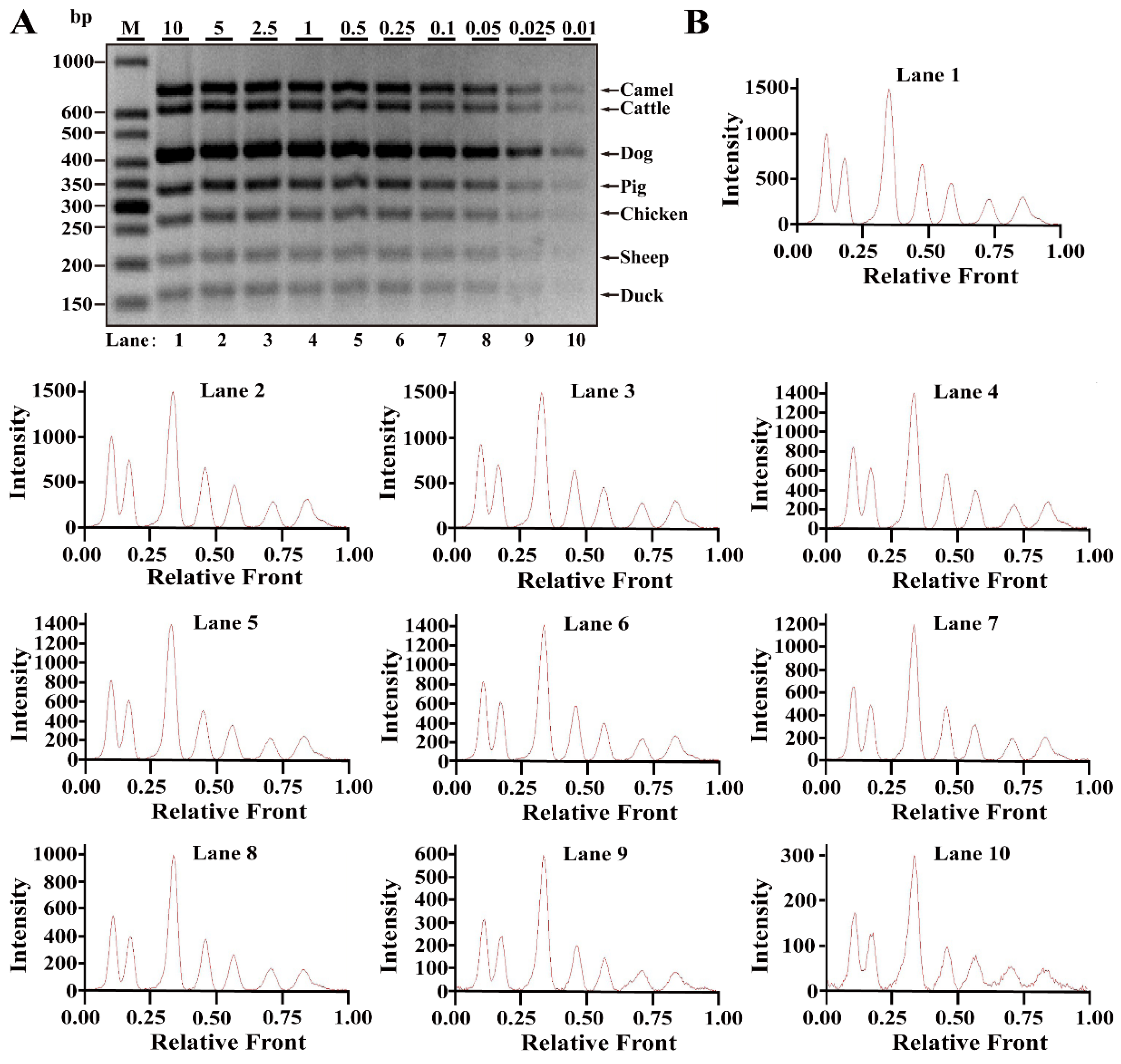
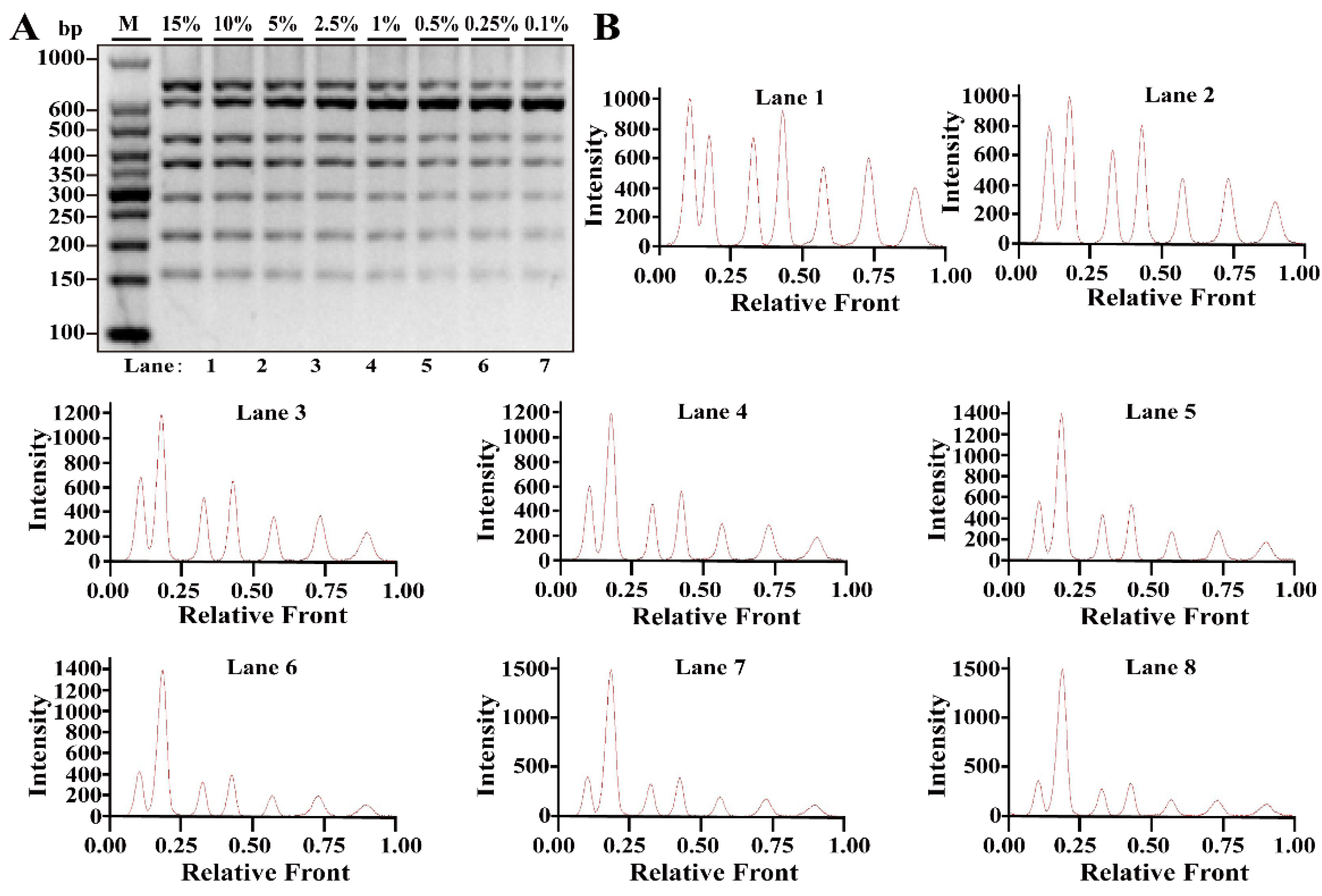

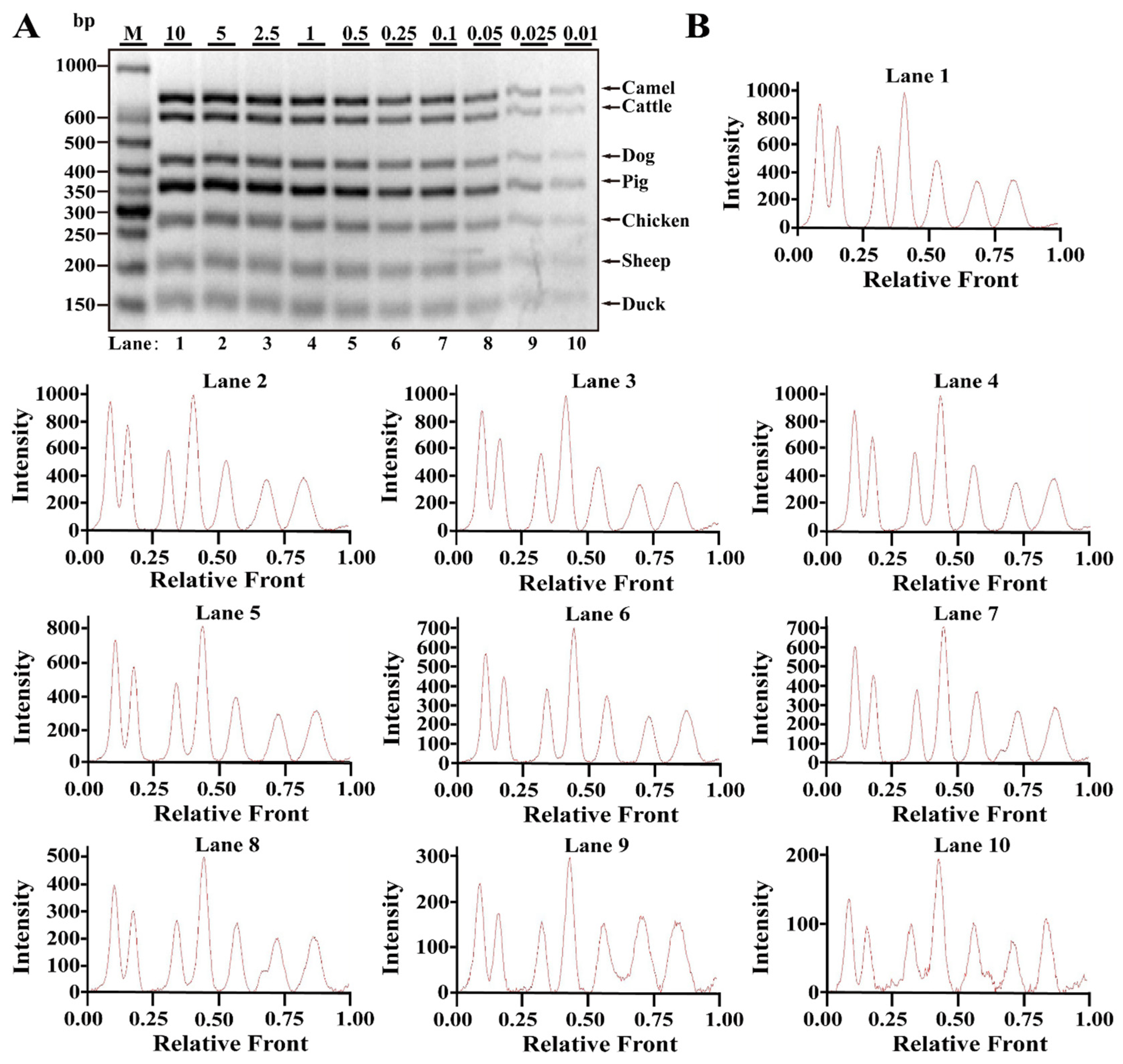
| Primers | Genes | Sequence (5′-3′ Direction) | Amplicons (bp) | Reference or Source |
|---|---|---|---|---|
| Camel | NADH dehydrogenase subunit 4 | TCGCCAGCCAATCTCACCTCT | 726 | this study |
| CATGGGCAACTATAAGGGTCGTA | ||||
| Cattle | Cytochrome c oxidase subunit I | ATGAGCCCACCATATATTCACT | 610 | this study |
| TGTCGTGGTTAAGTCTACAGTCA | ||||
| Dog | NADH dehydrogenase subunit 5 | TGGCCTATTTAAAGTCCTTCCCT | 428 | this study |
| CTAGTGCCAGGATGAAACCCAA | ||||
| Pig | NADH dehydrogenase subunit 4 | AGCCTATCCATTCCTCATGCTTT | 332 | this study |
| CTAGGTTTGTGAGGCTTGCTAC | ||||
| Chicken | 16S rRNA | GAGTGCGTCAAAGCTCCCTC | 268 | this study |
| GTTTGCCGAGTTCCTTCTGT | ||||
| Sheep | NADH dehydrogenase subunit 2 | ATCCAATAGCCTCCATACTCA | 205 | this study |
| ATTGATAGGGTTAGGATCAGGTC | ||||
| Duck | Cytochrome c oxidase subunit III | TCCACGCCCTAACATTGACGATT | 163 | this study |
| AAGGTGGATCCGATGATCACT | ||||
| Eukaryotes | 12S rRNA | CAACTGGGATTAGATACCCCACTAT | 456 | [20] |
| GAGGGTGACGGGCGGTGTGT | ||||
| Eukaryotes | 16S rRNA | AAGACGAGAAGACCCTATGGA | 240 | [21] |
| GATTGCGCTGTTATCCCTAGGGTA | ||||
| Eukaryotes | 18S rRNA | AGGATCCATTGGAGGGCAAGT | 99 | [22] |
| TCCAACTACGAGCTTTTTAACTGCA |
| Products | Number | Labelled | Detected Species | Adulteration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Camel | Cattle | Dog | Pig | Chicken | Sheep | Duck | ||||
| Sheep | 15 | 5 (33.3%) | ||||||||
| meat balls | 5 | mutton | 1/5 a, 1/5 b | 1/5 a | 5/5 | 1/5 b | ||||
| meat slices | 5 | mutton | 1/5 | 5/5 | ||||||
| kebab | 5 | mutton | 2/5 | 5/5 | ||||||
| Cattle | 15 | 4 (26.7%) | ||||||||
| meat balls | beef | 5/5 | 1/5 a, 1/5 b | 1/5 c | 1/5 a | |||||
| meat slices | beef | 5/5 | ||||||||
| kebab | beef | 5/5 | 1/5 | |||||||
| Camel | 10 | 1 (10%) | ||||||||
| drysaltery | 5 | camel | 5/5 | 1/5 | ||||||
| jerky | 5 | camel | 5/5 | |||||||
| Multiplex PCR Type | Species Number | Detection Items | Detection Limit | Detection Method | Reference or Source |
|---|---|---|---|---|---|
| Heptaplex | 7 | camel, cattle, dog, pig, chicken, sheep and duck | 0.025 ng DNA or 0.1% for each species | Gel | This study |
| Multiplex | 3 | chicken, turkey, duck | 1 pg for each species | Gel | [29] |
| Tetraplex | 3 | pig, cattle, fish | 0.001–0.1 ng DNA | Gel | [30] |
| Multiplex | 4 | ruminant, poultry, pork, donkey | 0.01–0.1 ng/μL DNA | Gel | [1] |
| Multiplex | 4 | pork, chicken, duck, cattle | 0.1% for each species | Gel | [31] |
| tetraplex | 4 | horse, soybean, poultry, pork | 0.01% for each species | Gel | [32] |
| Multiplex | 4 | chicken, duck, pork, beef | 0.05% for each species | Gel | [33] |
| Multiplex | 4 | buffalo, cattle, pork, duck | 1 pg DNA, 0.1% for each species | Gel | [34] |
| Quadruplex | 4 | chicken, mutton, beef, pork | 16 pg DNA, 0.01% of each species | Gel | [35] |
| Multiplex | 4 | rat, fox, duck, sheep | 0.05 ng/μL DNA | Gel | [36] |
| Multiplex | 5 | cat, dog, pig, monkey, rat | 0.01–0.02 ng DNA | chip | [19] |
| Multiplex | 5 | sheep/goat, bovine, chicken, duck, pig | 0.5 ng DNA | Gel | [26] |
| Hexaplex | 6 | horse, soybean, sheep, poultry, pork, cow | 0.01% for each species | Gel | [37] |
| Hexaplex | 6 | chicken, cow/buffalo, sheep/goat, horse/donkey, pork, dog | 0.03–0.05 ng DNA | Gel | [25] |
| Multiplex | 6 | goat, chicken, cattle, sheep, pig, horse | 0.25 ng DNA | Gel | [38] |
| Multiplex | 6 | mutton, pork, duck, chicken, horse, cat | 9.1% of each species | Gel | [39] |
| Septuple | 7 | turkey, goose, pig, sheep, beef, chicken, duck | 0.01–0.05 ng DNA | Gel | [17] |
| Heptaplex | 7 | pig, beef, horse, duck, chicken, pigeon, camel | 0.01–0.025 ng DNA or 0.1% for each species | Gel | [3] |
| Octuplex | 8 | dog, chicken, cattle, pig, horse, donkey, fox, rabbit | 0.05 ng/μL DNA | Gel | [21] |
| Multiplex (two-tube) | 10 | beef, sheep, pork, chicken, turkey; cat, dog, mouse, rat, human | 30 pg DNA | Gel | [24] |
| Multiplex (two-tube) | 12 | horse, pigeon, camel, rabbit, ostrich, beef; turkey, dog, chicken, duck, cat, goose | 0.05–0.1 ng DNA | Gel | [28] |
| Multiplex (two-tube) | 14 | cattle, donkey, canidae (dog, fox, raccoon-dog), deer, horse; pig, ovis (sheep, goat), poultry (chicken, duck), cat, mouse | 0.02–0.2 ng DNA | Chip | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Wang, S.; Ju, S.; Zhou, S.; Zeng, X.; Wu, Z.; Pan, D.; Zhong, G.; Cai, Z. Heat-Treated Meat Origin Tracing and Authenticity through a Practical Multiplex Polymerase Chain Reaction Approach. Nutrients 2022, 14, 4727. https://doi.org/10.3390/nu14224727
Cheng Y, Wang S, Ju S, Zhou S, Zeng X, Wu Z, Pan D, Zhong G, Cai Z. Heat-Treated Meat Origin Tracing and Authenticity through a Practical Multiplex Polymerase Chain Reaction Approach. Nutrients. 2022; 14(22):4727. https://doi.org/10.3390/nu14224727
Chicago/Turabian StyleCheng, Yan, Sha Wang, Shilong Ju, Song Zhou, Xiaoqun Zeng, Zhen Wu, Daodong Pan, Guowei Zhong, and Zhendong Cai. 2022. "Heat-Treated Meat Origin Tracing and Authenticity through a Practical Multiplex Polymerase Chain Reaction Approach" Nutrients 14, no. 22: 4727. https://doi.org/10.3390/nu14224727
APA StyleCheng, Y., Wang, S., Ju, S., Zhou, S., Zeng, X., Wu, Z., Pan, D., Zhong, G., & Cai, Z. (2022). Heat-Treated Meat Origin Tracing and Authenticity through a Practical Multiplex Polymerase Chain Reaction Approach. Nutrients, 14(22), 4727. https://doi.org/10.3390/nu14224727






