A Scoping Review of the Skeletal Effects of Naringenin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Article Selection
2.3. Data Extraction
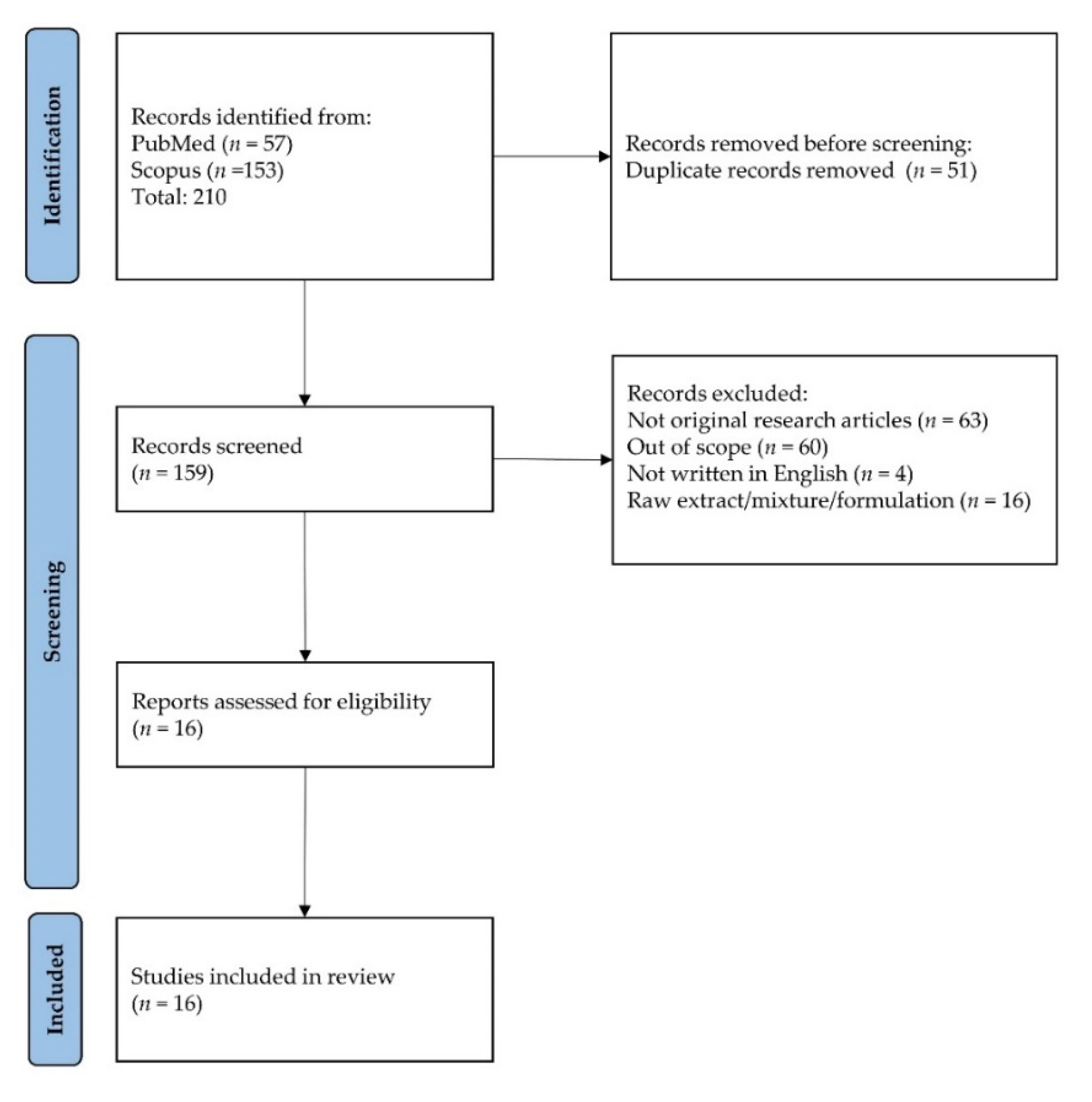
3. Results
3.1. Article Selection
3.2. Study Characteristics
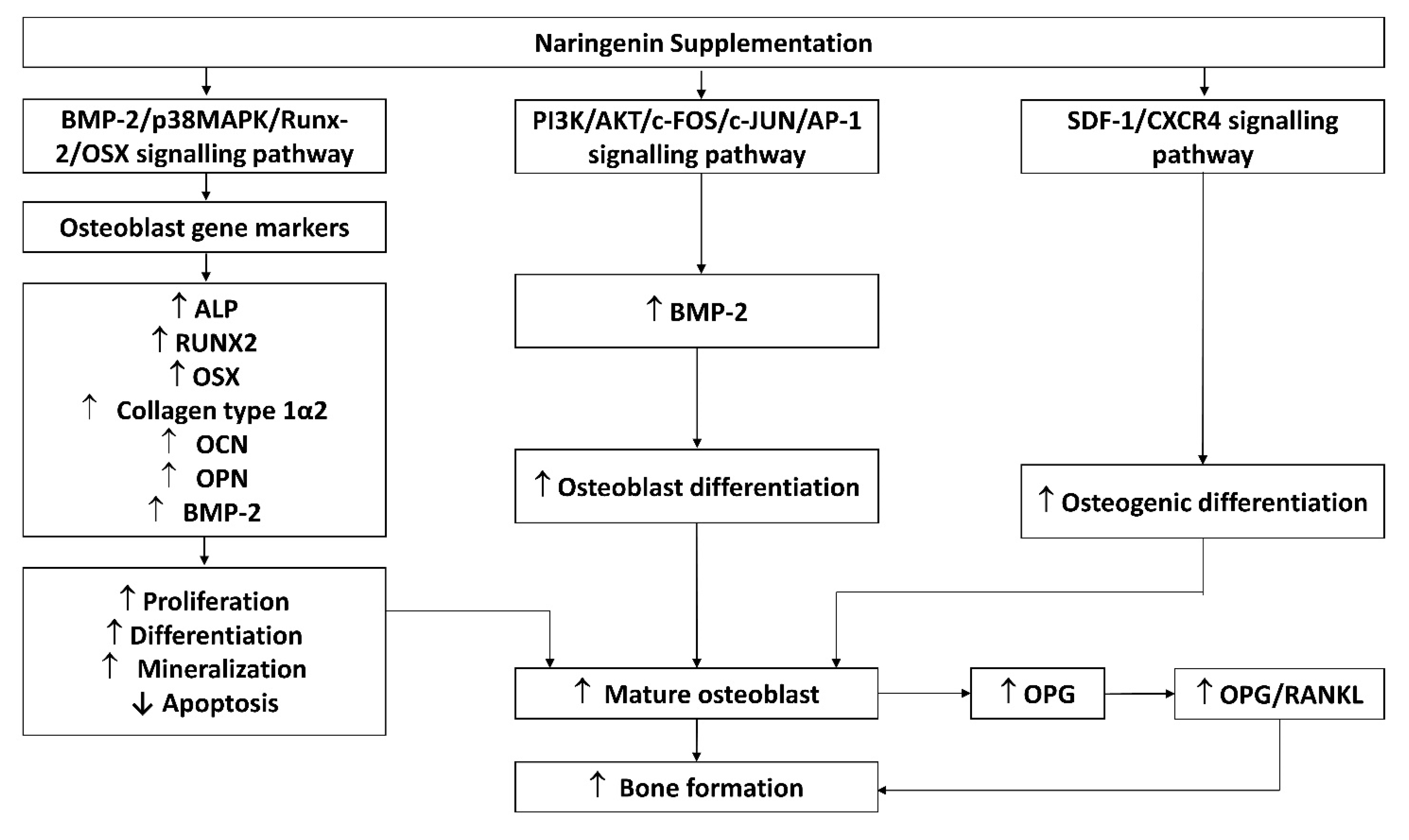
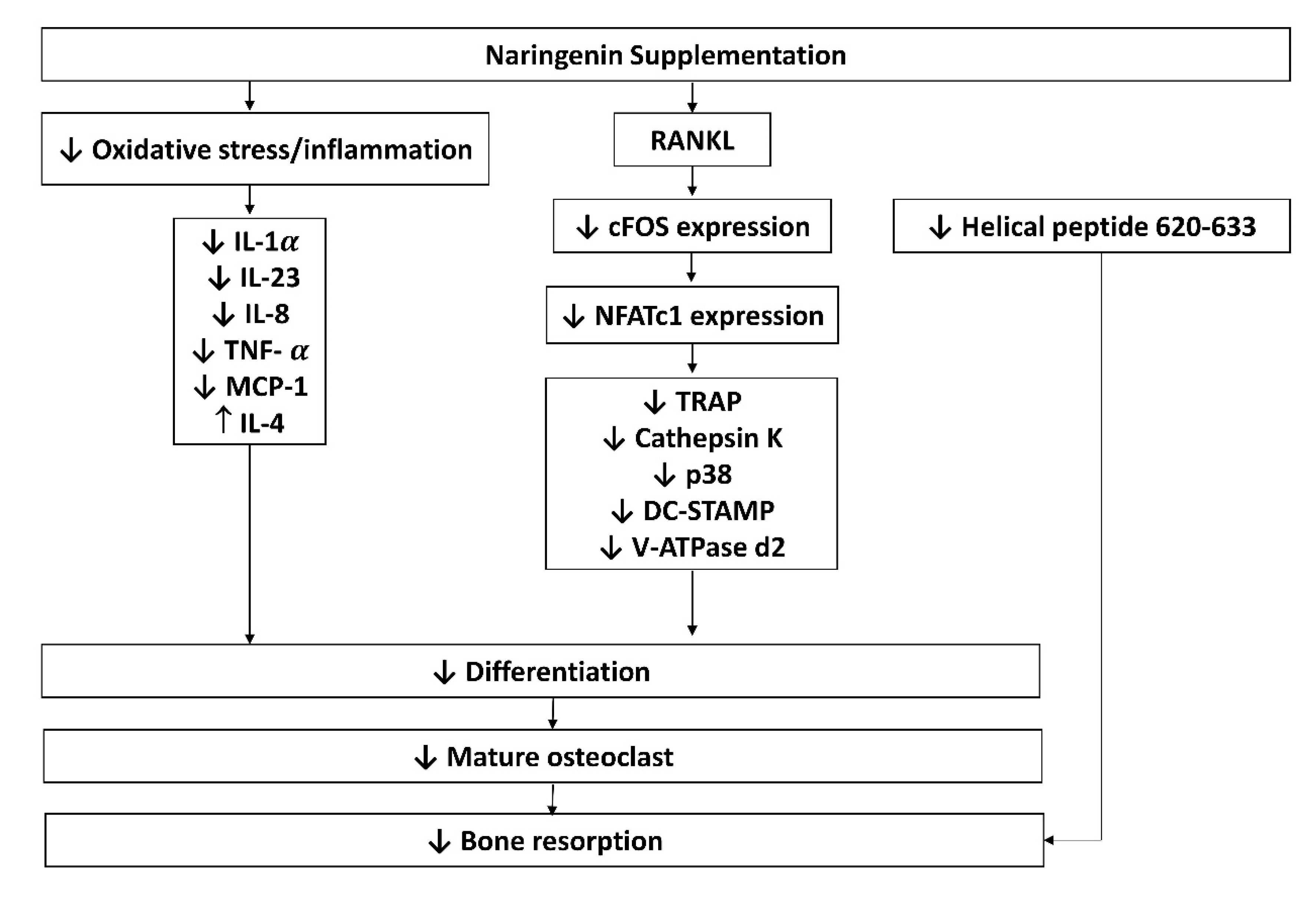
3.3. Findings from In Vitro Studies
3.4. Findings from In Vivo Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sozen, T.; Ozisik, L.; Calik Basaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, E.D. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs Aging 2018, 35, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Baber, R.J.; Panay, N.; Fenton, A. 2016 IMS Recommendations on womens midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef]
- Shoback, D.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Eastell, R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J. Clin. Endocrinol. Metab. 2020, 105, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, A.; Clements, J.N. Abaloparatide: A new pharmacological option for osteoporosis. Am. J. Health-Syst. Pharm. 2019, 76, 130–135. [Google Scholar] [CrossRef]
- Lacativa, P.G.S.; de Farias, M.L.F. Osteoporose e inflamação. Arq. Bras. Endocrinol. Metabol. 2010, 54, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Tang, B.M.; Eslick, G.D.; Nowson, C.; Smith, C.; Bensoussan, A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: A meta-analysis. Lancet 2007, 370, 657–666. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of caffeic acid and its derivatives on bone: A systematic review. Drug Des. Dev. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Dai, J.; Liu, H.; Li, R.R.; Sun, P.L.; Du, Q.; Pang, L.L.; Chen, Z.; Yin, K.S. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-κB activity in a murine model of asthma. Can. J. Physiol. Pharmacol. 2009, 87, 729–735. [Google Scholar] [CrossRef]
- Tsai, S.J.; Huang, C.S.; Mong, M.C.; Kam, W.Y.; Huang, H.Y.; Yin, M.C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2012, 60, 514–521. [Google Scholar] [CrossRef]
- Chung, T.W.; Li, S.; Lin, C.C.; Tsai, S.W. Antinociceptive and anti-inflammatory effects of the citrus flavanone naringenin. Tzu Chi Med. J. 2019, 31, 81–85. [Google Scholar] [CrossRef]
- Annadurai, T.; Muralidharan, A.R.; Joseph, T.; Hsu, M.J.; Thomas, P.A.; Geraldine, P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012, 68, 307–318. [Google Scholar] [CrossRef]
- Rashmi, R.; Magesh, S.B.; Ramkumar, K.M.; Suryanarayanan, S.; SubbaRao, M.V. Antioxidant potential of naringenin helps to protect liver tissue from streptozotocin-induced damage. Rep. Biochem. Mol. Biol. 2017, 7, 76–84. [Google Scholar]
- La, V.D.; Tanabe, S.; Grenier, D. Naringenin inhibits human osteoclastogenesis and osteoclastic bone resorption. J. Periodontal Res. 2009, 44, 193–198. [Google Scholar] [CrossRef]
- Ming, L.G.; Ge, B.F.; Wang, M.G.; Chen, K.M. Comparison between 8-prenylnarigenin and narigenin concerning their activities on promotion of rat bone marrow stromal cells’ osteogenic differentiation in vitro. Cell Prolif. 2012, 45, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.-G.; Lv, X.; Ma, X.-N.; Ge, B.-F.; Zhen, P.; Song, P.; Zhou, J.; Ma, H.-P.; Xian, C.J.; Chen, K.-M. The Prenyl Group Contributes to Activities of Phytoestrogen 8-Prenynaringenin in Enhancing Bone Formation and Inhibiting Bone Resorption In Vitro. Endocrinology 2013, 154, 1202–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarnkar, G.; Sharan, K.; Siddiqui, J.A.; Mishra, J.S.; Khan, K.; Khan, M.P.; Gupta, V.; Rawat, P.; Maurya, R.; Dwivedi, A.K.; et al. A naturally occurring naringenin derivative exerts potent bone anabolic effects by mimicking oestrogen action on osteoblasts. Br. J. Pharmacol. 2012, 165, 1526–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Li, M.; Luo, M.; Shen, M.; Xu, C.; Xu, G.; Chen, Y.; Xia, L. Naringenin inhibits osteoclastogenesis through modulation of helper T cells-secreted IL-4. J. Cell. Biochem. 2018, 119, 2084–2093. [Google Scholar] [CrossRef]
- Wang, W.; Wu, C.; Tian, B.; Liu, X.; Zhai, Z.; Qu, X.; Jiang, C.; Ouyang, Z.; Mao, Y.; Tang, T.; et al. The inhibition of RANKL-induced osteoclastogenesis through the suppression of p38 signaling pathway by naringenin and attenuation of titanium-particle-induced osteolysis. Int. J. Mol. Sci. 2014, 15, 21913–21934. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bai, S.; Cheng, Q.; Zeng, Y.; Xu, X.; Guan, G. Naringenin promotes sdf-1/cxcr4 signaling pathway in bmscs osteogenic differentiation. Folia Histochem. Cytobiol. 2021, 59, 66–73. [Google Scholar] [CrossRef]
- Wu, J.-B.; Fong, Y.C.; Tsai, H.Y.; Chen, Y.F.; Tsuzuki, M.; Tang, C.H. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur. J. Pharmacol. 2008, 588, 333–341. [Google Scholar] [CrossRef]
- Zhang, L.; He, H.; Zhang, M.; Wu, Y.; Xu, X.; Yang, M.; Mei, L. Assessing the effect and related mechanism of naringenin on the proliferation, osteogenic differentiation and endothelial differentiation of human periodontal ligament stem cells. Biochem. Biophys. Res. Commun. 2021, 534, 337–342. [Google Scholar] [CrossRef]
- Gera, S.; Sampathi, S.; Maddukuri, S.; Dodoala, S.; Junnuthula, V.; Dyawanapelly, S. Therapeutic Potential of Naringenin Nanosuspension: In Vitro and In Vivo Anti-Osteoporotic Studies. Pharmaceutics 2022, 14, 1449. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Chen, S.-C.; Lai, W.-F.T.; Chen, Y.-C.; Tsai, Y.-H. Screening of flavonoids for effective osteoclastogenesis suppression. Anal. Biochem. 2013, 433, 48–55. [Google Scholar] [CrossRef]
- Kaczmarczyk-Sedlak, I.; Wojnar, W.; Zych, M.; Ozimina-Kamińska, E.; Bońka, A. Effect of dietary flavonoid naringenin on bones in rats with ovariectomy-induced osteoporosis. Acta Pol. Pharm.-Drug Res. 2016, 73, 1073–1081. [Google Scholar]
- Oršolić, N.; Goluža, E.; Dikić, D.; Lisičić, D.; Sašilo, K.; Rodak, E.; Jeleč, Ž.; Lazarus, M.V.; Orct, T. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 2014, 53, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Wood, N. The effects of dietary naringenin supplementation on physiological changes in molar crestal alveolar bone-cemento-enamel junction distance in young rats. J. Med. Food 2005, 8, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Nemrava, J.; Jeleč, Ž.; Kukolj, M.; Odeh, D.; Jakopović, B.; Jazvinšćak Jembrek, M.; Bagatin, T.; Fureš, R.; Bagatin, D. Antioxidative and Anti-Inflammatory Activities of Chrysin and Naringenin in a Drug-Induced Bone Loss Model in Rats. Int. J. Mol. Sci. 2022, 23, 2872. [Google Scholar] [CrossRef]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res. Ther. 2007, 9, 203. [Google Scholar] [CrossRef] [Green Version]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Prim. 2016, 2, 16069. [Google Scholar] [CrossRef]
- Wei, M.; Yang, Z.; Li, P.; Zhang, Y.; Sse, W.C. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am. J. Chin. Med. 2007, 35, 663–667. [Google Scholar] [CrossRef]
- Saleh, K.J.; Thongtrangan, I.; Schwarz, E.M. Osteolysis: Medical and surgical approaches. Clin. Orthop. Relat. Res. 2004, 427, 138–147. [Google Scholar] [CrossRef]
- Sringkarnboriboon, S.; Matsumoto, Y.; Soma, K. Root resorption related to hypofunctional periodontium in experimental tooth movement. J. Dent. Res. 2003, 82, 486–490. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Gopalakrishnan, R.; Jiang, D.; Reith, E.; Benson, M.D.; Franceschi, R.T. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J. Bone Miner. Res. 2002, 17, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J. Biol. Chem. 2002, 277, 5330–5338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Wang, X.F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.C.; Zang, H.Y.; Guo, L.X.; Xue, H.B.; Liu, X.D.; Bai, Y.B.; Ma, Y.Z. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J. Recept. Signal Transduct. 2015, 35, 640–645. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Liu, Y.; Zhang, J.; Kang, Q.; Ho, K.; Chai, Y.; Li, G. Effect of SDF-1/Cxcr4 Signaling Antagonist AMD3100 on Bone Mineralization in Distraction Osteogenesis. Calcif. Tissue Int. 2017, 100, 641–652. [Google Scholar] [CrossRef]
- Luan, J.; Cui, Y.; Zhang, Y.; Zhou, X.; Zhang, G.; Han, J. Effect of CXCR4 inhibitor AMD3100 on alkaline phosphatase activity and mineralization in osteoblastic MC3T3-E1 cells. Biosci. Trends 2012, 6, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Soysa, N.S.; Alles, N. Osteoclast function and bone-resorbing activity: An overview. Biochem. Biophys. Res. Commun. 2016, 476, 115–120. [Google Scholar] [CrossRef]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.H. The M-CSF receptor in osteoclasts and beyond. Exp. Mol. Med. 2020, 52, 1239–1254. [Google Scholar] [CrossRef]
- Bharti, A.C.; Takada, Y.; Shishodia, S.; Aggarwal, B.B. Evidence That Receptor Activator of Nuclear Factor (NF)-κB Ligand Can Suppress Cell Proliferation and Induce Apoptosis through Activation of a NF-κB-independent and TRAF6-dependent Mechanism. J. Biol. Chem. 2004, 279, 6065–6076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Ishida, N.; Hayashi, K.; Hoshijima, M.; Ogawa, T.; Koga, S.; Miyatake, Y.; Kumegawa, M.; Kimura, T.; Takeya, T. Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J. Biol. Chem. 2002, 277, 41147–41156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Ma, S.; Feng, W.; Wei, Y.; Lu, H.; Zhong, G.; Wu, Z.; Wang, H.; Su, W.; Li, J. Tanshinone IIA protects against polyethylene particle-induced osteolysis response in a mouse calvarial model. Int. J. Clin. Exp. Pathol. 2018, 11, 4461–4471. [Google Scholar]
- Kumar, R.P.; Abraham, A. PVP- coated naringenin nanoparticles for biomedical applications—In vivo toxicological evaluations. Chem. Biol. Interact. 2016, 257, 110–118. [Google Scholar] [CrossRef]
- Shpigelman, A.; Shoham, Y.; Israeli-Lev, G.; Livney, Y.D. β-Lactoglobulin-naringenin complexes: Nano-vehicles for the delivery of a hydrophobic nutraceutical. Food Hydrocoll. 2014, 40, 214–224. [Google Scholar] [CrossRef]
- Yang, L.J.; Ma, S.X.; Zhou, S.Y.; Chen, W.; Yuan, M.W.; Yin, Y.Q.; Yang, X.D. Preparation and characterization of inclusion complexes of naringenin with β-cyclodextrin or its derivative. Carbohydr. Polym. 2013, 98, 861–869. [Google Scholar] [CrossRef]
- Martinez, S.E.; Lillico, R.; Lakowski, T.M.; Martinez, S.A.; Davies, N.M. Pharmacokinetic analysis of an oral multicomponent joint dietary supplement (Phycox®) in dogs. Pharmaceutics 2017, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropoyl-β-cyclodextrin. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanaze, F.I.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Dissolution enhancement of flavonoids by solid dispersion in PVP and PEG matrixes: A comparative study. J. Appl. Polym. Sci. 2006, 102, 460–471. [Google Scholar] [CrossRef]
- Bali, V.; Ali, M.; Ali, J. Nanocarrier for the enhanced bioavailability of a cardiovascular agent: In vitro, pharmacodynamic, pharmacokinetic and stability assessment. Int. J. Pharm. 2011, 403, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Zheng, Y.; Cao, H.; Huang, Q.; Xiao, J.; Chen, L. Enhancement of bioavailability and bioactivity of diet-derived flavonoids by application of nanotechnology: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef]
- Rodríguez-Fragoso, L.; Martínez-Arismendi, J.L.; Orozco-Bustos, D.; Reyes-Esparza, J.; Torres, E.; Burchiel, S.W. Potential Risks Resulting from Fruit/Vegetable-Drug Interactions: Effects on Drug-Metabolizing Enzymes and Drug Transporters. J. Food Sci. 2011, 76, R112–R124. [Google Scholar] [CrossRef]
- Selvam, M.; Kaliyaperumal, M. Determination of LD 50 of Naringenin for its effects on diabetic nephropathy in rats-A pilot study. J. Chem. Pharm. Res. 2015, 7, 550–553. [Google Scholar]
- Shin, J.; Seol, I.; Son, C. Interpretation of Animal Dose and Human Equivalent Dose for Drug Development. J. Korean Orient. Med. 2010, 31, 1–7. [Google Scholar]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]

| Cell Culture Studies | Major Findings (Changes vs. Negative Control) | Conclusion | |||
| Reference | Study Design | Indices Increased | Indices Decreased | Indices Unchanged | |
| [20] | Cell: Human primary osteoclast precursor cells Induction: RANKL- and M-CSF-induced osteoclastogenesis Treatment: 36.7, 91.8, and 183.6 μM of naringenin (2 μL per well) for 2 and 6 days. Control: Negative: No treatment Positive: No |
|
|
| Naringenin inhibits osteoclast formation and bone resorption. |
| [21] | Cell: rBMSCs from femur and tibia of Wistar rats Induction: Dexamethasone-induced osteogenesis Treatment: 10 μM of naringenin or 8-prenylnaringenin for 16 days Control Negative: No treatment Positive: n.a |
| Both prenylnaringenin and naringenin promote osteoblast differentiation and mineralization. Effects of prenylnaringenin are better than naringenin. | ||
| [22] | Cell: Calvarial osteoblasts from newborn Wistar rats and osteoclasts cells from femur and tibia of rabbit Induction: n.a Treatment: 10 μM of naringenin and 8-prenylnaringenin for 3, 6, 9, and 12 days Control: Negative: No treatment Positive: n.a |
|
|
| Both prenylnaringenin and naringenin suppress osteoclast formation and survival and promote osteoblast differentiation. Effects of prenylnaringenin are better than naringenin. |
| [23] | Cell: Mouse calvarial osteoblasts from Balb/cByJ mice Induction: n.a Treatment: Naringenin or naringenin-6-C-glucoside (0.001,0.01 and 0.1, 1, 10, 25, and 50 μM) for 21 days Control: Negative: No treatment Positive: 17β-oestradiol (1 nM) for 21 days |
| Both naringenin or naringenin-6-C-glucoside promote osteoblast differentiation and bone formation. Naringenin-6-C-glucoside is more potent than naringenin. | ||
| [24] | Cell: co-culture of T-cells from male BALB/c mice and BMMs Induction: M-CSF- and RANKL-induced osteoclastogenesis Treatment: Naringenin (0–800 μM) for 1, 2, and 10 days. Control: Negative: no treatment Positive: n. a |
|
| The anti-osteoclastogenesis effects of naringenin are exerted through IL-4 release by T-cells. | |
| [25] | Cell: BMMs from femur and tibia of C-57/BL6 mice and RAW 264.7 cells. Induction: M-CSF- and RANKL-induced osteoclastogenesis. Treatment: Naringenin (0–800 μM) for 2 days. Control: Negative: no treatment Positive: n. a |
|
| Naringenin inhibits osteoclast formation through suppression of p38 signaling. | |
| [26] | Cell: BMSCs from femur and tibia of Sprague Dawley rats Induction: dexamethasone-induced osteogenesis Treatment: 734.6 μM of naringenin for 14 days Control: Negative: No treatment Positive: n. a |
|
| Naringenin stimulates bone formation via upregulation of the SDF-1a through the SDF-1/CXCR4 signaling pathway | |
| [27] | Cell: MC3T3-E1, hOB and pOB cell line Induction: n.a Treatment: 0.3, 1, 3, and 10 μM of naringenin for 1–7 days Control: Negative: no treatment Positive: n.a |
|
| The osteogenic effects of naringin are exerted through upregulation of BMP-2 expression via the PI3K, Akt, c-Fos/c-Jun and AP-1-dependent signaling pathway | |
| [28] | Cell: hPDLSCs Induction: osteogenic differentiation Treatment: 0.1, 1 and 10 mM of naringenin for 0–72 h Control: Negative: no treatment Positive: n.a |
| Naringenin increases the osteogenic potential of hPDLSCs. | ||
| [29] | Cell: MG-63 cell lines Induction: n.a. Treatment: 100 μL of 0.15–10 μg/mL naringenin nanosuspension for 48 h Control: Negative: no treatment Positive: n.a |
| Naringenin nanosuspension may have pro-osteogenic effects. | ||
| [30] | Cell: mouse MBMMφ and RAW 264.7 cells Induction: M-CSF- and RANKL-induced osteoclastogenesis Treatment: 2.5, 5, and 10 μg/mL naringenin for 72 h Control: Negative: no treatment Positive: n.a |
| Naringenin suppresses osteoclast formation and resorption activity. | ||
| Animal Studies | Major Findings (Changes vs. Negative Control) | ||||
| Researchers (Year) | Study Design | Indices Increased | Indices Decreased | Indices Unchanged | |
| [31] | Animal: 28 Female Wistar rats (3 months old) Induction: OVX-induced osteoporosis Treatment: 50 mg/kg of naringenin for 4 weeks Control: Negative: no treatment Positive: 0.2 mg/kg of estradiol oestrogen for 4 weeks |
|
|
| Naringenin is safe for the skeleton and may have marginal skeletal beneficial effects on the bone. |
| [32] | Animal: 100 Y59 Female rats (3 months old, 200–250 g) Induction: Retinoic acid-induced bone loss Treatment: 100 mg/kg of naringenin for 14 days Control: Negative: no treatment Positive: 40 mg/kg of alendronate for 14 days |
|
| Naringenin protects against retinoic acid-induced bone loss via antioxidant effects. | |
| [23] | Animal: 15 Balb/cByJ mice Induction: OVX-induced osteoporosis Treatment: Preliminary studies—1 and 5 mg/kg/day of naringenin (25 μL) for 3 days in newborn mice Preventive studies—5 mg/kg/day of naringenin for 5 weeks in OVX mice Therapeutic studies—5 mg/kg/day of naringenin (i.p. injection) for 6 weeks in OVX mice Control: Negative: no treatment Positive: Preventive studies—17β estradiol (5 μg/kg/day for 5 weeks Therapeutic studies—40 μg/kg/day of human 1-34PTH (i.p. injection) for 6 weeks |
|
| Naringenin is less effective than Naringenin-6-C-glucoside in preventing bone loss. | |
| [25] | Animals: 20 C-57/BL6 mice (8 weeks old Induction: Ti-particle-induced osteolysis. Treatment: Naringenin (10 mg/kg and 25 mg/kg) for 2 weeks Control: Negative: no treatment Positive: n.a |
|
| Naringenin prevents titanium particle-induced osteolysis caused by excessive osteoclast formation and activity. | |
| [33] | Animal: 40 male Sprague-Dawley rats (46–54 g, 21-day-old) Induction: Soft diet-induced periodontal hypofunction. Treatment: 0.09%, 0.18%, 0.36%, and 0.72% of naringenin for 42 days Control: Negative: no treatment Positive: n.a |
| Naringenin decreases the molar CAB-CEJ distance during alveolar development in young male rats. | ||
| [27] | Animal: Female ICR mice (4 weeks old, 23–29 g) Induction: OVX-induced osteoporosis Treatment: 3 and 10 mg/mL of naringenin every 2 days for 4 weeks Control: Negative: no treatment Positive: 17β-estradiol (0.1 mg/mL every 2 days for 4 weeks) |
| Naringenin prevents bone loss due to ovariectomy. | ||
| [29] | Animal: 48 adult female adult Wistar rats (200–220 g) Induction: OVX-induced osteoporosis Treatment: 20 mg/kg naringenin for 60 days Control: Negative: no treatment Positive: 5.4 mg/kg raloxifene for 60 days |
|
| Oral naringenin nanosuspension prevents bone loss due to ovariectomy. | |
| [34] | Animal: 50 Y59 Female rats (3 months old) Induction: Retinoic acid-induced bone loss Treatment: 100 mg/kg of naringenin for 14 days Control: Negative: no treatment Positive: 40 mg/kg of alendronate for 14 days |
|
| Naringenin prevents bone loss through antioxidant and anti-inflammatory effects. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nor Muhamad, M.L.; Ekeuku, S.O.; Wong, S.-K.; Chin, K.-Y. A Scoping Review of the Skeletal Effects of Naringenin. Nutrients 2022, 14, 4851. https://doi.org/10.3390/nu14224851
Nor Muhamad ML, Ekeuku SO, Wong S-K, Chin K-Y. A Scoping Review of the Skeletal Effects of Naringenin. Nutrients. 2022; 14(22):4851. https://doi.org/10.3390/nu14224851
Chicago/Turabian StyleNor Muhamad, Muhamed Lahtif, Sophia Ogechi Ekeuku, Sok-Kuan Wong, and Kok-Yong Chin. 2022. "A Scoping Review of the Skeletal Effects of Naringenin" Nutrients 14, no. 22: 4851. https://doi.org/10.3390/nu14224851
APA StyleNor Muhamad, M. L., Ekeuku, S. O., Wong, S. -K., & Chin, K. -Y. (2022). A Scoping Review of the Skeletal Effects of Naringenin. Nutrients, 14(22), 4851. https://doi.org/10.3390/nu14224851








