Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Selection of Studies and Data Extraction
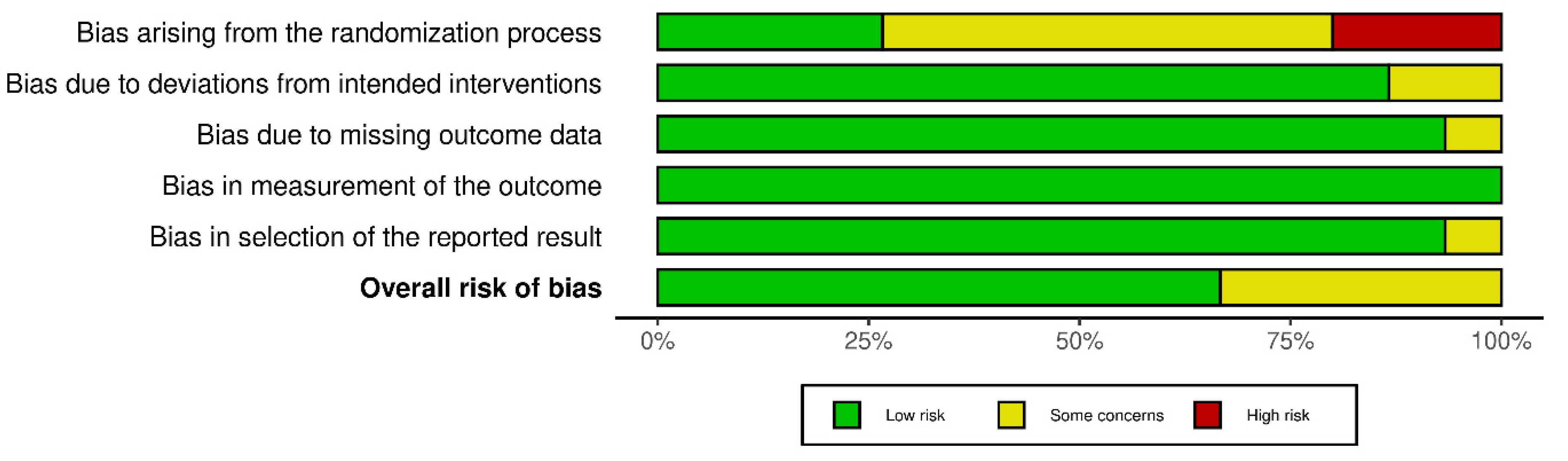
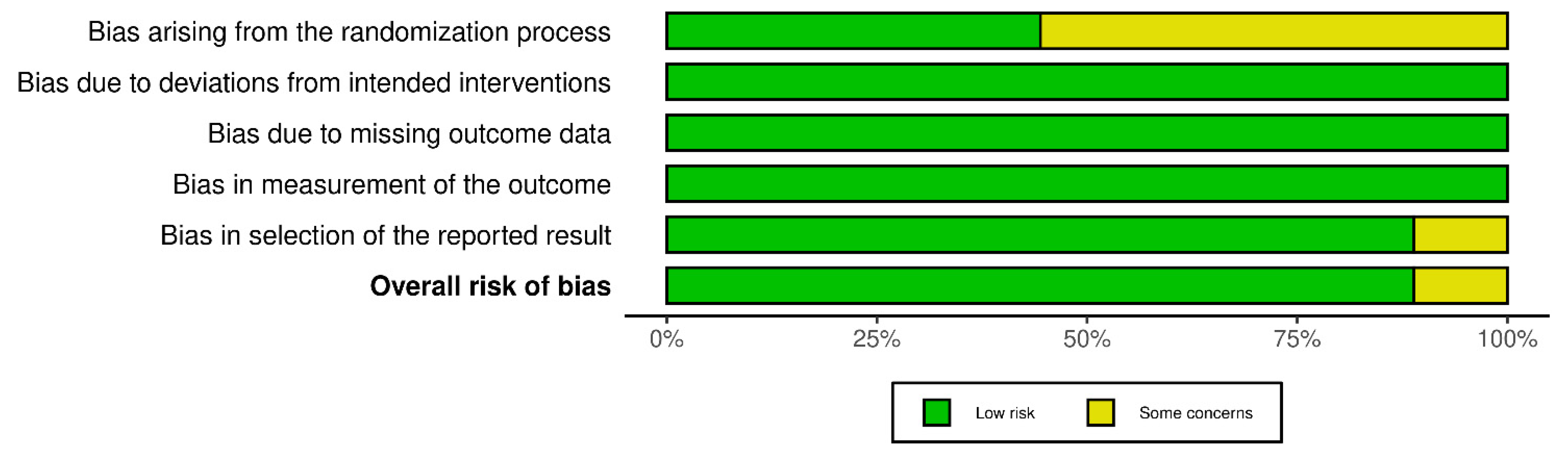
2.4. Risk of Bias
3. Results
3.1. Study Characteristics
| Reference | Country | Duration of Intervention and Study Type | Sample Size | Health Status Age (Years) Sex BMI (kg/m2) | Description of Groups | Dietary Intervention | Body Composition | Glucose Metabolism |
|---|---|---|---|---|---|---|---|---|
| a. Time-restricted feeding protocols | ||||||||
| Andriessen et al., 2022 [54] | Europe | 6 exp. weeks RX | 14 | Adults with T2DM 67.5 ± 5.2 7M:7F 30.5 ± 4.2 | TRF: (14 h fasting:10 h feeding)—last meal not later than 18:00 C: ≥14 h feeding window | TRF, C: habitual diet CGM attached | NA | TRF: ND in hepatic and peripheral Ins sensitivity TRF vs. C: Δ = 2.9 ± 0.3 h/day time spent in normoglycemic range Δ = −2.0 ± 0.2 h/day time spent in high Glu range ND in time spent in hypoglycemia Δ = 1.0 ± 0.0 mg/dL in FG Δ = −14.4 ± 1.8 mg/dL in 24-h mean Glu levels Lower nocturnal Glu levels ND in plasma Ins |
| Bao et al., 2022 [55] | East Asia | 6 exp. days RX | 12 | Healthy volunteers 24.0 ± 2.3 5M:7F 21.9 ± 1.71 | TRF: (18.5 h fasting:5.5 h feeding)—08:00–13:30 C: (13 h fasting:11 h feeding)—08:00–19:00 | Isocaloric diet (55% CHO, 15% Pr, 30% L) CGM attached 1 week washout period | NA | TRF vs. C: Δ = −4.86 ± 0.3 mg/dL 24-h mean blood Glu Δ = −5.94 ± 1.08 mg/dL diurnal blood Glu Δ = −20.88 ± 1.44 mg/dL MAGE Δ = −0.06 ± 0.01% coefficient variation |
| Cai et al., 2019 [39] | East Asia | 12 exp. weeks RCT | 264 | Individuals with Non-Alcoholic Fatty Liver Disease TRF: 33.56 ± 6.23 29M:66F 26.76 ± 1.59 ADF: 35.50 ± 4.417 35M:60F 26.12 ± 2.21 C: 34.54 ± 6.96 23M:79F 26.34 ± 2.73 | TRF: (16 h fasting:8h feeding) ADF: alternation between a feed day with ad libitum food intake and a fast day with an energy restriction of 75% C: no time restriction | TRF: ad libitum feeding and self-selected feeding window ADF: 25% of baseline energy needs through meals prepared in the metabolic kitchen and consumed between 12:00–14:00 C: consumption 80% of energy needs every day without any recommendations for or restrictions on usual lifestyle patterns | TRF vs. C: ↓3.25 ± 0.67 vs. ↓1.85 ± 0.65 kg BW ↓2.91 ± 0.41 vs. ↓1.15 ± 0.11 kg FM [4 wk] ND in FFM ND in BW between TRF and ADF | TRF vs. C: ND in FG and fasting Ins |
| Che et al., 2021 [40] | East Asia | 12 exp. weeks RCT | 104 | Overweight adults with Type 2 Diabetes TRF: 48.21 ± 9.32 31M:29F 26.42 ± 1.96 C: 48.78 ± 9.56 34M:26F 26.08 ± 2.14 | TRF: (14 h fasting:10 h feeding)—08:00–18:00 C: no time restriction | TRF: ad libitum feeding C: Maintenance of usual diet—ad libitum intake 2 weeks baseline weight stabilization period | TRF vs. C: ↓2.98 ± 0.43 vs. ↑0.83 ± 0.32 kg (↓4 vs. ↑1%) BW ↓1.64 ± 0.38 vs. ↑0.42 ± 0.24 kg/m2 BMI | TRF vs. C: ↓1.54 ± 0.19 vs. ↓0.66 ± 0.16% HbA1c ↓26.46 ± 4.5 vs. ↓14.04 ± 3.78 mg/dL FG ↓0.51 ± 0.08 vs. ↓0.12 ± 0.06 HOMA-IR ↑0.73 ± 0.21 vs. ↑0.27 ± 0.1 HOMA-β |
| Chow et al., 2020 [41] | North America | 12 exp. weeks RCT | 20 | Overweight humans 45.5 ± 12.1 3M:17F 34.1 ± 7.5 | TRF: (16 h fasting:8 h feeding) C: unrestricted feeding | Pre-intervention phase: 14 days with CGM TRF: self-selected feeding window, ad libitum feeding C: usual habits | TRF: ↓3.7 ± 1.8% (3.6 kg) BW ↓4.0 ± 2.9% (1.7 kg) BF ↓3.0 ± 2.7% (1.4 kg) LBM ↓11.1 ± 13.4% (0.3 kg) visceral fat TRF vs. C: ↓3.6 vs. ↓1.5 (Δ = −2.1) kg BW ↓0.3 vs. ↓0 (Δ = −0.3) kg visceral fat ↓1.4 vs. ↓0.1 (Δ = −1.3) kg LBM | TRF: ↓7.7 ± 6.9% FG ↑4.1 ± 5.5% TIR |
| Cienfuegos et al., 2020 [42] | North America | 8 exp. weeks RCT | 49 | Adults with obesity 4h-TRF: 49 ± 2 2M:14F 36 ± 1 6h-TRF: 46 ± 3 1M:18F 37 ± 1 C: 45 ± 2 2M:12F 36 ± 1 | 4h-TRF: (20 h fasting:4 h feeding)—15:00–19:00 6h-TRF: (18 h fasting:6 h feeding)—13:00–19:00 C: no meal timing restriction | Phase one: 2 weeks weight stabilization Phase two: intervention 4h-TRF: ad libitum intake 6h-TRF: ad libitum intake C: Maintenance of usual diet pattern | 4h-TRF: ↓3.2 ± 0.4% BW 6h-TRF: ↓3.2 ± 0.4% BW ND in weight loss between 4h-TRF and 6h-TRF Intention-to-treat analysis: 4h-TRF: ↓3.9 ± 0.4% BW 6h-TRF: ↓3.4 ± 0.4% BW ND in weight loss between 4h-TRF and 6h-TRF 4h-TRF: ↓2.8 ± 0.4 kg FM 6h-TRF: ↓1.4 ± 0.3 kg FM ND in FM loss between 4h-TRF and 6h-TRF 4h-TRF: ↓0.8 ± 0.4 kg LBM 6h-TRF: ↓1.5 ± 0.2 kg LBM 6h-TRF vs. 4h-TRF: Δ= −0.7 kg LBM ND in visceral fat loss between groups | ND in FG between groups 4h-TRF: ↓2.3 ± 1.5 μIU/mL fasting Ins 6h-TRF: ↓1.9 ± 1.1 μIU/mL fasting Ins ND in fasting Ins between 4h-TRF and 6h-TRF 4h-TRF: ↓29% IR 6h-TRF: ↓12% IR ND in IR between 4h-TRF and 6h-TRF |
| Domaszewski et al., 2020 [43] | Central Europe | 6 exp. weeks RCT | 42 | Non-smoking women >60 years 65 ± 5 42F TRF: 28.99 ± 5.18/C: 29.99 ± 4.20 | TRF: (16 h fasting:8 h feeding)—12:00–20:00 C: unrestricted time | Ad libitum feeding Usual physical activity | TRF (vs. C): ↓1.36 ± 0.09 (vs. ↑0.55 ± 0.15) kg BW ↓1.29 ± 0.08 (vs. ↑0.62 ± 0.45) kg/m2 BMI ↓1.5 ± 0.12 (vs. ↑1.15 ± 0.37) kg relative FM ↓1.66 ± 0.09 kg absolute FM | NA |
| Hutchison et al., 2019 [56] | Australia | 14 exp. days RX | 15 | Men at Risk for Type 2 Diabetes 55 ± 3 15M 33.9 ± 0.8 | eTRF: (15 h fasting:9 h feeding)—08:00–17:00 dTRF: (15 h fasting:9 h feeding)—12:00–21:00 | 2 weeks baseline period with CGM 2 × 7 days intervention periods with CGM Usual diet and sleep patterns in both groups 2 weeks washout period | eTRF: ↓1.3 ± 0.2 kg BW dTRF: ↓0.8 ± 0.3 kg BW ND in BW between groups | eTRF: ↓mean FG by CGM ↓mean 3-h PPG aftethe r first meal ND in FG by CGM between groups TRF: ↓36% (−28.8 ± 7.2 mg/dL/h) Glu iAUC Meal at 12:00 vs. 08:00: ↑21% (15.66 ± 7.2 mg/dL/h) Glu iAUC |
| Jamshed et al., 2019 [57] | North America | 8 exp. days RX | 11 | Healthy 32 ± 7 7M:4F 30.1 ± 2.7 | eTRF: (18 h fasting:6 h feeding)—08:00–14:00 C: (12 h fasting:12 h feeding)—08:00–20:00 | 2 days: regular feeding schedule, sleep, and exercise 2 days: provided meals (50% CHO, 35% F, 15% Pr) for weight maintenance under sedentary conditions, refrained from caffeine, no other food and beverages containing calories allowed, all food intake was matched across arms, with no weigh-backs allowed CGM attached eTRF: B = 08:00 (33%), L = 11:00 (33%), D = 14:00 (33%) C: B = 08:00 (33%), L = 14:00 (33%), D = 20:00 (33%), Sleeping 22:30–06:30 3.5–5 weeks washout | NA | eTRF vs. C (Δ): ↓3 ± 1 mg/dL mean 3-h PPG levels after B ↓7 ± 2 mg/dL mean Glu levels while sleeping ↓4 ± 1 mg/dL 24-h Glu levels ND in mean Glu levels while awake ↓12 ± 3 mg/dL MAGE ↓2 ± 1 mg/dL FG ↓2.9 ± 0.4 mU/L fasting Ins ↓0.73 ± 0.11 HOMA-IR ↑1.09 ± 0.43 evening HOMA-IR ↑4.5 ± 1.6 mU/L evening fasting Ins ↑25 ± 9% IRS2 gene expression ↑4 ± 1% AKT2 gene expression |
| Jamshed et al., 2022 [44] | North America | 14 exp. weeks RCT | 59 | Adults with obesity 43 ± 11 18M:72F 39.6 ± 6.7 | eTRF: (16 h fasting:8 h feeding)—07:00–15:00 + energy restriction C: ≥12 h window + energy restriction | eTRF, C: One-to-one counseling Hypocaloric diet (↓500 kcal/day) | eTRF vs. C: ↓6.3 (5.7%) vs. ↓4 (4.2%) kg BW ND in FM, FFM, ALM, WC, trunk fat and visceral fat eTRF: ↓6.3 kg (5.7%) BW ↓4.7 kg FM ↓1.5 kg FFM ↓2.8 kg trunk fat ↓0.3 kg visceral fat ↓5.3 cm WC ↓0.9 kg ALM | eTRF vs. C: ND in Glu levels, Ins levels, HOMA-IR, HOMA-β and HbA1c eTRF: ↓8 mg/dL FG ↓6.4 μIU/mL Ins ↓2 HOMA-IR |
| Lin et al., 2022 [45] | East Asia | 8 exp. weeks RCT | 63 | TRF: 50.1 ± 7.5 30F 25.9 ± 3.7 C: 54.2 ± 7.9 33F 25.7 ± 3.8 | TRF: (16 h fasting:8 h feeding)—10:00–18:00 or 12:00–20:00 C: no time restriction | 1 week washout TRF, C: daily low-calorie diet of 1400 kcal | TRF: ↓4.1 ± 2.8% BW ↓5.0 ± 5.8% BMI ↓2.1 ± 1.1% WC ↓1.9 ±1.5 %BF ↓12.8 ± 9.6% visceral fat mass ↓1.88 ± 2.2% FFM ↓0.01 ± 0.03% WHR TRF vs. C (Δ): ↓4.1 ± 2.8 vs. ↓2.4 ± 2.5 (Δ= −1.7 ± 0.3) % BW ↓5.0 ± 5.8 vs. ↓2.2 ± 2.9 (Δ= −2.8 ± 2.9) % BMI | TRF: ↑5.2 ± 8.9% (4.3 mg/dL) FG ↑27.3 ± 67.9% HOMA-IR |
| Liu et al., 2022 [46] | East Asia | 12 exp. months RCT | 118 | Obese individuals 31.9 ± 9.1 71M:68F TRF: 31.8 ± 2.9/C: 31.3 ± 2.6 | TRF: (16 h fasting:8 h feeding)—08:00–16:00 + energy restriction C: no time restriction + energy restriction | TRF, C: daily low-calorie diet (75% of daily energy needs)—1500–1800 kcal/day for males and 1200–1500 kcal/day for females—40–55% CHO, 15–20% Pr, 20–30% F | TRF: ↓8.0 kg BW ↓5.9 kg FM ↓2.9 kg/m2 BMI ↓4.3 %BF ↓1.7 kg LBM ↓8.8 cm WC TRF vs. C: ND in BW, BMI, WC, FM, LBM, visceral fat and ALM | TRF: ↓3.5 mg/dL FG ↓10.8 mg/dL 2-h PPG ↓1 HOMA-IR TRF vs. C: ND in FG, 2-h PPG and HOMA-IR |
| Lowe et al., 2020 [47] | North America | 12 exp. weeks RCT | 105 (via mobile app) 46 (in-person cohort) | In total (n = 105) 46.5 ± 10.5 70M:46F 32.7 ± 4.2 In-person (n = 46) 43.8 ± 11.2 28M:22F 31.4 ± 4.0 | TRF: (16 h fasting:8 h feeding)—12:00–20:00 C: no time restriction | TRF: ad libitum feeding C: 3 structured meals, snacks permitted No recommendation for calorie and macronutrient intake or physical activity | In total (n = 105) TRF: ↓0.94 kg (1.17%) BW ND in BW between groups In-person (n = 46) TRF: ↓1.7 kg (1.81%) BW ↓0.56 kg/m2 BMI ↓1.10 kg LBM ↓0.64 kg ALM ↓0.22 kg ALMI ↓0.47 kg trunk lean mass ND in FM TRF vs. C: ND in LBM, trunk lean mass and FM ↓0.64 vs. ↓0.17 (Δ = −0.47) kg in ALM ↓0.22 vs. ↓0.06 (Δ = −0.16) kg in ALMI | ND in FG, fasting Ins, HbA1c and HOMA-IR in TRF or between groups |
| Martens et al., 2020 [58] | North America | 12 exp. weeks RX | 22 | Healthy non-obese adults 67 ± 1 10M:12F 24.7 ± 0.6 | TRF: (16 h fasting:8 h feeding)—from 10:00–11:00 until 18:00–19:00 C: no time restriction | 1 week lead-in period TRF, C: normal feeding pattern | TRF: ND in BW | ND in FG and Ins levels at any time TRF vs. C: ↓Glu AUC |
| Nakamura et al., 2021 [59] | East Asia | 6 exp. days RX | 12 | Non-smokers healthy participants 3M:11F | TRF: (14 h fasting:10 h feeding)—08:00–18:00 C: 08:00—21:00 | 3 days each protocol CGM attached Day 1: prescribed diet, feeding at the designated time Day 2,3: prescribed diet and feeding at a set time under laboratory conditions Refrain from exercising from the day before the 3-day experimental period At least 3 days washout | ND in BW between groups | TRF: ↓8 ± 9 mg/dL MAGE Differences in the mean blood Glu levels between the two groups throughout the day and during the 12-h period from night to early morning ND in average blood Glu levels during the day between the two groups TRF vs. C: ↓mean Glu throughout the day and from 18:00 to 06:00 ↓PPG iAUC (D) |
| Parr et al., 2020 [7] | Australia | 10 exp. days RX | 11 | Sedentary men with overweight/obesity 38 ± 5 11M 32.2 ± 2 | TRF: (16 h fasting:8 h feeding)—10:00–18:00 C: (15 h extended feeding)—07:00–21:00 | 2 × 5 days isoenergetic diet (50% F, 30% CHO, 20% Pr) Provided foods CGMs attached 10 days washout | NA | TRF vs. C: ↓75.6 ± 104.4 mg/dL/h nocturnal Glu AUC ↓18.0 ± 16.2 mg/dL peak venous Glu (B) ↓16.2 ± 12.6 mg/dL peak venous Glu (L) ↓43.2 ± 28.5 mg/dL/h venous Glu iAUC (L) ↓18.0 ± 12.6 mg/dL peak interstitial Glu (B) ↓18.0 ± 21.6 mg/dL/h interstitial Glu iAUC (B) ↓57.6 ± 61.2 mg/dL/h interstitial Glu iAUC (D) ↓42.0 ± 61.0 μIU/mL peak Ins (B) ↓35.0 ± 43.0 μIU/mL peak Ins (L) ↑33.0 ± 44.0 μIU/mL peak Ins (D) ↓134.0 ± 96.0 μIU/h/mL Ins iAUC (L) |
| Phillips et al., 2021 [49] | Central Europe | 6 exp. months RCT | 45 | Adults with at least one component of Metabolic Syndrome (according to the International Diabetes Foundation) ≥18 years TRF: 28.0 ± 4.1/C: 27.0 ± 4.0 | TRF: (12 h fasting:12 h feeding)—self-selected feeding window C: no time restriction | TRF: no advice on nutrition quality, the quantity of macronutrients, or calorie intake C: standard dietary advice (food pyramid and Swiss recommendations for a balanced and healthy diet)—no advice on calorie intake | TRF: ↓1.6 ± 2.9 % BW ↓0.5 ± 0.2 kg/m2 BMI ↓1.5 ± 0.8 cm WC TRF vs. C: ND in BW, BMI, and WC | TRF: ND in FG and HbA1c TRF vs. C: ND in FG and HbA1c |
| Pureza et al., 2020 [50] | South America | 12 exp. months RCT | 27 | Low-income women with obesity TRF: 31.8 ± 6.96 31F 33.53 ± 4.53 C: 31.03 ± 7.16 27F 33.12 ± 3.63 | TRF: (12 h fasting:12 h feeding) C: no time restriction | TRF, C: Personalized hypoenergetic diet (↓500–1000 kcal/day) | ND in BW between groups TRF vs. C: ↓0.97 vs. ↑0.66 %BF | NA |
| Ravussin et al., 2019 [60] | North America | 8 exp. days RX | 11 | Healthy overweight adults 32 ± 7 7M:4F 30.1 ± 2.7 | eTRF: (18 h fasting:6 h feeding)—08:00–14:00 C: (12 h fasting:12 h feeding)—08:00–20:00 | 2 × 4 days isocaloric feeding Day 1–2: free-living Day 3–4: provided meals (50% CHO, 35% F, 15% Pr) Matched meals across arms 3.5–5 weeks washout | ND in BW | NA |
| Sutton et al., 2018 [61] | North America | 10 exp. weeks RX | 8 | Prediabetes 56 ± 9 8M 32.2 ± 4.4 | eTRF: (18 h fasting:6 h feeding)—from 06:30–08:30 until 12:30–14:30 C: (12 h fasting:12 h feeding) | Isocaloric and eucaloric feeding 2 × 5 weeks intervention eTRF: 3 provided meals every 3 h C: 3 provided meals every 6 h Matched food intake on a meal-by-meal basis Consistent physical activity and sleep patterns Day 1: all participants 3 meals over a 10-h period Days 2–36: assigned schedule 7 weeks washout | ND in BW in TRF or between groups | eTRF: ND in FG, Glu levels at any time of OGTT and mean Glu levels ↓3.4 ± 1.6 mU/L fasting Ins ↓Ins levels at t = 60min and 90min post-meal ↓26 ± 9 mU/L mean Ins values ↓35 ± 13 mU/L peak Ins values ↑14 ± 7 U/mg insulinogenic index (β-cell responsiveness) ↓36 ± 10 U/mg iAUC ratio |
| Thomas et al., 2022 [51] | North America | 39 exp. weeks RCT | 63 | Adults with obesity 38.0 ± 7.8 12M:69F 34.1 ± 5.7 | eTRF: (14 h fasting:10 h feeding) + daily caloric restriction C: daily caloric restriction, no time restriction | Behavioral weight loss intervention with a personalized calorie goal | eTRF: ↓6.2 ± 4.1 kg (↓6.3 ± 4.1%) BW by clinic (week-12) ↓4.4 ± 2.6 kg (4.6 ± 2.7%) BW by DXA (week-12) ↓4.9 ± 5.3 kg (5.2 ± 5.9%) BW by DXA (week-39) ↓3.5 ± 4.0 kg FM by DXA (week-39) ↓1.2 ± 2.3 kg FFM by DXA (week-39) ND in BW, FM and FFM between groups | ND in HbA1c in TRF or between groups |
| Xie et al., 2022 [52] | East Asia | 5 exp. weeks RCT | 82 | Healthy individuals eTRF: 28.68 ± 9.707 4M:24F 22.7 ± 3.1 mTRF: 31.08 ± 8.438 7M:19F 21.4 ± 2.2 C: 33.57 ± 11.6 7M:21F 21.5 ± 2.9 | eTRF: (16 h fasting:8 h feeding)—self-selected feeding window between 06:00–15:00 mTRF: (16 h fasting:8 h feeding)—self-selected feeding window between 11:00–20:00 C: no time restriction | eTRF, mTRF, C: ad libitum feeding, usual feeding regimens | eTRF vs. C: ↓1.6 ± 1.4 vs. ↑0.3 ± 1.2 kg BW ↓0.76 ± 1.01 vs. ↓0.41 ± 0.89 kg FM ↓0.6 ± 1.22 vs. ↓0.42 ± 1.16 %BF ND in BW, FM and %BF between mTRF and eTRF or between mTRF and C | eTRF vs. mTRF/C: ↓1.08 ± 1.59 vs. ↑0.39 ± 0.71/↑0.05 ± 0.75 HOMA-IR eTRF vs. C: ↓10.62 ± 15.12 vs. ↑2.88 ± 6.84 mg/dL FG ND in FG between eTRF and mTRF |
| b. Ramadan fasting protocols | ||||||||
| Lum et al., 2020 [48] | Southeast Asia | 30 exp. days (mean) RCT | 97 | Individuals with Type 2 Diabetes 59.5 ± 11.2 39M:58F 30.3 ± 5.5 | RF: 13.5 h fasting C: usual care—non-fasters | All participants: workshop which addressed Ramadan-specific self-care practices with lifestyle counseling, appropriate glucose monitoring, glucose-lowering medication management, and management of acute diabetes complications | NA | RF: ↓4.4 mmol/mol (0.4%) HbA1c (post-RF) ↓1.3 ± 12.6 mg/dL FG (post-RF) ↓3.6 mg/dL FG (3-month follow-up post-RF) ↓16.4 mg/dL PPG RF vs. C: ↓0.4 vs. ↓0.1% HbA1c ↓3.6 vs. ↑20.9 mg/dL FG (3-month follow-up post-RF) ND in PPG and % glycemic variability between groups |
| Zouhal et al., 2020 [53] | North Africa | 30 exp. days RCT | 30 | Sedentary males with obesity RF: 24.5 ± 3.8 15M 33.3 ± 1.3 C: 23.8 ± 3.7 15M 33.5 ± 2.7 | RF: (15–16 h fasting:8–9 h feeding) C: unrestricted time | C: normal daily habits | RF: ↓3.2% BW ↓3.1% (1.0 ± 0.1 kg/m2) BMI ↓5.8 %BF ↓6.2% (0.06 ± 0.03) WHR | NA |
3.2. Main Exposures
3.3. TRF Studies on Body Weight and Body Composition
3.3.1. Healthy Individuals
Normal Weight
Overweight and Obesity
3.3.2. Individuals with Metabolic Abnormalities
3.4. TRF Studies on Glycemic Responses and Insulin Sensitivity
3.4.1. Healthy Individuals
Normal Weight
Overweight and Obesity
3.4.2. Individuals with Metabolic Abnormalities
3.5. RF Studies on Body Weight, Body Composition, Glycemic Responses, and Insulin Sensitivity
4. Discussion
4.1. Effects of TRF on Body Weight and Body Composition
4.1.1. Mechanisms of Action
4.1.2. Energy Restriction
4.1.3. Distribution of Eating Windows
4.2. Effects of TRF on Glycemic Responses and Insulin Sensitivity
4.2.1. Mechanisms of Action
4.2.2. Meal Timing
4.3. Effects of TRF on Clock Genes Expression
4.4. Effects of RF on Body Weight, Body Composition, and Glycemic Responses
4.5. Areas of Future Scientific Interest
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henry, C.J.; Kaur, B.; Quek, R.Y.C. Chrononutrition in the Management of Diabetes. Nutr. Diabetes 2020, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harfmann, B.D.; Schroder, E.A.; Esser, K.A. Circadian Rhythms, the Molecular Clock, and Skeletal Muscle. J. Biol. Rhythms 2015, 30, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Reinke, H.; Asher, G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Oikonomou, C.; Nychas, G.; Dimitriadis, G.D. Effects of Diet, Lifestyle, Chrononutrition and Alternative Dietary Interventions on Postprandial Glycemia and Insulin Resistance. Nutrients 2022, 14, 823. [Google Scholar] [CrossRef]
- Charlot, A.; Hutt, F.; Sabatier, E.; Zoll, J. Beneficial Effects of Early Time-Restricted Feeding on Metabolic Diseases: Importance of Aligning Food Habits with the Circadian Clock. Nutrients 2021, 13, 1405. [Google Scholar] [CrossRef]
- Laermans, J.; Depoortere, I. Chronobesity: Role of the Circadian System in the Obesity Epidemic: Role of Circadian Clocks in Obesity. Obes. Rev. 2016, 17, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Radford, B.E.; Hawley, J.A. A Delayed Morning and Earlier Evening Time-Restricted Feeding Protocol for Improving Glycemic Control and Dietary Adherence in Men with Overweight/Obesity: A Randomized Controlled Trial. Nutrients 2020, 12, 505. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Hawley, J.A.; Sassone-Corsi, P.; Zierath, J.R. Chrono-Nutrition for the Prevention and Treatment of Obesity and Type 2 Diabetes: From Mice to Men. Diabetologia 2020, 63, 2253–2259. [Google Scholar] [CrossRef]
- Schuppelius, B.; Peters, B.; Ottawa, A.; Pivovarova-Ramich, O. Time Restricted Eating: A Dietary Strategy to Prevent and Treat Metabolic Disturbances. Front. Endocrinol. 2021, 12, 683140. [Google Scholar] [CrossRef]
- Gu, L.; Fu, R.; Hong, J.; Ni, H.; Yu, K.; Lou, H. Effects of Intermittent Fasting in Human Compared to a Non-Intervention Diet and Caloric Restriction: A Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 871682. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A. Intermittent versus Daily Calorie Restriction: Which Diet Regimen Is More Effective for Weight Loss?: Weight Loss by Calorie Restriction Diets. Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef] [PubMed]
- Hoddy, K.K.; Marlatt, K.L.; Çetinkaya, H.; Ravussin, E. Intermittent Fasting and Metabolic Health: From Religious Fast to Time-Restricted Feeding. Obesity 2020, 28 (Suppl. 1), S29–S37. [Google Scholar] [CrossRef]
- Clayton, D.J.; Mode, W.J.A.; Slater, T. Optimising Intermittent Fasting: Evaluating the Behavioural and Metabolic Effects of Extended Morning and Evening Fasting. Nutr. Bull. 2020, 45, 444–455. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M. Effect of Various Types of Intermittent Fasting (IF) on Weight Loss and Improvement of Diabetic Parameters in Human. Curr. Nutr. Rep. 2021, 10, 146–154. [Google Scholar] [CrossRef]
- Tinsley, G.M.; La Bounty, P.M. Effects of Intermittent Fasting on Body Composition and Clinical Health Markers in Humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef]
- Persynaki, A.; Karras, S.; Pichard, C. Unraveling the Metabolic Health Benefits of Fasting Related to Religious Beliefs: A Narrative Review. Nutrition 2017, 35, 14–20. [Google Scholar] [CrossRef]
- Al-Barha, N.S.; Aljaloud, K.S. The Effect of Ramadan Fasting on Body Composition and Metabolic Syndrome in Apparently Healthy Men. Am. J. Men’s Health 2019, 13, 1557988318816925. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Acar-Tek, N.; Bozkurt, O. Effect of Intermittent Fasting (18/6) on Energy Expenditure, Nutritional Status, and Body Composition in Healthy Adults. Evid. Based Complement. Altern. Med. 2021, 2021, 780961. [Google Scholar] [CrossRef]
- Johnstone, A.M. Fasting? The Ultimate Diet? Obes. Rev. 2007, 8, 211–222. [Google Scholar] [CrossRef]
- Osman, F.; Haldar, S.; Henry, C.J. Effects of Time-Restricted Feeding during Ramadan on Dietary Intake, Body Composition and Metabolic Outcomes. Nutrients 2020, 12, 2478. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Simmons, N.; Awan, S.; Chamari, K.; Ahmed, I. Intermittent Fasting: Eating by the Clock for Health and Exercise Performance. BMJ Open Sport Exerc. Med. 2022, 8, e001206. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Hawley, J.A. Perspective: Time-Restricted Eating—Integrating the What with the When. Adv. Nutr. 2022, 13, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Lustig, E.; Shubrook, J.H.; Pfotenhauer, K.M. Time-Restricted Feeding and Potential for Type 2 Diabetes Mellitus: A Narrative Review. J. Osteopath. Assoc. 2020, 120, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Arnason, T.G.; Bowen, M.W.; Mansell, K.D. Effects of Intermittent Fasting on Health Markers in Those with Type 2 Diabetes: A Pilot Study. World J. Diabetes 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Kesztyüs, D.; Fuchs, M.; Cermak, P.; Kesztyüs, T. Associations of Time-Restricted Eating with Health-Related Quality of Life and Sleep in Adults: A Secondary Analysis of Two Pre-Post Pilot Studies. BMC Nutr. 2020, 6, 76. [Google Scholar] [CrossRef]
- Crose, A.; Alvear, A.; Singroy, S.; Wang, Q.; Manoogian, E.; Panda, S.; Mashek, D.G.; Chow, L.S. Time-Restricted Eating Improves Quality of Life Measures in Overweight Humans. Nutrients 2021, 13, 1430. [Google Scholar] [CrossRef]
- Bitsanis, D.; Giannakou, K.; Hadjimbei, E.; Chrysostomou, S. The Effect of Early Time-Restricted Feeding on Glycemic Profile in Adults: A Systematic Review of Interventional Studies. Rev. Diabet. Stud. 2022, 18, 10–19. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef]
- Leung, G.K.W.; Huggins, C.E.; Ware, R.S.; Bonham, M.P. Time of Day Difference in Postprandial Glucose and Insulin Responses: Systematic Review and Meta-Analysis of Acute Postprandial Studies. Chronobiol. Int. 2020, 37, 311–326. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P. Timing of Food Intake and Obesity: A Novel Association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Karatzi, K.; Moschonis, G.; Choupi, E.; Manios, Y.; Manios, Y.; Moschonis, G.; Skenderi, K.P.; Grammatikaki, E.; Androutsos, O.; Tanagra, S.; et al. Late-Night Overeating Is Associated with Smaller Breakfast, Breakfast Skipping, and Obesity in Children: The Healthy Growth Study. Nutrition 2017, 33, 141–144. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Wainstein, J.; Tsameret, S.; Landau, Z. Role of High Energy Breakfast “Big Breakfast Diet” in Clock Gene Regulation of Postprandial Hyperglycemia and Weight Loss in Type 2 Diabetes. Nutrients 2021, 13, 1558. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, Y.; Hata, S.; Sone, Y. Effects of a Late Supper on Digestion and the Absorption of Dietary Carbohydrates in the Following Morning. J. Physiol. Anthropol. 2013, 32, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Minguez, J.; Saxena, R.; Bandín, C.; Scheer, F.A.; Garaulet, M. Late Dinner Impairs Glucose Tolerance in MTNR1B Risk Allele Carriers: A Randomized, Cross-over Study. Clin. Nutr. 2018, 37, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 2, 55–61. [Google Scholar] [CrossRef]
- Cai, H.; Qin, Y.-L.; Shi, Z.-Y.; Chen, J.-H.; Zeng, M.-J.; Zhou, W.; Chen, R.-Q.; Chen, Z.-Y. Effects of Alternate-Day Fasting on Body Weight and Dyslipidaemia in Patients with Non-Alcoholic Fatty Liver Disease: A Randomised Controlled Trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-Restricted Feeding Improves Blood Glucose and Insulin Sensitivity in Overweight Patients with Type 2 Diabetes: A Randomised Controlled Trial. Nutr. Metab. 2021, 18, 88. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans Who Are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef] [PubMed]
- Domaszewski, P.; Konieczny, M.; Pakosz, P.; Baczkowicz, D.; Sadowska-Krępa, E. Effect of a Six-Week Intermittent Fasting Intervention Program on the Composition of the Human Body in Women over 60 Years of Age. Int. J. Environ. Res. Public Health 2020, 17, 4138. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.-J.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults With Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Wang, Y.-T.; Chan, L.-C.; Chu, N.-F. Effect of Time-Restricted Feeding on Body Composition and Cardio-Metabolic Risk in Middle-Aged Women in Taiwan. Nutrition 2022, 93, 111504. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Lum, Z.K.; Khoo, Z.R.; Toh, W.Y.S.; Kamaldeen, S.A.K.; Shakoor, A.; Tsou, K.Y.K.; Chew, D.E.K.; Dalan, R.; Kwek, S.C.; Othman, N.; et al. Efficacy and Safety of Use of the Fasting Algorithm for Singaporeans With Type 2 Diabetes (FAST) During Ramadan: A Prospective, Multicenter, Randomized Controlled Trial. Ann. Fam. Med. 2020, 18, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Phillips, N.; Mareschal, J.; Schwab, N.; Manoogian, E.; Borloz, S.; Ostinelli, G.; Gauthier-Jaques, A.; Umwali, S.; Gonzalez Rodriguez, E.; Aeberli, D.; et al. The Effects of Time-Restricted Eating versus Standard Dietary Advice on Weight, Metabolic Health and the Consumption of Processed Food: A Pragmatic Randomised Controlled Trial in Community-Based Adults. Nutrients 2021, 13, 1042. [Google Scholar] [CrossRef]
- Pureza, I.R.O.M.; Melo, I.S.V.; Macena, M.L.; Praxedes, D.R.S.; Vasconcelos, L.G.L.; Silva-Júnior, A.E.; Florêncio, T.M.M.T.; Bueno, N.B. Acute Effects of Time-Restricted Feeding in Low-Income Women with Obesity Placed on Hypoenergetic Diets: Randomized Trial. Nutrition 2020, 77, 110796. [Google Scholar] [CrossRef]
- Thomas, E.A.; Zaman, A.; Sloggett, K.J.; Steinke, S.; Grau, L.; Catenacci, V.A.; Cornier, M.-A.; Rynders, C.A. Early Time-Restricted Eating Compared with Daily Caloric Restriction: A Randomized Trial in Adults with Obesity. Obesity 2022, 30, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized Controlled Trial for Time-Restricted Eating in Healthy Volunteers without Obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef] [PubMed]
- Zouhal, H.; Bagheri, R.; Triki, R.; Saeidi, A.; Wong, A.; Hackney, A.C.; Laher, I.; Suzuki, K.; Ben Abderrahman, A. Effects of Ramadan Intermittent Fasting on Gut Hormones and Body Composition in Males with Obesity. Int. J. Environ. Res. Public Health 2020, 17, 5600. [Google Scholar] [CrossRef] [PubMed]
- Andriessen, C.; Fealy, C.E.; Veelen, A.; van Beek, S.M.M.; Roumans, K.H.M.; Connell, N.J.; Mevenkamp, J.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; et al. Three Weeks of Time-Restricted Eating Improves Glucose Homeostasis in Adults with Type 2 Diabetes but Does Not Improve Insulin Sensitivity: A Randomised Crossover Trial. Diabetologia 2022, 65, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Sun, Y.; Jiang, Y.; Ye, L.; Hong, J.; Wang, W. Effects of Time-Restricted Feeding on Energy Balance: A Cross-Over Trial in Healthy Subjects. Front. Endocrinol. 2022, 13, 870054. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-Restricted Feeding Improves Glucose Tolerance in Men at Risk for Type 2 Diabetes: A Randomized Crossover Trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Manna, D.L.D.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [Green Version]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-Term Time-Restricted Feeding Is Safe and Feasible in Non-Obese Healthy Midlife and Older Adults. GeroScience 2020, 42, 667–686. [Google Scholar] [CrossRef]
- Nakamura, K.; Tajiri, E.; Hatamoto, Y.; Ando, T.; Shimoda, S.; Yoshimura, E. Eating Dinner Early Improves 24-h Blood Glucose Levels and Boosts Lipid Metabolism after Breakfast the next Day: A Randomized Cross-over Trial. Nutrients 2021, 13, 2424. [Google Scholar] [CrossRef]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Ratamess, N.A.; Faigenbaum, A.D.; Bush, J.A.; Beller, N.; Vargas, A.; Fardman, B.; Andriopoulos, T. Effect of Time-Restricted Feeding on Anthropometric, Metabolic, and Fitness Parameters: A Systematic Review. J. Am. Coll. Nutr. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; McStay, M.; Gabel, K.; Varady, K.A. Time Restricted Eating for the Prevention of Type 2 Diabetes. J. Physiol. 2022, 600, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Roekenes, J.; Martins, C. Ketogenic Diets and Appetite Regulation. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [Green Version]
- Elibol, B.; Kilic, U. High Levels of SIRT1 Expression as a Protective Mechanism Against Disease-Related Conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Cioffi, I.; Evangelista, A.; Ponzo, V.; Ciccone, G.; Soldati, L.; Santarpia, L.; Contaldo, F.; Pasanisi, F.; Ghigo, E.; Bo, S. Intermittent versus Continuous Energy Restriction on Weight Loss and Cardiometabolic Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Transl. Med. 2018, 16, 371. [Google Scholar] [CrossRef] [Green Version]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Mat Ludin, A.F. The Association between Chronotype and Dietary Pattern among Adults: A Scoping Review. Int. J. Environ. Res. Public Health 2019, 17, 68. [Google Scholar] [CrossRef] [Green Version]
- Van Der Merwe, M.; Sharma, S.; Caldwell, J.L.; Smith, N.J.; Gomes, C.K.; Bloomer, R.J.; Buddington, R.K.; Pierre, J.F. Time of Feeding Alters Obesity-Associated Parameters and Gut Bacterial Communities, but Not Fungal Populations, in C57BL/6 Male Mice. Curr. Dev. Nutr. 2020, 4, nzz145. [Google Scholar] [CrossRef] [Green Version]
- Ruddick-Collins, L.C.; Morgan, P.J.; Fyfe, C.L.; Filipe, J.A.N.; Horgan, G.W.; Westerterp, K.R.; Johnston, J.D.; Johnstone, A.M. Timing of Daily Calorie Loading Affects Appetite and Hunger Responses without Changes in Energy Metabolism in Healthy Subjects with Obesity. Cell Metab. 2022, 34, 1472–1485.e6. [Google Scholar] [CrossRef]
- Vujović, N.; Piron, M.J.; Qian, J.; Chellappa, S.L.; Nedeltcheva, A.; Barr, D.; Heng, S.W.; Kerlin, K.; Srivastav, S.; Wang, W.; et al. Late Isocaloric Eating Increases Hunger, Decreases Energy Expenditure, and Modifies Metabolic Pathways in Adults with Overweight and Obesity. Cell Metab. 2022, 34, 1486–1498.e7. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. 1), S113–S124. [Google Scholar] [CrossRef] [PubMed]
- Churuangsuk, C.; Hall, J.; Reynolds, A.; Griffin, S.J.; Combet, E.; Lean, M.E.J. Diets for Weight Management in Adults with Type 2 Diabetes: An Umbrella Review of Published Meta-Analyses and Systematic Review of Trials of Diets for Diabetes Remission. Diabetologia 2022, 65, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Grams, J.; Garvey, W.T. Weight Loss and the Prevention and Treatment of Type 2 Diabetes Using Lifestyle Therapy, Pharmacotherapy, and Bariatric Surgery: Mechanisms of Action. Curr. Obes. Rep. 2015, 4, 287–302. [Google Scholar] [CrossRef]
- Katsarou, A.L.; Katsilambros, N.L.; Koliaki, C.C. Intermittent Energy Restriction, Weight Loss and Cardiometabolic Risk: A Critical Appraisal of Evidence in Humans. Healthcare 2021, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Heilbronn, L.K. Time-Restricted Eating: Benefits, Mechanisms, and Challenges in Translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Beccuti, G.; Monagheddu, C.; Evangelista, A.; Ciccone, G.; Broglio, F.; Soldati, L.; Bo, S. Timing of Food Intake: Sounding the Alarm about Metabolic Impairments? A Systematic Review. Pharmacol. Res. 2017, 125, 132–141. [Google Scholar] [CrossRef]
- Hatanaka, M.; Hatamoto, Y.; Tajiri, E.; Matsumoto, N.; Tanaka, S.; Yoshimura, E. An Earlier First Meal Timing Associates with Weight Loss Effectiveness in A 12-Week Weight Loss Support Program. Nutrients 2022, 14, 249. [Google Scholar] [CrossRef]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr. Biol. 2021, 31, 650–657.e3. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian Regulation of Glucose, Lipid, and Energy Metabolism in Humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, Z. Circadian Clock and Temporal Meal Pattern. Med. Rev. 2022. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundell, L.S.; Parr, E.B.; Devlin, B.L.; Ingerslev, L.R.; Altıntaş, A.; Sato, S.; Sassone-Corsi, P.; Barrès, R.; Zierath, J.R.; Hawley, J.A. Time-Restricted Feeding Alters Lipid and Amino Acid Metabolite Rhythmicity without Perturbing Clock Gene Expression. Nat. Commun. 2020, 11, 4643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hutchison, A.T.; Liu, B.; Wittert, G.A.; Thompson, C.H.; Nguyen, L.; Au, J.; Vincent, A.; Manoogian, E.N.C.; Le, H.D.; et al. Time–Restricted Eating Alters the 24-hour Profile of Adipose Tissue Transcriptome in Men with Obesity. Obesity 2022. [Google Scholar] [CrossRef]
- Tuvia, N.; Pivovarova-Ramich, O.; Murahovschi, V.; Lück, S.; Grudziecki, A.; Ost, A.-C.; Kruse, M.; Nikiforova, V.J.; Osterhoff, M.; Gottmann, P.; et al. Insulin Directly Regulates the Circadian Clock in Adipose Tissue. Diabetes 2021, 70, 1985–1999. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Fatima, S.; Zaman, M.H.; Khan, S.A.; Safdar, M.; Alam, I.; Feng, Q. Time-Restricted Feeding Regulates Molecular Mechanisms with Involvement of Circadian Rhythm to Prevent Metabolic Diseases. Nutrition 2021, 89, 111244. [Google Scholar] [CrossRef]
- Fernando, H.; Zibellini, J.; Harris, R.; Seimon, R.; Sainsbury, A. Effect of Ramadan Fasting on Weight and Body Composition in Healthy Non-Athlete Adults: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 478. [Google Scholar] [CrossRef] [Green Version]
- Salti, I.; Bénard, E.; Detournay, B.; Bianchi-Biscay, M.; Le Brigand, C.; Voinet, C.; Jabbar, A.; on behalf of the EPIDIAR Study Group. A Population-Based Study of Diabetes and Its Characteristics During the Fasting Month of Ramadan in 13 Countries. Diabetes Care 2004, 27, 2306–2311. [Google Scholar] [CrossRef]
- Muñoz, J.S.G.; Cañavate, R.; Hernández, C.M.; Cara-Salmerón, V.; Morante, J.J.H. The Association among Chronotype, Timing of Food Intake and Food Preferences Depends on Body Mass Status. Eur. J. Clin. Nutr. 2017, 71, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Phoi, Y.Y.; Rogers, M.; Bonham, M.P.; Dorrian, J.; Coates, A.M. A Scoping Review of Chronotype and Temporal Patterns of Eating of Adults: Tools Used, Findings, and Future Directions. Nutr. Res. Rev. 2022, 35, 112–135. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Pellegrini, M.; D’Eusebio, C.; Goitre, I.; Ponzo, V.; Fadda, M.; Rosato, R.; Mengozzi, G.; Beccuti, G.; Merlo, F.D.; et al. The Effects of Time-Restricted Eating on Metabolism and Gut Microbiota: A Real-Life Study. Nutrients 2022, 14, 2569. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.P.; Guimarães, K.C.; Soares, A.G.N.S.; Marqueze, E.C.; Moreno, C.R.C.; Mota, M.C.; Crispim, C.A. Role of Chronotype in Dietary Intake, Meal Timing, and Obesity: A Systematic Review. Nutr. Rev. 2022, nuac044. [Google Scholar] [CrossRef]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of Postabsorptive and Postprandial Glucose Metabolism by Insulin-Dependent and Insulin-Independent Mechanisms: An Integrative Approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef]
- Bonuccelli, S.; Muscelli, E.; Gastaldelli, A.; Barsotti, E.; Astiarraga, B.D.; Holst, J.J.; Mari, A.; Ferrannini, E. Improved Tolerance to Sequential Glucose Loading (Staub-Traugott Effect): Size and Mechanisms. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E532–E537. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, I.; Chandramouli, V.; Schumann, W.C.; Kumaran, K.; Wahren, J.; Landau, B.R. Pathways of Hepatic Glycogen Formation in Humans Following Ingestion of a Glucose Load in the Fed State. Metabolism 1989, 38, 583–585. [Google Scholar] [CrossRef]
- Shulman, G.I.; Cline, G.; Schumann, W.C.; Chandramouli, V.; Kumaran, K.; Landau, B.R. Quantitative Comparison of Pathways of Hepatic Glycogen Repletion in Fed and Fasted Humans. Am. J. Physiol. Endocrinol. Metab. 1990, 259, E335. [Google Scholar] [CrossRef]
- Miles, J.M.; Wooldridge, D.; Grellner, W.J.; Windsor, S.; Isley, W.L.; Klein, S.; Harris, W.S. Nocturnal and Postprandial Free Fatty Acid Kinetics in Normal and Type 2 Diabetic Subjects. Diabetes 2003, 52, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, D.; Wainstein, J.; Ahren, B.; Landau, Z.; Bar-Dayan, Y.; Froy, O. Fasting Until Noon Triggers Increased Postprandial Hyperglycemia and Impaired Insulin Response After Lunch and Dinner in Individuals With Type 2 Diabetes: A Randomized Clinical Trial. Diabetes Care 2015, 38, 1820–1826. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsitsou, S.; Zacharodimos, N.; Poulia, K.-A.; Karatzi, K.; Dimitriadis, G.; Papakonstantinou, E. Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials. Nutrients 2022, 14, 4778. https://doi.org/10.3390/nu14224778
Tsitsou S, Zacharodimos N, Poulia K-A, Karatzi K, Dimitriadis G, Papakonstantinou E. Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials. Nutrients. 2022; 14(22):4778. https://doi.org/10.3390/nu14224778
Chicago/Turabian StyleTsitsou, Sofia, Nikolaos Zacharodimos, Kalliopi-Anna Poulia, Kalliopi Karatzi, George Dimitriadis, and Emilia Papakonstantinou. 2022. "Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials" Nutrients 14, no. 22: 4778. https://doi.org/10.3390/nu14224778
APA StyleTsitsou, S., Zacharodimos, N., Poulia, K.-A., Karatzi, K., Dimitriadis, G., & Papakonstantinou, E. (2022). Effects of Time-Restricted Feeding and Ramadan Fasting on Body Weight, Body Composition, Glucose Responses, and Insulin Resistance: A Systematic Review of Randomized Controlled Trials. Nutrients, 14(22), 4778. https://doi.org/10.3390/nu14224778









