Abstract
Background: Osteoporosis is caused by the deterioration of bone density and microstructure, resulting in increased fracture risk. It transpires due to an imbalanced skeletal remodelling process favouring bone resorption. Various natural compounds can positively influence the skeletal remodelling process, of which naringenin is a candidate. Naringenin is an anti-inflammatory and antioxidant compound found in citrus fruits and grapefruit. This systematic review aims to present an overview of the available evidence on the skeletal protective effects of naringenin. Method: A systematic literature search was conducted using the PubMed and Scopus databases in August 2022. Original research articles using cells, animals, or humans to investigate the bone protective effects of naringenin were included. Results: Sixteen eligible articles were included in this review. The existing evidence suggested that naringenin enhanced osteoblastogenesis and bone formation through BMP-2/p38MAPK/Runx2/Osx, SDF-1/CXCR4, and PI3K/Akt/c-Fos/c-Jun/AP-1 signalling pathways. Naringenin also inhibited osteoclastogenesis and bone resorption by inhibiting inflammation and the RANKL pathway. Conclusions: Naringenin enhances bone formation while suppressing bone resorption, thus achieving its skeletal protective effects. It could be incorporated into the diet through fruit intake or supplements to prevent bone loss.
1. Introduction
Osteoporosis is a degenerative skeletal condition characterised by reduced bone mass and microstructural deterioration, which subsequently lead to decreased bone strength and an increased fragility fracture risk []. Osteoporosis is asymptomatic until it presents as low-trauma fractures of the hip, spinal, proximal humerus, pelvis, and/or wrist []. Osteoporosis is more prominent in postmenopausal women because estrogen insufficiency accelerates bone loss []. The use of antiresorptive (e.g., bisphosphonates, denosumab, and selective estrogen receptor modulators) and anabolic medications (e.g., teriparatide and abaloparatide) can improve bone mineral density (BMD) and reduce the fracture risk of patients with osteoporosis [,]. However, they come with various side effects [,].
Bone loss occurs when the rate of osteoblastic bone formation is lower than the rate of osteoclastic bone resorption []. Various factors could influence the bone turnover process. Inflammation, known to promote bone resorption, is a risk factor for osteoporosis []. Proinflammatory cytokines stimulate the expression of receptor activators of nuclear factor-B (RANK) and its functional ligand (RANKL), along with macrophage colony-stimulating factor (M-CSF), which enhance osteoclast formation and function []. Furthermore, modifications in redox systems have been linked to the pathogenesis of osteoporosis. Reactive oxygen species (ROS) inhibit osteoblast formation, stimulate apoptosis in osteoblasts and osteocytes, and encourage the formation of osteoclasts [], all of which result in bone loss and osteoporosis.
Apart from calcium and vitamin D routinely used in osteoporosis prevention [], dietary antioxidants and anti-inflammatory compounds may slow the progression of osteoporosis [,]. Naringenin (Figure 1) is a flavanone present in citrus fruits, grapes, and tomato skin []. Naringenin has been investigated for its antioxidant [] and anti-inflammatory properties []. Previous research found that naringenin suppressed nuclear factor-kappa B (NF-kB) p65 activity and expression in streptozotocin (STZ)-induced diabetic mice [] and carrageenan-induced paw oedema in rats []. In STZ and nicotinamide-induced diabetic rats, naringenin significantly increased the activities of pancreatic enzymatic antioxidants, plasma non-enzymatic antioxidant levels and decreased pancreatic tissue malondialdehyde levels []. Meanwhile, another report indicated that naringenin lowered lipid peroxidation and enhanced the activity of antioxidant enzymes, such as superoxide dismutase, catalase, glutathione-s-transferase, glutathione peroxidase and reduced glutathione in the liver of STZ-induced diabetic mice []. The studies mentioned above point to the potential of naringenin as an antioxidant and anti-inflammatory agent, which could help to suppress bone loss.
Figure 1.
The molecular structure of naringenin.
Considering the effects of inflammation and antioxidants in the pathogenesis of osteoporosis, naringenin may be positioned as a functional food component in preventing bone loss. The objective of this review is to encapsulate the protective effects of naringenin on bone as evidenced by currently available studies. Furthermore, naringenin may also exert specific mechanisms to enhance bone health, which would be discussed in the current review.
2. Materials and Methods
2.1. Literature Review
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for scoping review (Table S1). To discern studies on the potential benefits of naringenin on bone, a systematic literature search was conducted using the PubMed and Scopus databases in August 2021. The keywords used in the search were (1) naringenin AND (2) (bone OR osteoporosis OR osteoblasts OR osteoclasts OR osteocytes).
2.2. Article Selection
Articles with the following features were included: (1) original research articles investigating the skeletal effects of naringenin; (2) studies conducted using cell cultures, animal models, or human subjects. Articles with the following characteristics were rejected: (1) conference abstracts, reviews, letters or commentary, editorial and opinion articles lacking original data; (2) studies not using pure naringenin; (3) articles written in a language other than English. The search was executed by two authors (MLNM and SOE), using both databases and the keywords listed. The inclusion of an article was based on consensus by both authors. If no consensus could be obtained, the opinions of other authors were sought after (SKW and KYC) to determine the outcome of the article.
2.3. Data Extraction
The list of articles was organised using Mendeley (Elsevier, Amsterdam, The Netherlands). Identification of duplicated items was performed using Mendeley and manually. The authors’ names, publication year, study design, dose, treatment period, outcomes, and study limitations were all extracted using a standardised table by two authors (MLNM and SOE).
3. Results
3.1. Article Selection
The literature search resulted in 210 unique articles, which were from PubMed and Scopus. Following the removal of duplicates (n = 51), 159 articles were screened. One hundred and forty-three articles were excluded because they did not fit the inclusion criteria (not original article = 63, out of scope = 60, not written in English = 4, raw extract/mixture/formulation = 16). Eventually, the review included 16 articles that met all the criteria (Table 1). The selection process is depicted in Figure 2.

Table 1.
Effects of naringenin on bone health.
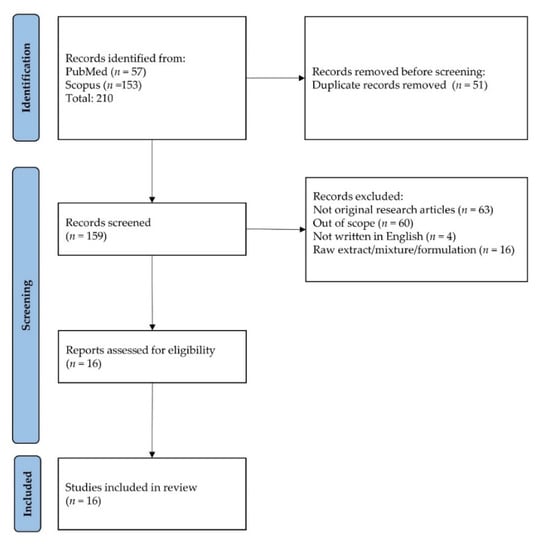
Figure 2.
Flowchart showing selection of articles.
3.2. Study Characteristics
The studies selected for this review were published from 2005 to 2021. There were eleven in vitro studies included in this review. The studies were either osteoblastogenesis/osteogenesis or osteoclastogenesis. For osteoblastogenesis/osteogenesis studies, cells used were rat bone marrow stroma cells (rBMSCs), mouse calvarial osteoblasts, bone marrow-derived mesenchymal stem cells (BMSCs), rat/mouse calvarial osteoblasts, MC3T3-E1, human foetal osteoblasts (hOB), primary osteoblast cells (pOB) and human periodontal ligament stem cells (hPDLSCs) [,,,,,,]. The osteogenic effect of naringenin was evaluated through dexamethasone-induced osteogenesis [,].
For osteoclastogenesis studies, cells used were rabbit osteoclasts, human primary osteoclasts precursor cells, co-culture of T-cells with bone marrow macrophages (BMMs), bone marrow monocytes (BMM), mouse MBMMφ and RAW 264.7 cell line [,,,,]. Macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-kB ligand (RANKL) were used to induce the conversion of osteoclast precursor cells into mature osteoclasts to investigate the effects of naringenin on osteoclast differentiation [,,,,]. Doses of naringenin used in the in vitro studies ranged from 0 to 800 µM. The treatment duration for osteoclastogenesis differentiation was about 2–6 days, while osteogenesis differentiation lasted 14–16 days.
Meanwhile, eight in vivo studies involved using rats (Wistar, Sprague Dawley, Y59) and mice (Balb/cByJ, C-57/BL6, and ICR) [,,,,,,,]. In animal studies, naringenin doses ranged from 0.005–25 mg/kg, 3–10 mg/mL, and 0.09–0.72% weight of diet [,,,,,,,]. The disease models used in these studies were ovariectomy (OVX)/oestrogen deficiency, retinoic acid-induced bone loss, titanium (Ti) particle-induced osteolysis, and soft diet-induced periodontal hypofunction. In these studies, the bone structure was determined using micro-computed tomography and histomorphometry. The bone remodelling process was investigated using circulating bone remodelling markers. The treatment duration for in vivo studies was between 2–6 weeks. There was no finding from human studies on this topic. The effects of naringenin on bone health are summed up in Table 1.
3.3. Findings from In Vitro Studies
Osteoblasts are mesenchymal cells that support bone formation by producing a bone matrix and subsequently mineralising it []. Naringenin was found to inhibit etoposide- and tumour necrosis factor-alpha (TNF-α)-induced osteoblast cell apoptosis in murine primary osteoblastic cells []. Naringenin improved osteoblast differentiation, mineralization, and osteogenic function in cultured rat calvarial osteoblasts [,,,], rBMSCs [], and hPDLSCs [] through the bone morphogenetic protein-2 (BMP-2)/p38 mitogen-activated protein kinase (p38 MAPK)/runt-related transcription factor2 (Runx2)/Osterix (Osx)/alkaline phosphatase (ALP) signalling pathway. Enhanced expressions of C-X-C chemokine receptor type 4 (CXCR4) and stromal cell-derived factor 1 (SDF-1a) levels were observed in naringenin-treated BMSCs [] and hPDLSCs [], suggesting that naringenin regulated osteogenic differentiation through the SDF-1/CXCR4 signalling pathway. Naringenin also exerted BMP-dependent osteogenic effects via the phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), c-Fos/c-Jun and activator protein 1 (AP-1) dependent signalling pathways []. Another study also found that naringenin increased OPG/RANKL ratio based on mRNA and protein expression from osteoblasts []. This alteration can potentially alter osteoclast formation. However, no changes in osteocalcin (OCN) were observed in MG-63 cell lines treated with naringenin. The possible molecular mechanisms of naringenin’s effect on osteoblasts are summarized in Figure 3.
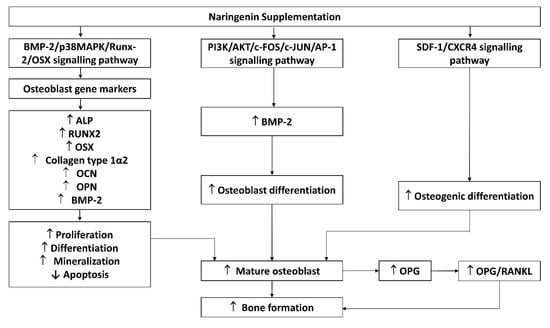
Figure 3.
Possible mechanism of naringenin effect on osteoblasts. Abbreviations: ALP, alkaline phosphatase; IL-1β, OPG, osteoprotegerin; OSX, osterix; RANK, receptor activator of nuclear factor-kappa B ligand; BMP-2; p38MAPK, phosphoinositide 38 mitogen-activated protein kinase; Runx2, runt-related transcription factor2; OCN, osteocalcin; OPN, osteopontin; P13K, phosphoinositide 3-kinase (PI3K); AKT, protein kinase B; c-FOS; c-JUN; AP-1, activator protein 1; SDF-1, stromal cell-derived factor 1; CXCR4, C-X-C chemokine receptor type 4.
Osteoclasts are multinucleated hematopoietic cells that serve as the main bone-resorbing cells and play an important role in bone remodelling []. Naringenin significantly inhibited osteoclastogenesis and secretion of interleukin (IL)-1α, IL-23 as well monocyte chemoattractant protein-1 in pre-osteoclast cultures. A significant decrease in helical peptide 620–633 release indicating bone resorption activity by naringenin, and an increase in tumour necrosis factor-α (TNF-α), IL-8, and IL-4 [] levels were observed in human pre-osteoclasts, but no changes were found on IL-6, IL-1b, IL-17, and osteoprotegerin (OPG) levels []. Besides, naringenin also was found to inhibit osteoclast formation and bone resorption activity, indicated by reduced numbers of osteoclasts, bone resorption pits, and area, as well as markers of osteoclast maturation, such as tartrate-resistant acid phosphatase (TRAP) and cathepsin-K [,,]. Naringenin was proven to minimize the number and size of F-actin rings, lower the expressions of cathepsin K, c-Fos, dendritic cell-specific transmembrane protein (DC-STAMP), nuclear factor of activated T-cell 1 (NFATc1), tartrate-resistant acid phosphatase (TRAP) and vacuolar type proton-translocating ATPase (V-ATPase d2) of osteoclasts at mRNA and protein levels [,]. Furthermore, naringenin has been shown to reduce the RANKL-induced p38 phosphorylation signalling in pre-osteoclasts and cause no changes in gene expression of nuclear factor-κB (NF-κB), extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), nuclear factor kappa light polypeptide gene enhancer B-cells inhibitor alpha (IκBα) and phosphorylated nuclear factor kappa light polypeptide gene enhancer B-cells inhibitor alpha (p-IκBα) []. The possible mechanism of actions of naringenin on osteoclasts are summarised in Figure 4.
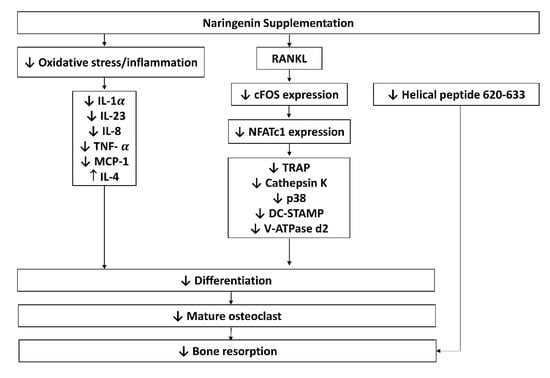
Figure 4.
Possible mechanism of actions of naringenin on osteoclasts. Abbreviations: IL-1 α, -23, -8, -4, interleukin-1 alpha, -23, -8, -4; NFATc1, nuclear factor of activated T- cells; OPG, osteoprotegerin; OSX, osterix; RANKL, receptor activator of nuclear factor-kappa B; ligand; TNF-α, tumour necrosis factor-alpha; TRAP, tartrate-resistant acid phosphatase; p38, phosphoinositide 38; DC-STAMP, dendritic cell-specific transmembrane protein; V-ATPase d2, vacuolar-type proton-translocating ATPase; MCP-1, monocyte chemoattractant protein-1.
3.4. Findings from In Vivo Studies
Postmenopausal bone loss due to oestrogen deficiency is caused by a high bone remodelling phenomenon that results in an imbalance between bone formation and resorption []. Gera et al., (2022) [] reported that naringenin supplementation (20 mg/kg for 60 days) in female Wistar rats with OVX-induced osteoporosis increased ALP and decreased acid phosphatase in the serum. They also reported improved cortical and trabecular bone architecture following naringenin supplementation. Kaczmarczyk-Sedlak et al., 2016 [] reported that naringenin supplementation (50 mg/kg for 4 weeks) in female Wistar rats with OVX-induced osteoporosis increased the width of trabecular in the epiphysis, decreased organic substances in the tibia, periosteal transverse growth of the diaphysis as well lower the ratio of the transverse cross-section area of the marrow cavity/diaphysis. However, the study showed no skeletal biomechanical and mineral (calcium and phosphate) changes probably because of the short duration of supplementation. The supplementation also did not alter the transverse cross-sectional area of cortical bone in the diaphysis and cartilage width compared to the negative group.
Swarnkar et al., (2012) [] discovered that naringenin (10 mg/kg for 6 weeks) treatment in Balb/cByJ mice mitigated OVX-induced trabecular bone changes by increasing the mineralized nodule, mineral apposition rate and bone formation rate/bone surface (BFR/BS) compared to negative group. This effect was probably attributed to improved osteoblastogenesis indicated by the mRNA gene expression of runt-related transcription factor 2 (Runx-2) and type I collagen. In a preventive model, the same group revealed a lack of changes in trabecular microstructure at the distal femoral epiphysis and tibial proximal metaphysis. In the therapeutic model (5 mg/kg/day of naringenin (i.p.) for 6 weeks), they reported decreased trabecular bone pattern factor but no changes in trabecular microstructure in the distal femoral epiphysis and tibial proximal metaphysis with supplementation. Wu et al., (2008) [] also claimed that treating OVX ICR mice with naringenin (10 mg/mL every 2 days for 4 weeks) could enhance BMD, bone mineral content, ALP activity, and BMP-2 expression.
Retinoic acid-induced bone loss in rats has been used to evaluate the skeletal impacts of multiple substances on the skeletal system []. According to Oršolić et al., (2014) [], the administration of naringenin (100 mg/kg for 14 days) in Y59 female rats with retinoic acid-induced bone loss resulted in increased calcium and phosphorus content in the femur, higher BMD in the proximal and distal femur and improved femur length. They attribute the protection to the improvement of the redox status of the rats, but the redox parameters, such as glutathione and malondialdehyde, were measured in the liver and kidney. Oršolić et al., (2022) [] also reported that the administration of naringenin (100 mg/kg for 14 days) in Y59 female rats with retinoic acid-induced bone loss resulted in decreased serum β-CTx, IL-1β, IL-6, TNFα, and chemokine ligand 5 (CCL5/RANTES). They also reported decreased MDA and increased GSH and CAT in the ovary and kidney. However, they reported a lack of changes in circulating bone formation marker (OCN) level, circulating, bone calcium, and phosphorus levels, densitometry, and cortical geometry in the supplemented group. A short duration of treatment might be responsible for the lack of skeletal effects of the treatment.
Osteolysis is the progressive degeneration of periprosthetic bony tissue, which appears on serial radiographs as progressive radiolucent lines and/or cavitation at the implant-bone or cement-bone interface. Osteolysis can progress to aseptic loosening and implant failure if not treated promptly []. Based on the study performed by Wang et al., (2014) [], naringenin supplementation (10 and 25 mg/kg for 2 weeks) reduced TRAP-positive multinucleated osteoclasts and lowered the number of pores and the percentage porosity in the calvarial region of interest in C-57/BL6 mice with Ti-particle induce osteolysis. Moreover, an increase in trabecular bone volume was reported compared to the negative group.
Periodontal hypofunction can occur as a consequence of disassociation with an opposing tooth in certain malocclusions, such as open bites and ectopic teeth []. Wood (2005) [] showed that naringenin supplementation (0.09%, 0.18%, 0.36%, and 0.72% for 42 days) reduced physiological molar crestal alveolar bone (CAB)-cemento-enamel junction (CEJ) distance in buccal maxilla and mandible as well as in lingua maxilla and mandible in Sprague Dawley rats with soft diet-induced periodontal hypofunction compared to the unsupplemented group.
4. Discussion
The currently available evidence shows that naringenin exerts stimulatory effects on osteoblasts through MAPK, PI3K/Akt, and CXCR4/SDF-1 pathways. Osteoblasts are responsible for osteogenesis by synthesising and mineralising organic bone matrix (osteoid) during skeleton construction and bone remodelling []. The MAPK cascades regulate Runx2 phosphorylation and transcription, which promote osteoblast differentiation. MAPK pathways and their components, JNK, ERK, and p38, which enforce osteoblastogenesis and establish the non-canonical BMP-2 signal transduction pathways [,,,]. Naringenin stimulates osteogenic gene activation, indicating that it has a stimulatory effect on osteogenic differentiation [,]. Activation of the PI3K/Akt signalling pathway also promotes osteoblast proliferation, differentiation, and bone formation activity []. Naringenin has been reported to stimulate BMP-2-dependent osteoblastogenesis through the activation of the PI3K/Akt signalling pathway []. Activation of the CXCR4/SDF-1 signalling pathway is critical in early osteoblastogenesis and its suppression leads to lower bone formation and mineralisation [,].
Osteoclasts are bone resorption cells originating from hematopoietic lineage cells []. Bone resorption is vital in bone remodelling, but excessive resorption can result in pathological bone loss. Osteoclast differentiation and activation are governed by various hormones and cytokines. The cytokines RANKL and M-CSF, in particular, are required for osteoclastic differentiation []. To promote osteoclast differentiation, preservation and bone resorption, the M-CSF binds to the colony-stimulating factor 1 receptor, whilst RANKL binds to the RANK receptor [,]. TRAF factors such as TRAF 6 are recruited by RANK-RANKL binding [], leading to the activation of NF-kB, Akt, and MAPKs (ERK/p38/JNK) pathways. Furthermore, the RANKL signalling stimulates c-Fos and then NFATc1, a major switch that plays a role in controlling osteoclast terminal differentiation [,]. Naringenin was reported to reduce M-CSF and RANKL-induced expression of critical markers of osteoclast differentiation markers such as cathepsin K, c-Fos, and NFATc1 [,].
The biological properties of naringenin suggest a broad range of clinical applications. Naringenin decreased CAB-CEJ distance in buccal maxilla and mandible as well as in lingua maxilla and mandible, indicating that naringenin supplementation protects against alveolar bone loss in rats with induced periodontal disease []. Supplementation of naringenin also improved bone mineral microstructure, mineral, and biomechanical strength [,,,]. However, the bone loss models that have been used to test the effects of naringenin have been limited to OVX and retinol-induced models. Thus, results from other models, such as testosterone deficiency and glucocorticoid models, are indispensable before it is tested on patients with other causes of osteoporosis. Following joint replacement, the abrasive particles initiated by the prosthesis are primarily responsible for osteolysis []. Naringenin supplementation prevented Ti-particle-induced osteolysis, implying that it may be preferable for treating periprosthetic osteolysis [].
Pharmacokinetics and safety issues of naringenin should be considered before it is used clinically. From the pharmacokinetic aspects, naringenin has very low in vivo bioavailability due to its hydrophobic nature, which limits its practical use. It has a short half-life and is easily converted to its crystalline form, and therefore it is poorly absorbed by the digestive system [,,,]. Previous researchers developed a variety of methods to improve naringenin absorption and low bioavailability, including particle size reduction, complexation with cyclodextrins [], salt formation, solid dispersions [], surfactant usage, nanoparticles, nanocarriers [], and self-emulsifying drug delivery system, as well as prodrug formation []. Nanotechnology proved to be an efficient way to improve the bioavailability of naringenin by multiple delivery routes to enhance its effectiveness in the treatment of cancer, inflammation, diabetes, liver, brain, and ocular diseases mostly through numerous in vitro and in vivo methods []. Meanwhile, Rodríguez-Fragoso et al., (2011) [] found out that naringenin inhibits some drug-metabolizing cytochrome P450 enzymes, including CYP3A4 and CYP1A2, potentially leading to drug-drug interactions in the intestine and liver, where phytochemical concentrations are higher. Modification in cytochrome P450 and other enzymatic activity may influence the outcome of drugs that go through extensive first-pass metabolism. An acute toxicity study using Wistar rats reported the lethal dose (LD50) value of naringenin to be 340 mg/kg body weight []. Using body surface ratio conversation [], the human equivalent dose is 64 mg/kg.
The term “naringenin” was searched for on https://clinicaltrials.gov/ (accessed on 31 August 2022) and the search revealed thirteen registered clinical trials on naringenin. The trials investigate the effects of naringenin on healthy subjects (NCT02627547, NCT04867655, NCT05073523, NCT02380144), hepatitis virus/HCV infection/chronic HCV/Hepatitis C (NCT01091077), energy expenditure/safety issues/glucose metabolism (NCT04697355), safety issues/pharmacokinetics (NCT03582553), subjective cognitive decline (NCT04744922), cardiovascular disease risk factors (NCT00539916), intestinal disease (NCT03032861), metabolic syndrome/vascular compliance/predisposition to cardiovascular disease (NCT04731987), pharmacokinetics of new curcumin formulations/safety of new curcumin formulations (NCT01982734) and cardiovascular risk factor/type-2 diabetes mellitus/insulin sensitivity/metabolic syndrome (NCT03527277). Seven of these clinical trials have been completed, four are still recruiting, one with an unknown status, and lastly one trial is still active but not recruiting. However, no attempt has been made to conduct a human clinical trial to evaluate the impact of naringenin on skeletal diseases. Since pure naringenin has only been studied in limited clinical trials, more research on free drug and naringenin-loaded nanosystems in humans is warranted. Further exploration into the interactions of these nanoformulations with the human body is required before they can be translated into pharmaceuticals and nutraceutical supplements [].
This review also has several limitations. We only include articles written in English in this review, which potentially excludes studies published in other languages. We did not exclude studies based on their quality because the number of studies is limited. Nevertheless, the current review provides an overview of the skeletal effects of naringenin and prospects of its clinical application as a functional food component to protect bone health.
5. Conclusions
Preclinical findings demonstrate that naringenin protects the skeleton by suppressing osteoclastogenesis and bone resorption while enhancing osteoblastogenesis and bone formation. Human clinical trials to justify naringenin’s skeletal effects are lacking. Hence, comprehensive clinical studies should be performed to validate naringenin’s skeletal properties and reveal the safety of this flavanone compound.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14224851/s1, Table S1: Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist. Reference [] is cited in the supplementary materials.
Author Contributions
Conceptualization, methodology, M.L.N.M., S.O.E. and K.-Y.C.; validation, S.-K.W. and K.-Y.C.; writing-original draft preparation, M.L.N.M.; writing—review and editing, S.O.E., S.-K.W., and K.-Y.C.; visualization, M.L.N.M. and S.O.E.; supervision, K.-Y.C.; funding acquisition, K.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Kebangsaan Malaysia through Research University Grant (GUP-2020-021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This manuscript does not contain original data.
Acknowledgments
The authors thank Universiti Kebangsaan Malaysia for the support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging 2018, 35, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Baber, R.J.; Panay, N.; Fenton, A. 2016 IMS Recommendations on womens midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, A.; Clements, J.N. Abaloparatide: A new pharmacological option for osteoporosis. Am. J. Health-Syst. Pharm. 2019, 76, 130–135. [Google Scholar] [CrossRef]
- Lacativa, P.G.S.; de Farias, M.L.F. Osteoporose e inflamação. Arq. Bras. Endocrinol. Metabol. 2010, 54, 123–132. [Google Scholar] [CrossRef]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Tang, B.M.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of caffeic acid and its derivatives on bone: A systematic review. Drug Des. Dev. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Dai, J.; Liu, H.; Li, R.R.; Sun, P.L.; Du, Q.; Pang, L.L.; Chen, Z.; Yin, K.S. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-κB activity in a murine model of asthma. Can. J. Physiol. Pharmacol. 2009, 87, 729–735. [Google Scholar] [CrossRef]
- Tsai, S.J.; Huang, C.S.; Mong, M.C.; Kam, W.Y.; Huang, H.Y.; Yin, M.C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2012, 60, 514–521. [Google Scholar] [CrossRef]
- Chung, T.W.; Li, S.; Lin, C.C.; Tsai, S.W. Antinociceptive and anti-inflammatory effects of the citrus flavanone naringenin. Tzu Chi Med. J. 2019, 31, 81–85. [Google Scholar] [CrossRef]
- Annadurai, T.; Muralidharan, A.R.; Joseph, T.; Hsu, M.J.; Thomas, P.A.; Geraldine, P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012, 68, 307–318. [Google Scholar] [CrossRef]
- Rashmi, R.; Magesh, S.B.; Ramkumar, K.M.; Suryanarayanan, S.; SubbaRao, M.V. Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep. Biochem. Mol. Biol. 2017, 7, 76–84. [Google Scholar]
- La, V.D.; Tanabe, S.; Grenier, D. Naringenin inhibits human osteoclastogenesis and osteoclastic bone resorption. J. Periodontal Res. 2009, 44, 193–198. [Google Scholar] [CrossRef]
- Ming, L.G.; Ge, B.F.; Wang, M.G.; Chen, K.M. Comparison between 8-prenylnarigenin and narigenin concerning their activities on promotion of rat bone marrow stromal cells’ osteogenic differentiation in vitro. Cell Prolif. 2012, 45, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.-G.; Lv, X.; Ma, X.-N.; Ge, B.-F.; Zhen, P.; Song, P.; Zhou, J.; Ma, H.-P.; Xian, C.J.; Chen, K.-M. The Prenyl Group Contributes to Activities of Phytoestrogen 8-Prenynaringenin in Enhancing Bone Formation and Inhibiting Bone Resorption In Vitro. Endocrinology 2013, 154, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, G.; Sharan, K.; Siddiqui, J.A.; Mishra, J.S.; Khan, K.; Khan, M.P.; Gupta, V.; Rawat, P.; Maurya, R.; Dwivedi, A.K.; et al. A naturally occurring naringenin derivative exerts potent bone anabolic effects by mimicking oestrogen action on osteoblasts. Br. J. Pharmacol. 2012, 165, 1526–1542. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, M.; Luo, M.; Shen, M.; Xu, C.; Xu, G.; Chen, Y.; Xia, L. Naringenin inhibits osteoclastogenesis through modulation of helper T cells-secreted IL-4. J. Cell. Biochem. 2018, 119, 2084–2093. [Google Scholar] [CrossRef]
- Wang, W.; Wu, C.; Tian, B.; Liu, X.; Zhai, Z.; Qu, X.; Jiang, C.; Ouyang, Z.; Mao, Y.; Tang, T.; et al. The inhibition of RANKL-induced osteoclastogenesis through the suppression of p38 signaling pathway by naringenin and attenuation of titanium-particle-induced osteolysis. Int. J. Mol. Sci. 2014, 15, 21913–21934. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, S.; Cheng, Q.; Zeng, Y.; Xu, X.; Guan, G. Naringenin promotes sdf-1/cxcr4 signaling pathway in bmscs osteogenic differentiation. Folia Histochem. Cytobiol. 2021, 59, 66–73. [Google Scholar] [CrossRef]
- Wu, J.-B.; Fong, Y.C.; Tsai, H.Y.; Chen, Y.F.; Tsuzuki, M.; Tang, C.H. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur. J. Pharmacol. 2008, 588, 333–341. [Google Scholar] [CrossRef]
- Zhang, L.; He, H.; Zhang, M.; Wu, Y.; Xu, X.; Yang, M.; Mei, L. Assessing the effect and related mechanism of naringenin on the proliferation, osteogenic differentiation and endothelial differentiation of human periodontal ligament stem cells. Biochem. Biophys. Res. Commun. 2021, 534, 337–342. [Google Scholar] [CrossRef]
- Gera, S.; Sampathi, S.; Maddukuri, S.; Dodoala, S.; Junnuthula, V.; Dyawanapelly, S. Therapeutic Potential of Naringenin Nanosuspension: In Vitro and In Vivo Anti-Osteoporotic Studies. Pharmaceutics 2022, 14, 1449. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Chen, S.-C.; Lai, W.-F.T.; Chen, Y.-C.; Tsai, Y.-H. Screening of flavonoids for effective osteoclastogenesis suppression. Anal. Biochem. 2013, 433, 48–55. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Wojnar, W.; Zych, M.; Ozimina-Kamińska, E.; Bońka, A. Effect of dietary flavonoid naringenin on bones in rats with ovariectomy-induced osteoporosis. Acta Pol. Pharm.-Drug Res. 2016, 73, 1073–1081. [Google Scholar]
- Oršolić, N.; Goluža, E.; Dikić, D.; Lisičić, D.; Sašilo, K.; Rodak, E.; Jeleč, Ž.; Lazarus, M.V.; Orct, T. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 2014, 53, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Wood, N. The effects of dietary naringenin supplementation on physiological changes in molar crestal alveolar bone-cemento-enamel junction distance in young rats. J. Med. Food 2005, 8, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Nemrava, J.; Jeleč, Ž.; Kukolj, M.; Odeh, D.; Jakopović, B.; Jazvinšćak Jembrek, M.; Bagatin, T.; Fureš, R.; Bagatin, D. Antioxidative and Anti-Inflammatory Activities of Chrysin and Naringenin in a Drug-Induced Bone Loss Model in Rats. Int. J. Mol. Sci. 2022, 23, 2872. [Google Scholar] [CrossRef]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res. Ther. 2007, 9, 203. [Google Scholar] [CrossRef]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Prim. 2016, 2, 16069. [Google Scholar] [CrossRef]
- Wei, M.; Yang, Z.; Li, P.; Zhang, Y.; Sse, W.C. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am. J. Chin. Med. 2007, 35, 663–667. [Google Scholar] [CrossRef]
- Saleh, K.J.; Thongtrangan, I.; Schwarz, E.M. Osteolysis: Medical and surgical approaches. Clin. Orthop. Relat. Res. 2004, 427, 138–147. [Google Scholar] [CrossRef]
- Sringkarnboriboon, S.; Matsumoto, Y.; Soma, K. Root resorption related to hypofunctional periodontium in experimental tooth movement. J. Dent. Res. 2003, 82, 486–490. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Gopalakrishnan, R.; Jiang, D.; Reith, E.; Benson, M.D.; Franceschi, R.T. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J. Bone Miner. Res. 2002, 17, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002, 277, 5330–5338. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.C.; Zang, H.Y.; Guo, L.X.; Xue, H.B.; Liu, X.D.; Bai, Y.B.; Ma, Y.Z. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J. Recept. Signal Transduct. 2015, 35, 640–645. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Liu, Y.; Zhang, J.; Kang, Q.; Ho, K.; Chai, Y.; Li, G. Effect of SDF-1/Cxcr4 Signaling Antagonist AMD3100 on Bone Mineralization in Distraction Osteogenesis. Calcif. Tissue Int. 2017, 100, 641–652. [Google Scholar] [CrossRef]
- Luan, J.; Cui, Y.; Zhang, Y.; Zhou, X.; Zhang, G.; Han, J. Effect of CXCR4 inhibitor AMD3100 on alkaline phosphatase activity and mineralization in osteoblastic MC3T3-E1 cells. Biosci. Trends 2012, 6, 63–69. [Google Scholar] [CrossRef]
- Soysa, N.S.; Alles, N. Osteoclast function and bone-resorbing activity: An overview. Biochem. Biophys. Res. Commun. 2016, 476, 115–120. [Google Scholar] [CrossRef]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med. 2020, 52, 1239–1254. [Google Scholar] [CrossRef]
- Bharti, A.C.; Takada, Y.; Shishodia, S.; Aggarwal, B.B. Evidence That Receptor Activator of Nuclear Factor (NF)-κB Ligand Can Suppress Cell Proliferation and Induce Apoptosis through Activation of a NF-κB-independent and TRAF6-dependent Mechanism. J. Biol. Chem. 2004, 279, 6065–6076. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Ishida, N.; Hayashi, K.; Hoshijima, M.; Ogawa, T.; Koga, S.; Miyatake, Y.; Kumegawa, M.; Kimura, T.; Takeya, T. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 2002, 277, 41147–41156. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, S.; Feng, W.; Wei, Y.; Lu, H.; Zhong, G.; Wu, Z.; Wang, H.; Su, W.; Li, J. Tanshinone IIA protects against polyethylene particle-induced osteolysis response in a mouse calvarial model. Int. J. Clin. Exp. Pathol. 2018, 11, 4461–4471. [Google Scholar]
- Kumar, R.P.; Abraham, A. PVP- coated naringenin nanoparticles for biomedical applications—In vivo toxicological evaluations. Chem. Biol. Interact. 2016, 257, 110–118. [Google Scholar] [CrossRef]
- Shpigelman, A.; Shoham, Y.; Israeli-Lev, G.; Livney, Y.D. β-Lactoglobulin-naringenin complexes: Nano-vehicles for the delivery of a hydrophobic nutraceutical. Food Hydrocoll. 2014, 40, 214–224. [Google Scholar] [CrossRef]
- Yang, L.J.; Ma, S.X.; Zhou, S.Y.; Chen, W.; Yuan, M.W.; Yin, Y.Q.; Yang, X.D. Preparation and characterization of inclusion complexes of naringenin with β-cyclodextrin or its derivative. Carbohydr. Polym. 2013, 98, 861–869. [Google Scholar] [CrossRef]
- Martinez, S.E.; Lillico, R.; Lakowski, T.M.; Martinez, S.A.; Davies, N.M. Pharmacokinetic analysis of an oral multicomponent joint dietary supplement (Phycox®) in dogs. Pharmaceutics 2017, 9, 30. [Google Scholar] [CrossRef]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef] [PubMed]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: A comparative study. J. Appl. Polym. Sci. 2006, 102, 460–471. [Google Scholar] [CrossRef]
- Bali, V.; Ali, M.; Ali, J. Nanocarrier for the enhanced bioavailability of a cardiovascular agent: In vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int. J. Pharm. 2011, 403, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Zheng, Y.; Cao, H.; Huang, Q.; Xiao, J.; Chen, L. Enhancement of bioavailability and bioactivity of diet-derived flavonoids by application of nanotechnology: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef]
- Rodríguez-Fragoso, L.; Martínez-Arismendi, J.L.; Orozco-Bustos, D.; Reyes-Esparza, J.; Torres, E.; Burchiel, S.W. Potential Risks Resulting from Fruit/Vegetable-Drug Interactions: Effects on Drug-Metabolizing Enzymes and Drug Transporters. J. Food Sci. 2011, 76, R112–R124. [Google Scholar] [CrossRef]
- Selvam, M.; Kaliyaperumal, M. Determination of LD 50 of Naringenin for its effects on diabetic nephropathy in rats-A pilot study. J. Chem. Pharm. Res. 2015, 7, 550–553. [Google Scholar]
- Shin, J.; Seol, I.; Son, C. Interpretation of Animal Dose and Human Equivalent Dose for Drug Development. J. Korean Orient. Med. 2010, 31, 1–7. [Google Scholar]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).