Abstract
Background: The association of meat intake with gastric adenocarcinoma is controversial. We examined the relation between white, red, and processed meat intake and gastric adenocarcinoma, considering doneness preference and cooking methods, by histological subtype and anatomical subsite. Methods: MCC-Spain is a multicase–control study that included 286 incident gastric adenocarcinoma cases and 2993 controls who answered a food-frequency questionnaire. The association of gastric adenocarcinoma with meat intake, doneness preference and cooking methods was assessed using binary multivariate logistic regression mixed models and a possible interaction with sex was considered. Multinomial logistic regression models were used to estimate risk by tumor subsite (cardia vs. non-cardia) and subtype (intestinal vs. diffuse). Sensitivity analyses were conducted comparing models with and without data on Helicobacter pylori infection. Results: The intake of red and processed meat increased gastric adenocarcinoma risk (OR for one serving/week increase (95% CI) = 1.11 (1.02;1.20) and 1.04 (1.00;1.08), respectively), specifically among men and for non-cardia and intestinal gastric adenocarcinoma. Those who consume well done white or red meat showed higher risk of non-cardia (white: RRR = 1.57 (1.14;2.16); red: RRR = 1.42 (1.00;2.02)) and intestinal tumors (white: RRR = 1.69 (1.10;2.59); red: RRR = 1.61 (1.02;2.53)) than those with a preference for rare/medium doneness. Stewing and griddling/barbequing red and white meat, and oven baking white meat, seemed to be the cooking methods with the greatest effect over gastric adenocarcinoma. The reported associations remained similar after considering Helicobacter pylori seropositivity. Conclusions: Reducing red and processed meat intake could decrease gastric adenocarcinoma risk, especially for intestinal and non-cardia tumors. Meat cooking practices could modify the risk of some gastric cancer subtypes.
1. Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide [1,2]. Despite some improvements in the diagnosis and treatment, 5-year survival rate is still lower than 30% in most countries in the world [3,4]. Fortunately, its incidence has been decreasing globally, approaching levels of a rare disease in some populations [5]. This decline has been attributed to changes in the prevalence of exposure to known risk factors for this pathology, associated with higher standards of hygiene, improvements in diet quality and food preservation, increased intake of fresh fruits and vegetables, decreased prevalence of Helicobacter pylori (H. pylori) infection, and interventions to control tobacco or alcohol consumption, among others [6,7,8]. Globally, geographic differences in the occurrence of this neoplasm have been documented [1]. In Spain, GC mortality also displays a striking geographic pattern [6], and this tumor represents the sixth cause of cancer death [7,8].
Gastric adenocarcinoma (GAC) is the most common histological type of GC (90%). Even though chronic H. pylori infection is the main known cause, GC etiology is multifaceted, and diverse risk factors have been associated with this neoplasm, including environmental and genetic factors [9,10]. Some of them, e.g., age, male sex, or GAC family history are not modifiable [11], while others, such as tobacco consumption, diet, or excessive alcohol drinking, are potentially modifiable [12]. Certain contextual and environmental factors are also associated with the development of GC, including parental socioeconomic status, water pollution, soil pollution or soil element content [13].
GAC can be further classified into two histological subtypes according to Lauren’s classification, i.e., intestinal (well-differentiated) and diffuse (undifferentiated) adenocarcinoma [12]. These subtypes differ in their microscopic and gross appearance, sex ratio, age at diagnosis, epidemiologic features, pathogenesis, and prognosis (8). The intestinal type, which accounts for approximately 54% of cases, is more common in elderly males, and shows a slower progression, whereas the diffuse type is more common in younger individuals and has a worse prognosis [14].
Anatomical location of the tumor also has implications in terms of etiology [15]. In recent decades, incidence of cardia gastric adenocarcinoma (CGA) has increased in some geographic areas, while non-cardia gastric adenocarcinoma (NCGA) has decreased [11,16,17]. The main recognized risk factor for NCGA is H. pylori chronic infection, and dietary factors are also involved [11], while CGA is more related to gastroesophageal reflux disease and obesity [15,16].
Dietary habits have long been considered as an important risk factor for GAC [10,18,19]. A low intake of fruits, vegetables (except starchy ones), and pulses, as well as a high consumption of salt, salted and smoked foods, chili pepper, processed and grilled/barbequed meats, alcohol, or a high adherence to the Western dietary pattern, have been associated with an increased risk of GAC [20,21]. In terms of meat, its worldwide intake has being steadily rising since the second half of the twentieth century [22]. Total, red, and processed meat intake has been associated with an increased risk of GAC in several studies [23,24,25,26,27,28], and potential underlying biological mechanisms have been identified for this association [23], but evidence is still limited [27,29]. Moreover, meat cooking practices and doneness preferences, which have been less studied, could independently increase the risk of GAC and might help in explaining the heterogeneity currently observed among results of epidemiological studies [23,25,30,31].
In this work, we aimed to elucidate the role of meat intake in the incidence of GAC, including type of meat, meat cooking methods and doneness preferences, overall and by histological subtypes and anatomical subsites, within the MCC-Spain multicase–control study.
2. Materials and Methods
2.1. Study Population
The MCC-Spain study was carried out between 2008 and 2013 with the objective of identifying etiological factors associated with breast, prostate, colorectal, gastric, and chronic lymphocytic leukemia. The design of the study has already been described [32]. Briefly, in 23 hospitals from 12 Spanish provinces cases of these tumors were prospectively recruited. To ensure a frequency matching by age and sex with the total distribution of cases in each province, controls were chosen at random from the general practitioners’ lists in the same regions. The response rate was 55% among gastric cancer cases and 53% among controls of the full sample of controls (77% among the selected sample of controls for the specific analysis of GC). Two of the provinces did not participate in the recruitment of gastric cancer cases. Participants were invited to join the study if they could complete the questionnaire, were 20 to 85 years old, and had lived in the study area for at least 6 months before the diagnosis/interview. Telephone calls were made to contact controls and those who agreed to participate attended a personal interview. Cases were invited to participate, as promptly as possible after diagnosis was made and were interviewed at their hospital. Histologically confirmed GAC incident cases with no history of the disease were included. Tumors were divided into cardia and non-cardia categories based on their location, and into intestinal and diffuse based on their tumor morphology following the Lauren’s classification [12]. The Ethics Committees of all the participating institutions approved MCC-Spain protocol. All participants signed an informed consent after being informed about the study objectives.
2.2. Data Collection and Diet Assessment
In a face-to-face interview, trained personnel administered a structured computerized epidemiological questionnaire to collect data on socio-demographic variables, personal/family medical history and lifestyle, among others. A 154-items semi-quantitative food frequency questionnaire (FFQ) based on a validated instrument [33] was used to assess dietary intake. Height and weight were self-reported. At the end of the interview, the participants received the FFQ and instructions to complete it at home or while in the hospital, and to send the filled form by mail. This FFQ also gathered information on meat cooking methods (griddled-grilled-barbequed, pan-fried/breaded-coated fried, stewed or oven-baked) and used pictures to determine doneness preferences (rare, medium, well-done). The consumption of total, white, red, and processed meat, as well as the consumption of red and white meat according to the frequency of use of each cooking method were reported in servings/week and then converted into grams/day, using sex-specific standard portion sizes. Chicken, turkey, duck, rabbit, and other game were classified as white meat while pork, beef, lamb, liver of any other animal entrails, as well as pork or beef hamburgers or meatballs were considered as red meat. Finally, bacon, sausages, paté/foie-gras, serrano ham and other cold meat were included in the processed meat category. The time reference of the FFQ was the year before cancer diagnosis or before interview in cases and controls, respectively.
Additionally, blood samples were donated by the 61% of cases and 64% of controls who accepted to do it. Samples were processed, aliquoted and stored at −80 °C in the first 48 h. Later, for the H. pylori multiplex serology assay, a serum aliquot from each participant was sent on dry ice to be analyzed at the German Cancer Research Centre (DKFZ), in Germany.
2.3. Statistical Analyses
Sociodemographic, anthropometric, and lifestyle characteristics were described separately for cases and controls, as well as by type of meat consumption, doneness preference, and cooking methods using basic descriptive statistics. Continuous variables with a normal distribution were described with the mean and the standard deviation (mean -SD-), and differences between cases and controls were tested using t-tests. For continuous variables that presented skewedness, median, and interquartile interval (median (IQI)) were used for description and rank-sum tests to assess differences between cases and controls. Frequencies and percentages were used to describe categorical variables and chi-square tests to assess differences between groups.
Adjusted associations between meat consumption and GAC, by type (white, red, or processed), doneness (rare-medium or well-done) and cooking methods (griddled-grilled-barbequed, pan-fried, stewed, or oven-baked) were evaluated with binary logistic regression mixed models with a random province-specific intercept. All the odds ratios (OR) and 95% confidence intervals (95% CI) estimated by these models were adjusted by education, sex, age, body mass index (BMI), physical activity (metabolic equivalents (METs)) during the previous year, smoking status, family history of gastric cancer and caloric, alcohol, fruits, salty fish, and olive intakes as potential confounders. Additionally, (1) models evaluating the effect of each type of meat (white, red, and processed) were additionally adjusted by the consumption of other types of meat (e.g., for white meat, models were adjusted by red and processed meat intake); (2) models evaluating the effect of doneness preference were also adjusted by total meat-specific intake; and (3) models evaluating the effect of cooking methods, were also adjusted by other meat specific cooking methods (e.g., for griddle-grilled/barbequed (BBQ) white meat, the models were adjusted by fried, stewed, and oven-baked white meat consumption).
A sensitivity analysis was conducted to determine whether the amount of missing values for H. pylori seropositivity (36% among participants with complete information on the rest of the variables of interest) influenced the final results. We compared the outcomes of five models, characterized by different sets of adjustment variables (with or without H. pylori seropositivity) and by different subsamples (i.e., all the participants, only participants with data on H. pylori serostatus or only H. pylori seropositive participants). Since the direction and magnitude of the associations found were similar in all the models, to optimize the statistical power we present the results of the analyses in the main text without considering H. pylori related information. Results considering data on H. pylori serostatus are shown in the Online Resource 1, Tables S1 and S2.
An interaction term between sex and meat consumption (by type, doneness, and cooking method) was included in the models to check for heterogeneity of the effects by sex. Models with and without the interaction term were compared using the likelihood ratio test to obtain the P value for heterogeneity.
The same associations were evaluated taking into account tumor location (no cancer; cardia GC; and non-cardia GC) and morphology (no cancer; intestinal GC and diffuse GC) using multinomial logistic regression models that provided relative risk ratio (RRR) and 95% CI.
Meat consumption was analyzed both as a continuous variable and grouping weekly intake in grams into quartiles of their distribution among controls. However, to improve interpretability, results are presented in servings/week, with one serving representing 125 g of total, red, or white meat, and as 50 g of processed meat. For the analyses by sex, weekly intake was grouped using sex-specific data distribution among controls. The analyses for doneness preference and cooking methods were restricted to group specific meat consumers. All analyses were performed with Stata/MP 16.0 (StataCorp, College Station, TX, USA).
3. Results
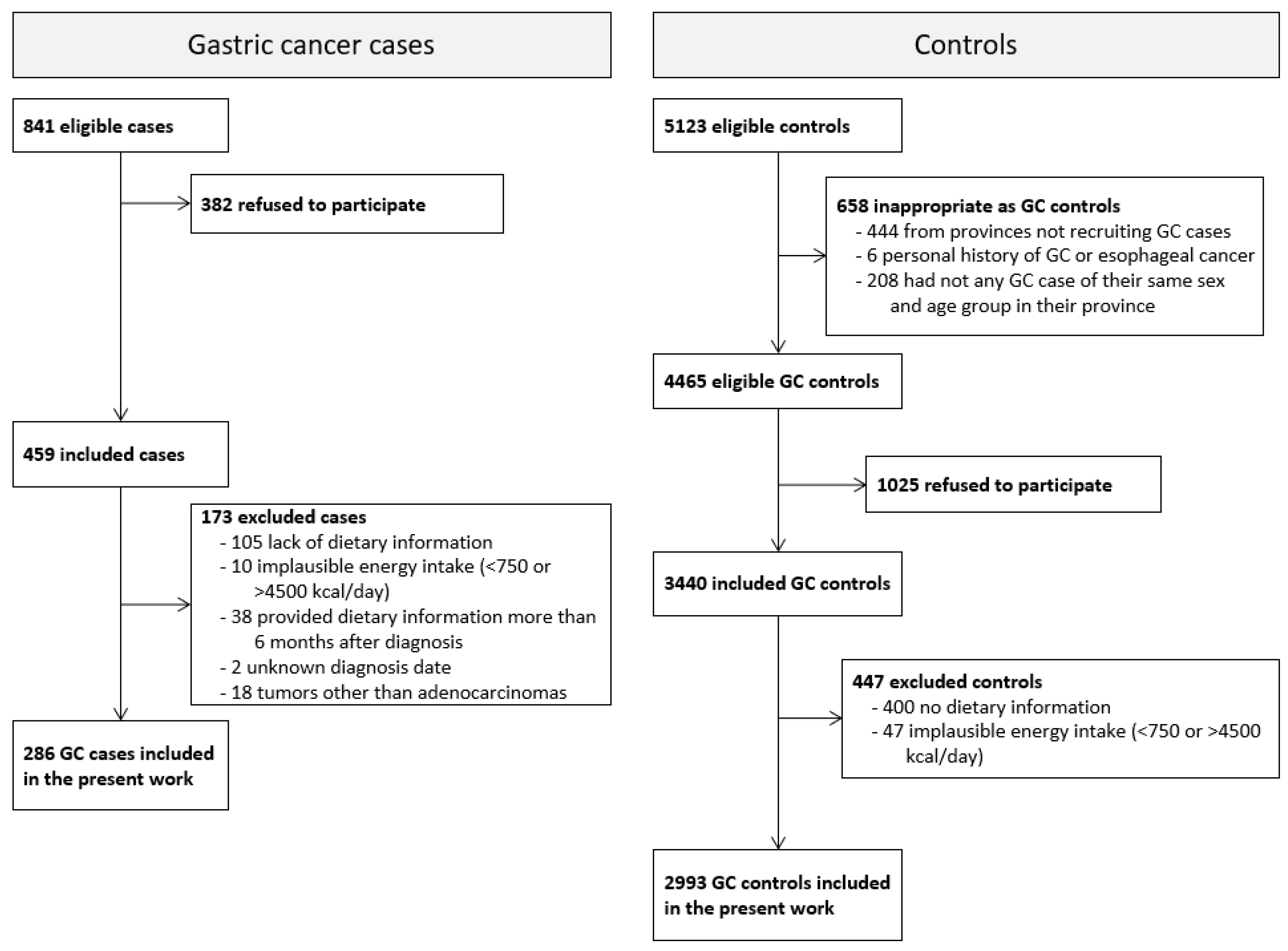
Initially, 459 cases of GC and 3440 controls were enrolled, with 344 (75%) cases and 2993 (87%) controls providing valid dietary information. Cases whose dietary information was collected more than 6 months after diagnosis (n = 38), with an uncertain diagnosis date (n = 2), or with tumors that were not adenocarcinomas (n = 18) were excluded from the analyses. Therefore, the present study included 286 GAC cases and 2993 controls aged 26 to 85 years (Figure 1).
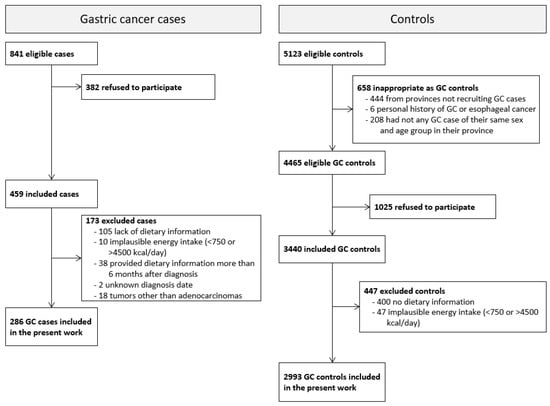
Figure 1.
Selection process of gastric cancer cases and controls in the MCC-Spain study from 2008 to 2013.
Bivariate analyses showed that cases were primarily men, older than the controls and with lower educational level. GAC cases also showed higher caloric and alcohol intake, lower physical activity, and higher prevalence of family history of gastric cancer (Table 1). In terms of meat consumption, cases showed higher consumption of all types of meat and reported a higher preference for well-done meat, and for pan-fried (red meat), stewed (white and red meat), and oven-baked (white meat) cooking methods (Table 2).

Table 1.
Description of socio-demographic and other baseline characteristics of controls and gastric adenocarcinoma cases in the MCC-Spain study.

Table 2.
Description of meat intake, doneness preference and cooking methods for controls and gastric adenocarcinoma cases in the MCC-Spain study.
Table 3 shows the association between GAC and total, white, red, and processed meat consumption for the whole population and by sex. Compared to individuals with a total meat intake under 3.0 servings/week, participants with an intake of 4.3–5.9 or over 5.9 servings/week showed a 58% (OR (95% CI) = 1.58 (1.02;2.42)) and a 73% (1.73 (1.10;2.71)) higher risk of GAC, respectively (p-trend = 0.01). This was mainly due to the effect of red and, to a lesser extent, processed meat intake. Individuals with a red meat consumption of 1.8–2.9 servings/week or over 2.9 servings/week showed a 73% (OR = 1.73 (1.12;2.66)) and 76% (OR = 1.76 (1.14;2.72)) higher risk of GAC, respectively, than those eating less than 1.1 servings/week. For processed meat, the risk of GAC increased by 4% (OR = 1.04 (1.00;1.08)) for each increase in one serving/week. The corresponding linear increase for total and red meat was 11%. When evaluating the effect separately by sex, the observed associations were mainly among men.

Table 3.
Association between gastric adenocarcinoma incidence and total, white, red, and processed meat consumption overall and by sex.
Similarly, analyses by tumor subsite (Table 4) revealed that associations, when present, were observed with total, red, and processed meat. Although no statistically significant heterogeneity was detected, no strong associations were found for cardia adenocarcinoma, whereas non-cardia adenocarcinoma was associated with a consumption of over 4.3 servings/week of total meat (p-trend = 0.013). Specifically, individuals with an intake of 4.3–5.9 servings/week showed a 70% (RRR (95% CI) = 1.70 (1.04;2.80)) and those over 5.9 servings/week a 95% (RRR = 1.95 (1.16;3.27)) higher risk of non-cardia adenocarcinoma than those eating meat less than 3.0 times per week. As for red meat, individuals with intakes between 1.8–2.9 and over 2.9 servings/week, showed a 72% (RRR = 1.72 (1.06;2.78)) and a 69% (RRR = 1.69 (1.04;2.76)) higher risk of non-cardia adenocarcinoma, respectively (p-trend = 0.023), than those with the lowest consumption (<1.1 servings/week). Finally, non-cardia cancer risk was a 79% higher (RRR = 1.79 (1.10;2.92)) among those eating 4.3 servings/week or more than among those consuming less than 1.4 servings/week of processed meat.

Table 4.
Association between gastric adenocarcinoma incidence and total, white, red, and processed meat consumption by tumor subsite (cardia, non-cardia) and morphology (intestinal, diffuse).
Analyses by tumor morphology (Table 4) showed more striking differences, with markedly stronger associations for intestinal tumors with red meat. Compared with individuals eating less than 1.1 servings/week of red meat, the risk of intestinal GAC was 2.77 (95% CI = 1.28;6.00) times bigger for those consuming between 1.1 and 1.8 servings/week, 3.62 (1.66;7.90) times bigger for 1.8–2.9 servings/week, and 5.60 (2.58;12.13) times bigger for intakes ≥ 2.9 servings/week.
Regarding meat doneness preference and cooking methods (Table 5), our results show an increased risk of GAC for the intake vs. non intake of white meat for all cooking methods, especially for stewing (OR (95% CI) = 1.71 (1.19;2.47)), oven-baking (OR = 1.62 (1.20;2.20)), and griddling/barbequing (OR = 1.49 (1.08;2.07)). By sex, differences were mainly observed among men (stewing: OR = 1.97 (1.26;3.08); oven-baking: OR = 2.03 (1.41;2.93); griddling/barbequing: OR = 1.56 (1.07;2.27)). The intake of stewed (OR (95% CI) = 1.62 (1.01;2.60)) and griddled/barbequed (OR = 1.59 (1.03;2.45)) red meat was also linked to a higher global GAC risk. When considering tumor subsite and morphology (Table 6) and referencing individuals with rare/medium doneness preference, those eating well-done white and red meat presented a 57% (RRR (95% CI) = 1.57 (1.14;2.16)) and a 42% (RRR = 1.42 (1.00;2.02)) higher risk of NCGA, and a 69% (RRR = 1.69 (1.10;2.59)) and 61% (RRR = 1.61 (1.02;2.53)) higher risk of intestinal GAC, respectively. As for the cooking methods, stewing and oven-baking white meat were associated with NCGA (stewed: RRR = 1.73 (1.14;2.63); oven-baked: RRR = 1.36 (0.98;1.88)), with cardia GAC (oven-baked: RRR = 2.14 (1.15–3.96)), and with intestinal tumors (stewed: RRR = 2.40 (1.28;4.49); oven-baked: RRR = 1.57 (1.01;2.44)). Griddling/barbequing red meat was also associated with these tumor subtypes (NCGA: RRR = 1.71 (1.02;2.85); intestinal: RRR = 2.16 (1.09;4.29)).

Table 5.
Association between gastric adenocarcinoma incidence and meat-type specific doneness preference and cooking methods, overall and by sex (restricted to consumers of each type of meat).

Table 6.
Association between gastric adenocarcinoma incidence and meat-type specific doneness preference and cooking methods by tumor subsite (cardia, non-cardia) and morphology (intestinal, diffuse) (restricted to consumers of each type of meat).
4. Discussion
Our results indicate that meat consumption may increase GAC risk, specifically among men and non-cardia and intestinal tumors, mainly due to the association with red and, to a lesser extent, processed meat. Increased GAC risk was observed for consumption of 1.8 or more 125 g-servings per week of red meat and 4.3 or more 50 g-servings per week of processed meat. Doneness preference might also influence GAC risk. In fact, participants who preferred well-done white or red meat presented a higher risk of non-cardia and intestinal tumors than those with a preference for rare/medium doneness. Additionally, stewing and griddling/barbequing red and white meat and oven baking white meat appeared to be the cooking methods with the greatest effect over GAC.
A positive dose–response association was observed between GAC and both total and red meat and, to a lesser extent, processed meat intake. In 2017, a systematic review found null results in cohort studies for the association between GAC risk with red and processed meat consumption, while case–control studies yielded positive associations [25]. In 2019, a meta-analysis including 43 studies (11 cohort studies and 32 case–control studies), concluded that red and processed meat consumption increased the risk of GAC by 41% and 57%, respectively [23]. More recently, the Stomach Cancer Pooling (StoP) Project consortium reported an elevated risk of gastric cancer for the consumption of total (OR: 1.30; 95% CI: 1.09–1.55), red (OR: 1.24; 95% CI: 1.00–1.53), and processed meat (OR: 1.23; 95% CI: 1.06–1.43) [26]. With regard to white meat intake, a reverse association with the risk of this malignancy has been found in some studies [23,34], but not in all [20,24]. In our study, consumption of white meat had no clear effect on GAC, although it was associated with higher risk of diffuse GAC, and specific cooking methods were also associated with increased risk. Variability in these aspects could underlie the inconsistent results found among published studies.
Regarding results by anatomical subsite, in agreement with our findings, in the European Prospective Investigation Into Cancer and Nutrition (EPIC) cohort, total, red, and processed meat consumption was associated with an elevated risk of non-cardia gastric adenocarcinoma, especially in H. pylori antibody-positive individuals, while not with cardia adenocarcinoma [24]. By histological subtype, apart from the EPIC study, we have only found a previous study, conducted in Italy, which studied the risk patterns for intestinal and diffuse adenocarcinoma subtypes [35]. In these studies, high meat intake was associated with an increased risk for both subtypes, with no remarkable differences between them. In our study, total and red meat intake were strongly and positively associated with the risk of having an intestinal type tumor, with a clear dose–response effect, while no association was observed for diffuse GAC.
When comparing the highest and lowest categories of total meat intake separately by sex, a positive association was observed mainly among men, mostly due to the effect of red and processed meat. Additionally, in the dose–response analysis, a linear increase for total, red, and processed meat was observed among men. These results support that consumption of processed meat increases the risk of gastric adenocarcinoma. However, the number of GAC cases among women was small, due to the lower incidence of this tumor among women, what resulted in less precise estimates. More research is warranted to explore possible differences in the effects of meat intake in gastric cancer between women and men.
Several studies have explored the biological mechanism that could explain the association between red and processed meat ingestion and GC. First, it has been hypothesized that high dietary iron intake and elevated body iron status increase the risk of several cancers, including GAC [36,37,38]. Iron contained in red and processed meat may cause oxidative DNA damage by catalyzing the formation of reactive oxygen species, and involving the endogenous formation of carcinogenic N-nitroso compounds (NOCs) [39]. In addition, heme iron is considered a critical factor for bacterial growth, such as H. pylori, the main established risk factor of non-cardia gastric cancer [40,41]. Furthermore, independently of iron, consumption of processed foods contributes to an increased intake of salt, saturated fats, cholesterol, polycyclic aromatic hydrocarbons (PAHs), and heterocyclic amines (HCAs), compounds that have also been described as potential carcinogens [42], that may affect different intracellular pathways related to proliferation, angiogenesis, inflammatory responses, or apoptosis [43]. Salt irritates the gastric mucosa rendering it more susceptible to chemical carcinogenesis and to H. pylori colonization [19,44]. As for white meat, compared to red meat, it contains less cholesterol, less saturated fats, and also a lower content of heme iron, reducing the endogenous formation of NOCs [45]. Moreover, white meat is a source of polyunsaturated fatty acids (PUFAs), some of which possess anti-inflammatory activity and induce apoptosis [46]. Thus, white meat intake could contribute to decreasing the risk of gastric adenocarcinoma by limiting chronic mucosal inflammation, which is a major risk factor for gastric carcinogenesis. Nevertheless, residual confounding cannot be completely discarded, since overall healthier dietary patterns might be associated with higher intake of white meat [47,48,49].
The methods used to cook, process, and preserve meat may also influence the risk of GAC. According to our findings, an increased risk of NCGA and intestinal tumors was observed among consumers of well-done white or red meat. Moreover, stewing and oven-baking seemed to be the cooking methods with the greatest effects over GAC for white meat. Griddling/barbequing and stewing red meat were also associated with GAC. A previous case–control study [30] exploring the association between different methods of cooking meat (roasting/grilling; boiling/stewing; frying/pan frying) and cancer risk, also reported a statistically significant association with gastric cancer risk only for boiled/stewed red meat (OR = 1.86; 95% CI: 1.20–2.87). Depending on the type of meat, cooking method, and level of doneness, red and white meats cooked at high temperature or for a long period of time (e.g., griddling, barbequing, stewing) may form high levels of mutagens, including HCAs and PAHs, which could have an important role in GAC pathogenesis [50,51,52,53,54,55]. Stewed dishes might have higher amounts of these carcinogens, since these substances may remain in the sauce [56]. The use of cooking method or doneness preference as a measure of exposure, rather than individual compounds, may reflect the carcinogenicity of all known and unknown elements present in cooked meat.
Although many factors contribute to the development of gastric cancer, the dominant risk factor for NCGC is chronic infection with H. pylori [57,58]. However, adjusting our models by H. pylori antibody status had no significant effect in the results (Online Resource 1, Tables S1 and S2). H. pylori infection is highly prevalent worldwide, and, in our study, 89% of participants were seropositive [59]. With this high prevalence of infection, there was insufficient heterogeneity to observe a possible confounding or modification effect of infection status on the association between GAC and meat consumption.
Several limitations should be considered before drawing conclusions. Firstly, as is always the case with case–control studies, particularly when assessing the effect of self-reported dietary data, we are concerned about the possible recall bias. Anticipating the possible presence of this bias, we included in the questionnaire specific questions on general eating habits that were subsequently used to adjust the responses of the FFQ [60], and we excluded all cases that answered the questionnaire more than 6 months after diagnosis. Moreover, carcinogenesis may be associated with long-term lifestyles, and the ability of our FFQ to assess long-term dietary habits may be of concern. However, several studies have found a strong agreement between recall of past diet and current diet, suggesting that if remote diet is of interest, focusing questions on the period of interest generally provides appropriate information up to approximately 10 years [61]. Recall bias can also affect self-reported height and weight information, which would influence the calculation of the BMI in the year prior to GC diagnosis or interview. Additionally, participation rate was 55% for cases and 53% for controls, and though it could appear to be low, there is general consensus that a 50% rate might be adequate [62], especially when biological samples are collected.
As for the representativeness of the meat intake collected between 2008 and 2013, meat consumption has decreased in Spain in the last 10 years, especially for red and processed meat [63]. However, this does not reduce the validity of the results obtained in this study. Cut points are relative, therefore, general changes in meat consumption would result in different cut points for quartiles but similar distribution of individuals and therefore similar associations. Differences in the estimates would be observed if changes occur differently among cases and controls. In this case, we would expect the largest decrease in meat intake to occur among controls, which would lead to stronger effects. Moreover, the main results were not adjusted by H. pylori infection. However, the sensitivity analyses performed (Tables S1 and S2) did not show differences in the effect estimates when considering this factor, thus supporting that the main results are not confounded by H. pylori infection status. Finally, since cases and controls are frequency matched for the whole sample that includes all five cancer locations investigated in the MCC-Spain study, some imbalance in the characteristics of cases and controls are observed when analyzing one specific tumor. However, to correct the possible bias introduced by this misbalance, all multivariable analyses were adjusted by age and sex, as well as for gastric cancer risk factors.
Our study also has some notable strengths. Incident GAC cases were recruited, and all of them had histologic confirmation. We were able to analyze different subgroups, according to tumor location and histological subtype, which have been scarcely addressed in previous studies on meat intake and gastric cancer, and to test interactions with sex. We collected information on main possible confounders, such as BMI, alcohol and tobacco consumption, physical activity, and family history of gastric cancer, and all our estimates were adjusted for these factors. Another important asset is the use of a detailed FFQ to collect information on type of meat consumption, doneness preference, and cooking methods, which also included pictures to ensure the correct classification of doneness preference. In addition, the recruitment of cases and controls in 10 provinces from the North, South, Centre, West, and East of the country allowed us to capture the geographical variability in the consumption of meat in Spain.
5. Conclusions
This work supports the hypothesis of an association between the intake of different types of meat and GAC. A differential role of doneness preferences and cooking methods in this relationship was also suggested. Reducing red and processed meat consumption and avoiding overcooking and preparing meat at high temperatures for long periods of time, could help to reduce the risk of GAC, especially intestinal and non-cardia tumors. Further research is warranted to reinforce the strength of the evidence and to improve our understanding of the possible underlying mechanisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14224852/s1. Since data on H. pylori seropositivity was missing for 36% of the participants with complete information on the rest of the variables, a sensitivity analysis was carried out to choose the best modelling strategy. We compared the results of five models, characterized by different sets of adjusting variables (with or without H. pylori seropositivity) and by different subsamples (i.e., all the participants, only participants with data on H. pylori serostatus or only H. pylori seropositive participants). Given that the direction and magnitude of the associations found were similar in all the models, in order to optimize the statistical power, we present in the main text the results of the analyses not taking into account H. pylori related information. Results considering data on H. pylori serostatus are shown in the Online Resource 1, Tables S1 and S2. Table S1: Sensitivity analysis to explore the role of Helicobacter pylori (HP) infection in the association between gastric adenocarcinoma incidence and total, white, red and processed meat consumption; Table S2: Sensitivity analysis to explore the role of Helicobacter pylori (HP) infection in the association between gastric adenocarcinoma incidence and meat-type specific doneness preference and cooking methods.
Author Contributions
This research has been conducted by a multicentre group (MCC-Spain Group). E.B., A.C. and N.A. carried out the study concept and design, database curation, analysis, interpretation of the data, and drafting of the manuscript. N.F.d.L. participated in the interpretation of the data and critical revision of the manuscript. M.P., M.K., G.C.-V., V.M., B.P.-G., M.O.-S., M.G., J.M.C., I.G.-A., G.F.-T., M.V.-E., R.O.-R., J.A. and M.D.C. participated in the study concept and design, interpretation of the data, and critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Carlos III Institute of Health, co-funded by FEDER funds—a way to build Europe (PI08/1770, PI09/00773, PS09/01286, PI09/1903, PI09/1662, and PI09/2078, PI11/01403), the Spanish Ministry of Economy and Competitiveness (IJCI-2014-20900), by Fundación Marqués de Valdecilla (grant API 10/09), by Junta de Castilla y León (LE22A10-2), by Acción Transversal del Cancer, approved by the Spanish Ministry Council on October 11, 2007, by the CIBER of Epidemiology and Public Health (CIBERESP), by the Catalan Government DURSI (grants 2014SGR647 and 2014SGR756), by the Consejería de Salud of the Junta de Andalucía (grant 2009-S0143), by the Conselleria de Sanitat of the Generalitat Valenciana (grant AP061/10), by the University of Oviedo, IUOPA and Fundación Caja de Asturias. ISGlobal acknowledges support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program. Elena Boldo was supported by a grant from the Ministry of Economy and Competitiveness (Bolsa de Ampliación de Estudios. Acción Estratégica en Salud del Plan Nacional I+D+i 2008–2011). None of the funders played any role in conducting the research or writing the paper. M. Obón-Santacana received a post-doctoral fellowship from the Spanish Association Against Cancer Scientific Foundation (AECC; POSTD037OBÓN). Biological samples were stored in the biobanks supported by Instituto de Salud Carlos III- FEDER: Parc de Salut MAR Biobank (MARBiobanc) (RD09/0076/00036), “Biobanco La Fe” (RD 09 0076/00021) and FISABIO Biobank (RD09 0076/00058). Also, in the Public Health Laboratory of Gipuzkoa, the Basque Biobank, the ICOBIOBANC (sponsored by the Catalan Institute of Oncology), the IUOPA Biobank of the University of Oviedo and the ISCIII Biobank.
Institutional Review Board Statement
The protocol of MCC-Spain was approved by each of the Ethics Committees of the participating institutions. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed Consent Statement
Informed consent was obtained from all patients.
Data Availability Statement
The datasets generated for this paper are not publicly available due to restrictions imposed by the Carlos III Committee for Ethical Research, but are available from the principal investigator on reasonable request.
Conflicts of Interest
The authors declare that they have no conflict of interest. This article presents independent research. The views expressed are those of the authors and not necessarily those of the Carlos III Institute of Health.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet Lond. Engl. 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Guevara, M.; Molinuevo, A.; Salmerón, D.; Marcos-Gragera, R.; Carulla, M.; Chirlaque, M.-D.; Rodríguez Camblor, M.; Alemán, A.; Rojas, D.; Vizcaíno Batllés, A.; et al. Cancer Survival in Adults in Spain: A Population-Based Study of the Spanish Network of Cancer Registries (REDECAN). Cancers 2022, 14, 2441. [Google Scholar] [CrossRef]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is Gastric Cancer Becoming a Rare Disease? A Global Assessment of Predicted Incidence Trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Aragonés, N.; Pérez-Gómez, B.; Pollán, M.; Ramis, R.; Vidal, E.; Lope, V.; García-Pérez, J.; Boldo, E.; López-Abente, G. The Striking Geographical Pattern of Gastric Cancer Mortality in Spain: Environmental Hypotheses Revisited. BMC Cancer 2009, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Martín-Richard, M.; Carmona-Bayonas, A.; Custodio, A.B.; Gallego, J.; Jiménez-Fonseca, P.; Reina, J.J.; Richart, P.; Rivera, F.; Alsina, M.; Sastre, J. SEOM Clinical Guideline for the Diagnosis and Treatment of Gastric Cancer (GC) and Gastroesophageal Junction Adenocarcinoma (GEJA) (2019). Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Instituto de Salud Carlos III Mortalidad de Cáncer En España—Tabla de Datos. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesCronicas/Paginas/Tablas-de-datos.aspx (accessed on 8 November 2022).
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.-I.; Kook, M.-C.; Park, B.; Joo, J. Family History of Gastric Cancer and Helicobacter Pylori Treatment. N. Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef]
- Poorolajal, J.; Moradi, L.; Mohammadi, Y.; Cheraghi, Z.; Gohari-Ensaf, F. Risk Factors for Stomach Cancer: A Systematic Review and Meta-Analysis. Epidemiol. Health 2020, 42, e2020004. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and so-Called Intestinal-Type Carcinoma, an Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, X.; Li, S.; Li, C.; Guo, Z. Impact of Environmental Factors on Gastric Cancer: A Review of the Scientific Evidence, Human Prevention and Adaptation. J. Environ. Sci. Chin. 2020, 89, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric Cancer: Classification, Histology and Application of Molecular Pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Ottavia, C.; Serena, S.; Chiara, M.; Alberto, B.; Antonio, N.; Gioacchino, L.; Tiziana, M.; de’ Angelis, G.L.; Francesco, D.M. Epidemiology of Gastric Cancer and Risk Factors. Acta Bio. Med. Atenei Parm. 2018, 89, 82–87. [Google Scholar] [CrossRef]
- Abdi, E.; Latifi-Navid, S.; Zahri, S.; Yazdanbod, A.; Pourfarzi, F. Risk Factors Predisposing to Cardia Gastric Adenocarcinoma: Insights and New Perspectives. Cancer Med. 2019, 8, 6114–6126. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, F.; Xie, S.-H.; Mattsson, F.; Lagergren, J. Updated Incidence Trends in Cardia and Non-Cardia Gastric Adenocarcinoma in Sweden. Acta Oncol. Stockh. Swed. 2018, 57, 1173–1178. [Google Scholar] [CrossRef]
- Vahid, F.; Davoodi, S.H. Nutritional Factors Involved in the Etiology of Gastric Cancer: A Systematic Review. Nutr. Cancer 2021, 73, 376–390. [Google Scholar] [CrossRef]
- Maddineni, G.; Xie, J.J.; Brahmbhatt, B.; Mutha, P. Diet and Carcinogenesis of Gastric Cancer. Curr. Opin. Gastroenterol. 2022, 38, 588–591. [Google Scholar] [CrossRef]
- Fang, X.; Wei, J.; He, X.; An, P.; Wang, H.; Jiang, L.; Shao, D.; Liang, H.; Li, Y.; Wang, F.; et al. Landscape of Dietary Factors Associated with Risk of Gastric Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Cancer Oxf. Engl. 1990 2015, 51, 2820–2832. [Google Scholar] [CrossRef]
- Castelló, A.; Fernández de Larrea, N.; Martín, V.; Dávila-Batista, V.; Boldo, E.; Guevara, M.; Moreno, V.; Castaño-Vinyals, G.; Gómez-Acebo, I.; Fernández-Tardón, G.; et al. High Adherence to the Western, Prudent, and Mediterranean Dietary Patterns and Risk of Gastric Adenocarcinoma: MCC-Spain Study. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 372–382. [Google Scholar] [CrossRef]
- Daniel, C.R.; Cross, A.J.; Koebnick, C.; Sinha, R. Trends in Meat Consumption in the United States. Public Health Nutr. 2011, 14, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kim, K.; Lee, S.A.; Kwon, S.O.; Lee, J.-K.; Keum, N.; Park, S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose−Response Meta-Analysis. Nutrients 2019, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Jakszyn, P.; Pera, G.; Agudo, A.; Bingham, S.; Palli, D.; Ferrari, P.; Boeing, H.; del Giudice, G.; Plebani, M.; et al. Meat Intake and Risk of Stomach and Esophageal Adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2006, 98, 345–354. [Google Scholar] [CrossRef]
- Zhao, Z.; Yin, Z.; Zhao, Q. Red and Processed Meat Consumption and Gastric Cancer Risk: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 30563–30575. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Rosato, V.; Rota, M.; Costa, A.R.; Morais, S.; Pelucchi, C.; Johnson, K.C.; Hu, J.; Palli, D.; Ferraroni, M.; et al. Meat Intake and Risk of Gastric Cancer in the Stomach Cancer Pooling (StoP) Project. Int. J. Cancer 2020, 147, 45–55. [Google Scholar] [CrossRef] [PubMed]
- IARC. Red Meat and Processed Meat; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2018; Volume 114, ISBN 978-92-832-0152-6. [Google Scholar]
- Huang, Y.; Cao, D.; Chen, Z.; Chen, B.; Li, J.; Guo, J.; Dong, Q.; Liu, L.; Wei, Q. Red and Processed Meat Consumption and Cancer Outcomes: Umbrella Review. Food Chem. 2021, 356, 129697. [Google Scholar] [CrossRef] [PubMed]
- WCRF. Diet, Nutrition, Physical Activity and Stomach Cancer. In Continuous Update Project Report; World Cancer Research Fund/American Institute for Cancer Research: London, UK, 2016; Revised 2018. [Google Scholar]
- Di Maso, M.; Talamini, R.; Bosetti, C.; Montella, M.; Zucchetto, A.; Libra, M.; Negri, E.; Levi, F.; La Vecchia, C.; Franceschi, S.; et al. Red Meat and Cancer Risk in a Network of Case-Control Studies Focusing on Cooking Practices. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 3107–3112. [Google Scholar] [CrossRef]
- Song, P.; Lu, M.; Yin, Q.; Wu, L.; Zhang, D.; Fu, B.; Wang, B.; Zhao, Q. Red Meat Consumption and Stomach Cancer Risk: A Meta-Analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 979–992. [Google Scholar] [CrossRef]
- Castano-Vinyals, G.; Aragones, N.; Perez-Gomez, B.; Martin, V.; Llorca, J.; Moreno, V.; Altzibar, J.M.; Ardanaz, E.; de Sanjose, S.; Jimenez-Moleon, J.J.; et al. Population-Based Multicase-Control Study in Common Tumors in Spain (MCC-Spain): Rationale and Study Design. Gac. Sanit. 2015, 29, 308–315. [Google Scholar] [CrossRef]
- García-Closas, R.; García-Closas, M.; Kogevinas, M.; Malats, N.; Silverman, D.; Serra, C.; Tardón, A.; Carrato, A.; Castaño-Vinyals, G.; Dosemeci, M.; et al. Food, Nutrient and Heterocyclic Amine Intake and the Risk of Bladder Cancer. Eur. J. Cancer Oxf. Engl. 1990 2007, 43, 1731–1740. [Google Scholar] [CrossRef]
- Zamani, N.; Hajifaraji, M.; Fazel-tabar Malekshah, A.; Keshtkar, A.A.; Esmaillzadeh, A.; Malekzadeh, R. A Case-Control Study of the Relationship between Gastric Cancer and Meat Consumption in Iran. Arch. Iran. Med. 2013, 16, 324–329. [Google Scholar] [PubMed]
- Buiatti, E.; Palli, D.; Bianchi, S.; Decarli, A.; Amadori, D.; Avellini, C.; Cipriani, F.; Cocco, P.; Giacosa, A.; Lorenzini, L. A Case-Control Study of Gastric Cancer and Diet in Italy. III. Risk Patterns by Histologic Type. Int. J. Cancer 1991, 48, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a Central Player and Promising Target in Cancer Progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and Cancer: More Ore to Be Mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Ward, M.H.; Cross, A.J.; Abnet, C.C.; Sinha, R.; Markin, R.S.; Weisenburger, D.D. Heme Iron from Meat and Risk of Adenocarcinoma of the Esophagus and Stomach. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2012, 21, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Jakszyn, P.; Agudo, A.; Lujan-Barroso, L.; Bueno-de-Mesquita, H.B.; Jenab, M.; Navarro, C.; Palli, D.; Boeing, H.; Manjer, J.; Numans, M.E.; et al. Dietary Intake of Heme Iron and Risk of Gastric Cancer in the European Prospective Investigation into Cancer and Nutrition Study. Int. J. Cancer 2012, 130, 2654–2663. [Google Scholar] [CrossRef]
- Amieva, M.; Peek, R.M. Pathobiology of Helicobacter Pylori-Induced Gastric Cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef]
- Shimizu, T.; Marusawa, H.; Watanabe, N.; Chiba, T. Molecular Pathogenesis of Helicobacter Pylori-Related Gastric Cancer. Gastroenterol. Clin. North Am. 2015, 44, 625–638. [Google Scholar] [CrossRef]
- Rohrmann, S.; Linseisen, J. Processed Meat: The Real Villain? Proc. Nutr. Soc. 2016, 75, 233–241. [Google Scholar] [CrossRef]
- Patrad, E.; Khalighfard, S.; Amiriani, T.; Khori, V.; Alizadeh, A.M. Molecular Mechanisms Underlying the Action of Carcinogens in Gastric Cancer with a Glimpse into Targeted Therapy. Cell. Oncol. Dordr. 2022. [Google Scholar] [CrossRef]
- Wu, X.; Chen, L.; Cheng, J.; Qian, J.; Fang, Z.; Wu, J. Effect of Dietary Salt Intake on Risk of Gastric Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies. Nutrients 2022, 14, 4260. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Hughes, R.; Cross, A.J. Effect of White versus Red Meat on Endogenous N-Nitrosation in the Human Colon and Further Evidence of a Dose Response. J. Nutr. 2002, 132, 3522S–3525S. [Google Scholar] [CrossRef] [PubMed]
- Koumbi, L. Dietary Factors Can Protect against Liver Cancer Development. World J. Hepatol. 2017, 9, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.R.; Cross, A.J.; Graubard, B.I.; Hollenbeck, A.R.; Park, Y.; Sinha, R. Prospective Investigation of Poultry and Fish Intake in Relation to Cancer Risk. Cancer Prev. Res. Phila. 2011, 4, 1903–1911. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-Quality Scores and Plasma Concentrations of Markers of Inflammation and Endothelial Dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean Diet and Other Dietary Patterns and Breast Cancer Risk: Case-Control EpiGEICAM Study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, X.; Ma, Y.; Zhao, J.; Tang, Z. Concentrations and Distributions of Polycyclic Aromatic Hydrocarbon in Vegetables and Animal-Based Foods before and after Grilling: Implication for Human Exposure. Sci. Total Environ. 2019, 690, 965–972. [Google Scholar] [CrossRef]
- Ahmad Kamal, N.H.; Selamat, J.; Sanny, M. Simultaneous Formation of Polycyclic Aromatic Hydrocarbons (PAHs) and Heterocyclic Aromatic Amines (HCAs) in Gas-Grilled Beef Satay at Different Temperatures. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2018, 35, 848–869. [Google Scholar] [CrossRef]
- Sugimura, T. Nutrition and Dietary Carcinogens. Carcinogenesis 2000, 21, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Rothman, N.; Brown, E.D.; Salmon, C.P.; Knize, M.G.; Swanson, C.A.; Rossi, S.C.; Mark, S.D.; Levander, O.A.; Felton, J.S. High Concentrations of the Carcinogen 2-Amino-1-Methyl-6-Phenylimidazo-[4,5-b]Pyridine (PhIP) Occur in Chicken but Are Dependent on the Cooking Method. Cancer Res. 1995, 55, 4516–4519. [Google Scholar]
- Solyakov, A.; Skog, K. Screening for Heterocyclic Amines in Chicken Cooked in Various Ways. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2002, 40, 1205–1211. [Google Scholar] [CrossRef]
- Sinha, R.; Knize, M.G.; Salmon, C.P.; Brown, E.D.; Rhodes, D.; Felton, J.S.; Levander, O.A.; Rothman, N. Heterocyclic Amine Content of Pork Products Cooked by Different Methods and to Varying Degrees of Doneness. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1998, 36, 289–297. [Google Scholar] [CrossRef]
- de Batlle, J.; Gracia-Lavedan, E.; Romaguera, D.; Mendez, M.; Castaño-Vinyals, G.; Martín, V.; Aragonés, N.; Gómez-Acebo, I.; Olmedo-Requena, R.; Jimenez-Moleon, J.J.; et al. Meat Intake, Cooking Methods and Doneness and Risk of Colorectal Tumours in the Spanish Multicase-Control Study (MCC-Spain). Eur. J. Nutr. 2018, 57, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Helicobacter and Cancer Collaborative Group. Gastric Cancer and Helicobacter Pylori: A Combined Analysis of 12 Case Control Studies Nested within Prospective Cohorts. Gut 2001, 49, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Tsugawa, H.; Suzuki, H. Precision Medicine Approaches to Prevent Gastric Cancer. Gut Liver 2021, 15, 3–12. [Google Scholar] [CrossRef]
- Lorenzo, I.; Fernández-de-Larrea, N.; Michel, A.; Romero, B.; Lope, V.; Bessa, X.; Moreno, V.; Martín, V.; Amiano, P.; Castilla, J.; et al. Helicobacter Pylori Seroprevalence in Spain: Influence of Adult and Childhood Sociodemographic Factors. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2019, 28, 294–303. [Google Scholar] [CrossRef]
- Calvert, C.; Cade, J.; Barrett, J.H.; Woodhouse, A. Using Cross-Check Questions to Address the Problem of Mis-Reporting of Specific Food Groups on Food Frequency Questionnaires. UKWCS Steering Group. United Kingdom Women’s Cohort Study Steering Group. Eur. J. Clin. Nutr. 1997, 51, 708–712. [Google Scholar] [CrossRef]
- Willett, W. Recall of Remote Diet. In Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998; ISBN 978-0-19-512297-8. [Google Scholar]
- Draugalis, J.R.; Coons, S.J.; Plaza, C.M. Best Practices for Survey Research Reports: A Synopsis for Authors and Reviewers. Am. J. Pharm. Educ. 2008, 72, 11. [Google Scholar] [CrossRef]
- Spanish Statistical Office—INE. ¿Cuánta Carne Se Consume Cada Año En España? Gráficos y Evolución. Available online: https://www.epdata.es/datos/carne-consume-ano-espana-graficos-evolucion/605 (accessed on 7 April 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).