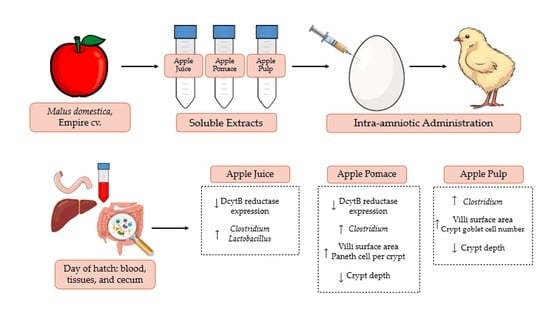
Empire Apple (Malus domestica) Juice, Pomace, and Pulp Modulate Intestinal Functionality, Morphology, and Bacterial Populations In Vivo (Gallus gallus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apple Preparation
2.2. Apple Analysis Sample Preparation
2.2.1. Polyphenol Analysis
2.2.2. Fibrous and Non-Fibrous Carbohydrate Analysis
2.3. Extraction of Soluble Apple Contents
2.4. Animals and Design
2.5. Blood Analysis
2.6. Pectoral Glycogen
2.7. Total RNA Extraction from Duodenum and Liver Tissue Samples
2.8. Real-Time Polymerase Chain Reaction (RT-PCR)
2.9. Microbial Samples and Intestinal Contents DNA Isolation
2.10. Primer Design and PCR Amplification of Bacterial 16S rRNA
2.11. Histomorphological Examination
2.12. Statistical Analysis
3. Results
3.1. Apple Polyphenol and Fiber Content
3.2. Body Weight, Blood Glucose, and Glycogen Content
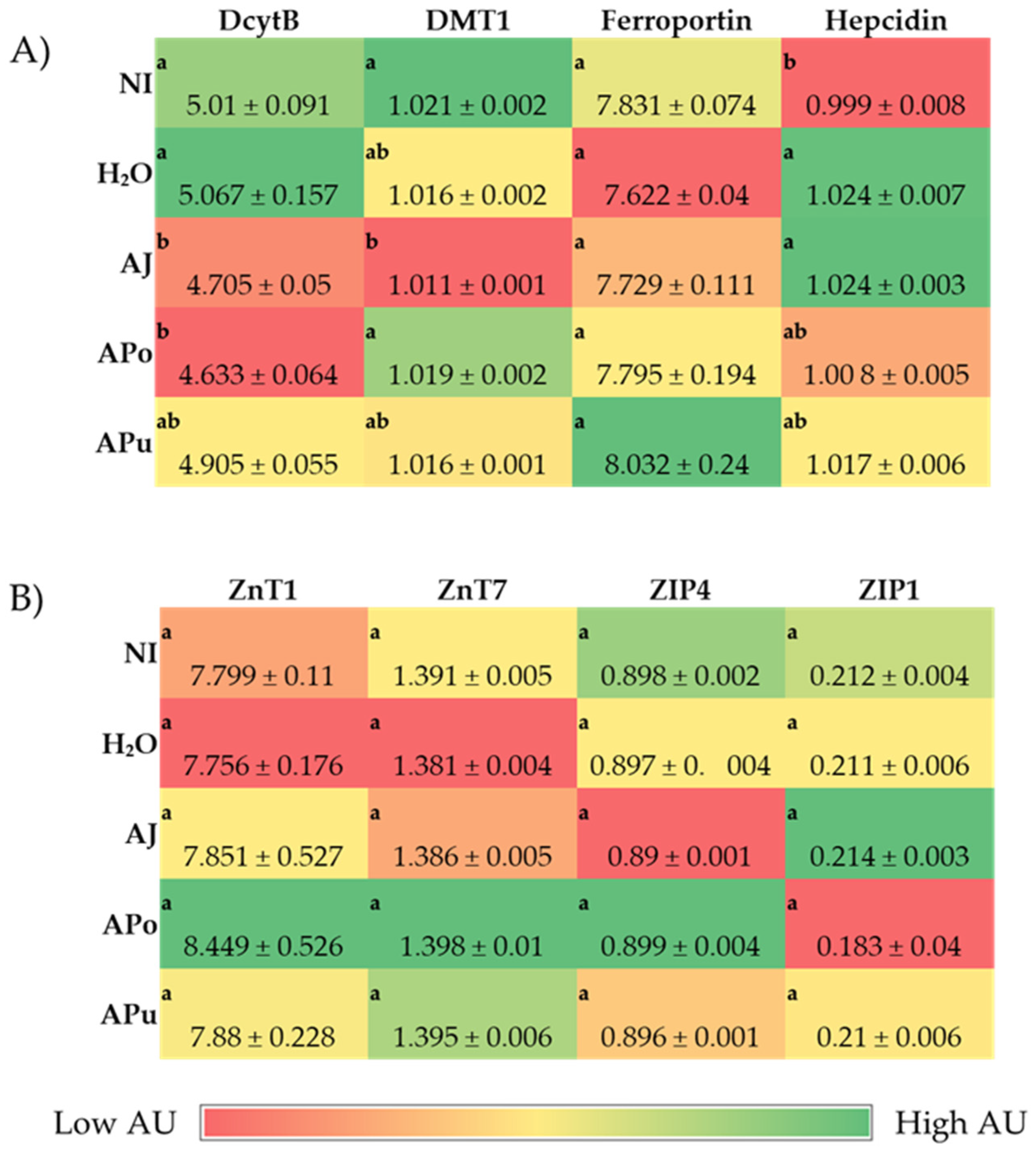
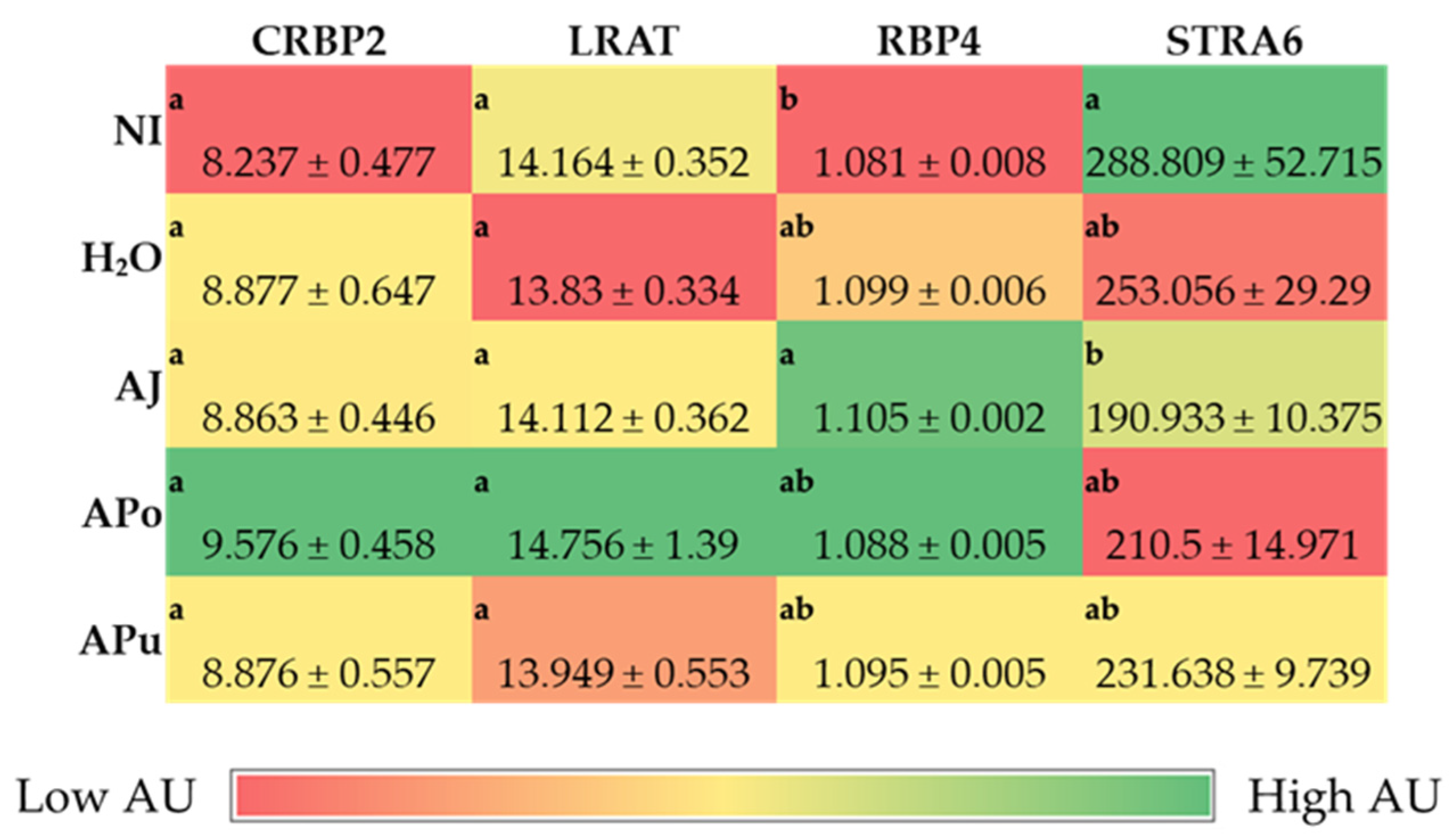
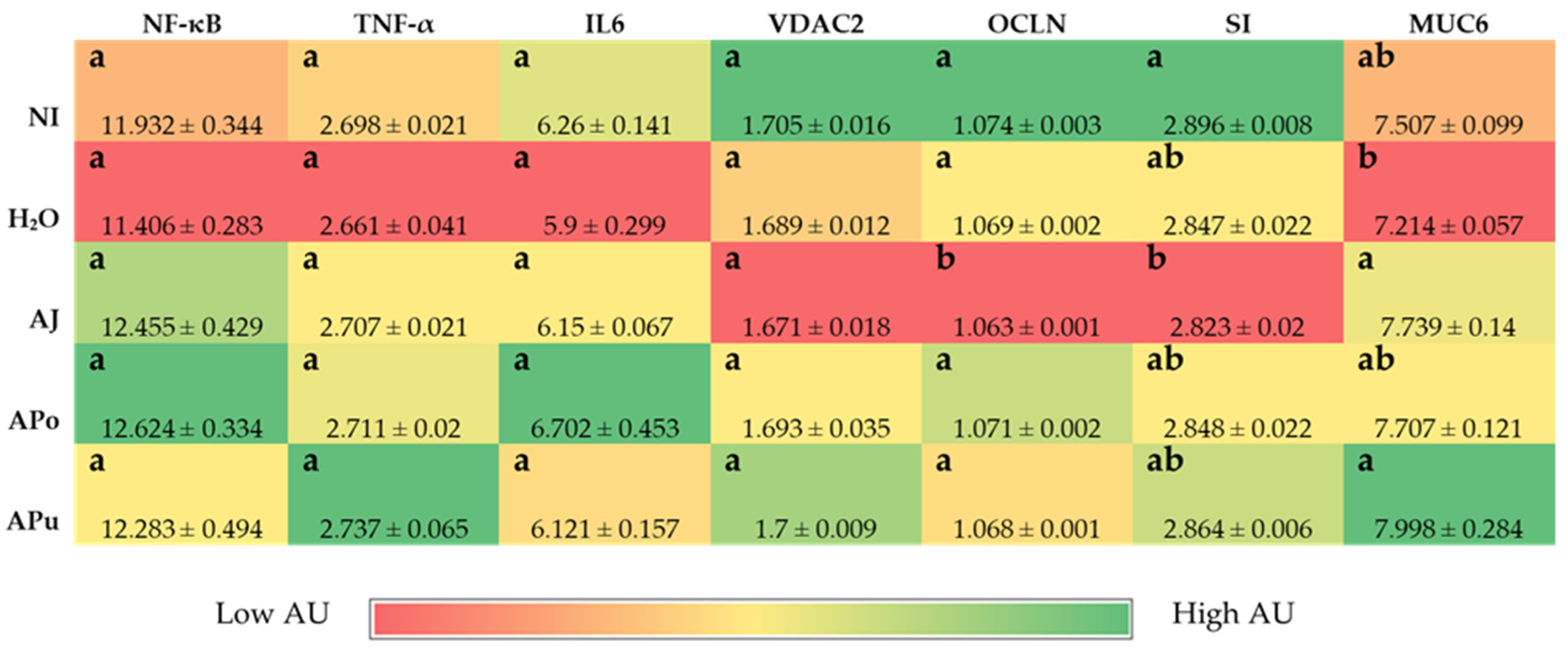
3.3. Duodenal and Hepatic Gene Expression of Related Proteins
3.3.1. Iron and Zinc-Related Proteins
3.3.2. Vitamin A-Related Proteins
3.3.3. Inflammatory and Functionality-Related Proteins
3.4. Morphometric Analysis
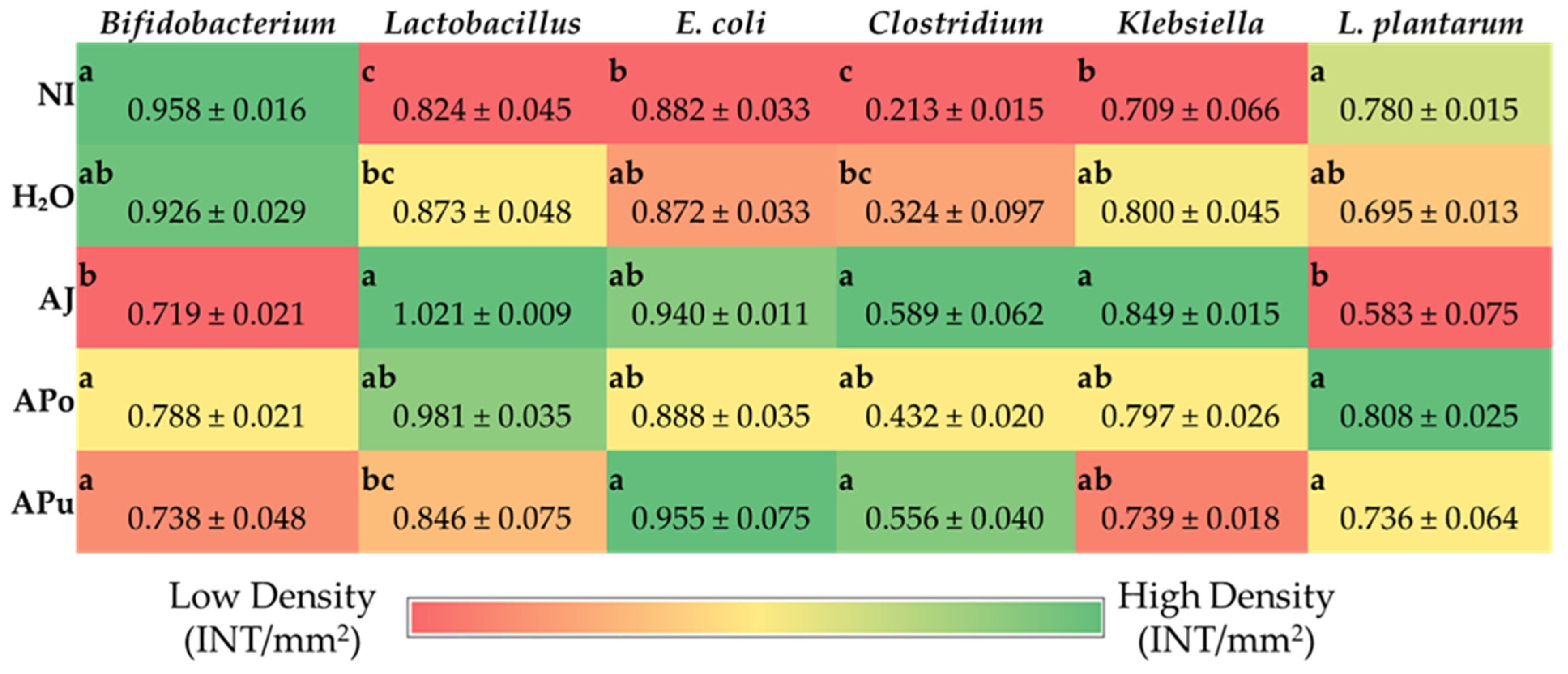
3.5. Microbial Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- FAO Apple Production Worldwide from 2010 to 2020 (in Million Metric Tons). Available online: https://www.statista.com/statistics/961248/production-of-apples-worldwide/ (accessed on 1 June 2022).
- US Department of Agriculture. Economic Research Service Leading Fruits in the United States in 2018, Based on Production Volume (in 1000 Tons). Available online: https://www.statista.com/statistics/631886/leading-fruits-united-states-based-on-production-quantity/ (accessed on 9 June 2022).
- Nicklas, T.A.; O’Neil, C.E.; Fulgoni, V.L. Consumption of Various Forms of Apples Is Associated with a Better Nutrient Intake and Improved Nutrient Adequacy in Diets of Children: National Health and Nutrition Examination Survey 2003–2010. Food Nutr. Res. 2015, 59, 25948. [Google Scholar] [CrossRef] [Green Version]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F.; Esposito, E.; Ninow, J.L. Apple Pomace: A Versatile Substrate for Biotechnological Applications. Crit. Rev. Biotechnol. 2008, 28, 1–12. [Google Scholar] [CrossRef]
- U.S. Apple Association. US Apple Industry Outlook 2021. 2021. Available online: https://usapple.org/wp-content/uploads/2021/08/USAppleIndustryOutlook2021.pdf (accessed on 22 October 2022).
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus Domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Crus, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple Pomace Extract as a Sustainable Food Ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Gimenez-Bastida, J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Vieira, F.G.K.; Borges, G.D.S.C.; Copetti, C.; Di Pietro, P.F.; da Costa Nunes, E.; Fett, R. Phenolic Compounds and Antioxidant Activity of the Apple Flesh and Peel of Eleven Cultivars Grown in Brazil. Sci. Hortic. 2011, 128, 261–266. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Laskowski, P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008, 56, 6520–6530. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Eggert, D.; Mukhtar, H.; Ahmad, N. Antiproliferative Effects of Apple Peel Extract against Cancer Cells. Nutr. Cancer 2010, 62, 517–524. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, P.; Li, S.; Shah, N.P. Antioxidant, Antibacterial, and Antiproliferative Activities of Free and Bound Phenolics from Peel and Flesh of Fuji Apple. J. Food Sci. 2016, 81, M1735–M1742. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. Phytochemical Profiles, Antioxidant, and Antiproliferative Activities of Red-Fleshed Apple as Affected by in Vitro Digestion. J. Food Sci. 2020, 85, 2952–2959. [Google Scholar] [CrossRef]
- He, X.; Liu, R.H. Phytochemicals of Apple Peels: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidant Activities. J. Agric. Food Chem. 2008, 56, 9905–9910. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.; Perry, N.B.; Laing, W.A. Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar] [CrossRef]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of Apple Polyphenols on Inflammatory Gene Expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Speisky, H.; Brunser, O.; Pastene, E.; Gotteland, M. Apple Peel Polyphenols Protect against Gastrointestinal Mucosa Alterations Induced by Indomethacin in Rats. J. Agric. Food Chem. 2011, 59, 6459–6466. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stockl, A.; Bohm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the in Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Boyer, J.; Liu, R.H. Apple Phytochemicals and Their Health Benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Fidelis, M.; de Moura, C.; Kabbas, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Kovačević, D.B.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from by-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible Carbohydrates, Butyrate, and Butyrate-Producing Bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in Diet: Interactions with the Human Microbiome, Role in Gut Homeostasis, and Nutrient-Drug Interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef]
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef]
- Campbell, J.M.; Fahey, G.C., Jr.; Wolf, B.W. Selected Indigestible Oligosaccharides Affect Large Bowel Mass, Cecal and Fecal Short-Chain Fatty Acids, PH, and Microflora in Rats. J. Nutr. 1997, 127, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Venegas, D.P.; de La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [Green Version]
- Alexander, C.; Swanson, K.S.; Fahey, G.C., Jr.; Garleb, K.A. Perspective: Physiologic Importance of Short-Chain Fatty Acids from Nondigestible Carbohydrtae Fermentation. Adv. Nutr. 2019, 10, 576–589. [Google Scholar] [CrossRef] [Green Version]
- Pearce, S.C.; Weber, G.J.; van Sambeek, D.M.; Soares, J.W.; Racicot, K.; Breault, D.T. Intestinal Enteroids Recapitulate the Effects of Short-Chain Fatty Acids on the Intestinal Epithelium. PLoS ONE 2020, 15, e0230231. [Google Scholar] [CrossRef]
- Hou, T.; Tako, E. The in Ovo Feeding Administration (Gallus Gallus)—An Emerging in Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients 2018, 10, 418. [Google Scholar] [CrossRef]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, 11.1.1–11.1.8. [Google Scholar] [CrossRef]
- Kornasio, R.; Halevy, O.; Kedar, O.; Uni, Z. Effect of in Ovo Feeding and Its Interaction with Timing of First Feed on Glycogen Reserves, Muscle Growth, and Body Weight. Poult. Sci. 2011, 90, 1467–1477. [Google Scholar] [CrossRef]
- Uni, Z.; Ferket, P.R.; Tako, E.; Kedar, O. In Ovo Feeding Improves Energy Status of Late-Term Chicken Embryos. Poult. Sci. 2005, 84, 764–770. [Google Scholar] [CrossRef]
- Dreiling, C.E.; Brown, D.E.; Casale, L.; Kelly, L. Muscle Glycogen: Comparison of Iodine Binding and Enzyme Digestion Assays and Application to Meat Samples. Meat Sci. 1987, 20, 167–177. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P. Intra-Amniotic Administration and Dietary Inulin Affect the Iron Status and Intestinal Functionality of Iron-Deficient Broiler Chickens. Poult. Sci. 2012, 91, 1361–1370. [Google Scholar] [CrossRef]
- Tako, E.; Beebe, S.; Reed, S.; Hart, J.; Glahn, R.P. Polyphenolic Compounds Appear to Limit the Nutritional Benefit of Biofortified Higher Iron Black Bean (Phaseolus vulgaris L.). Nutr. J. 2014, 13, 28. [Google Scholar] [CrossRef]
- Agrizzi Verediano, T.; Stampini Duarte Martino, H.; Kolba, N.; Fu, Y.; Cristina Dias Paes, M.; Tako, E. Black Corn (Zea mays L.) Soluble Extract Showed Anti-Inflammatory Effects and Improved the Intestinal Barrier Integrity in Vivo (Gallus Gallus). Food Res. Int. 2022, 157, 111227. [Google Scholar] [CrossRef]
- Agarwal, N.; Kolba, N.; Jung, Y.; Cheng, J.; Tako, E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology in Vivo (Gallus Gallus). Nutrients 2022, 14, 220. [Google Scholar] [CrossRef]
- da Silva, B.P.; Kolba, N.; Martino, H.S.D.; Hart, J.; Tako, E. Soluble Extracts from Chia Seed (Salvia hispanica L.) Affect Brush Border Membrane Functionality, Morphology and Intestinal Bacterial Populations in Vivo (Gallus Gallus). Nutrients 2019, 11, 2457. [Google Scholar] [CrossRef]
- Agarwal, N.; Kolba, N.; Khen, N.; Even, C.; Turjeman, S.; Koren, O.; Tako, E. Quinoa Soluble Fiber and Quercetin Alter the Composition of the Gut Microbiome and Improve Brush Border Membrane Morphology In Vivo (Gallus Gallus). Nutrients 2022, 14, 448. [Google Scholar] [CrossRef]
- Wang, X.; Kolba, N.; Liang, J.; Tako, E. Aleterations in Gut Microflora Populations and Brush Border Functionality Following Intra-Amniotic Administration (Gallus Gallus) of Wheat Bran Prebiotic Extracts. Food Funct. 2019, 10, 4834–4843. [Google Scholar] [CrossRef]
- Tako, E.; Glahn, R.P.; Welch, R.M.; Lei, X.; Yasuda, K.; Miller, D.D. Dietary Inulin Affects the Expression of Intestinal Enterocyte Iron Transporters, Receptors and Storage Protein and Alters the Microbiota in the Pig Intestine. Br. J. Nutr. 2008, 99, 472–480. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.J.C.; Martino, H.S.D.; Kolba, N.; Cheng, J.; Agarwal, N.; de Moura Rocha, M.; Tako, E. Zinc Biofortified Cowpea (Vigna unguiculata L. Walp.) Soluble Extracts Modulate Assessed Cecal Bacterial Populations and Gut Morphology In Vivo (Gallus Gallus). Front. Biosci.-Landmark 2022, 27, 140. [Google Scholar] [CrossRef]
- Dias, D.M.; Kolba, N.; Hart, J.J.; Ma, M.; Sha, S.T.; Lakshmanan, N.; Nutti, M.R.; Martino, H.S.D.; Glahn, R.P.; Tako, E. Soluble Extracts from Carioca Beans (Phaseolus vulgaris L.) Affect the Gut Microbiota and Iron Related Brush Border Membrane Protein Expression in Vivo (Gallus Gallus). Food Res. Int. 2019, 123, 172–180. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Kolba, N.; Tako, E. Yacon (Smallanthus sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology in Vivo (Gallus Gallus). Food Res. Int. 2020, 137, 109705. [Google Scholar] [CrossRef]
- Tako, E.; Ferket, P.R.; Uni, Z. Changes in Chicken Intestinal Zinc Exporter MRNA Expression and Small Intestinal Functionality Following Intra-Amniotic Zinc-Methionine Administration. J. Nutr. Biochem. 2005, 16, 339–346. [Google Scholar] [CrossRef]
- Uni, Z.; Noy, Y.; Sklan, D. Posthatch Development of Small Intestinal Function in the Poult. Poult. Sci. 1999, 78, 215–222. [Google Scholar] [CrossRef]
- New York Apple Association Apples from New York: Varieties-Empire. Available online: https://www.applesfromny.com/varieties/empire/ (accessed on 17 July 2022).
- US Apple Association. U.S. Apple Association: Apple Varieties. Available online: https://usapple.org/apple-varieties (accessed on 12 July 2022).
- Asgary, S.; Rastqar, A.; Keshvari, M. Weight Loss Associated With Consumption of Apples: A Review. J. Am. Coll. Nutr. 2018, 37, 627–639. [Google Scholar] [CrossRef]
- Cho, K.-D.; Han, C.-K.; Lee, B.-H. Loss of Body Weight and Fat and Improved Lipid Profiles in Obese Rats Fed Apple Pomace or Apple Juice Concentrate. J. Med. Food 2013, 16, 823–830. [Google Scholar] [CrossRef]
- Samout, N.; Bouzenna, H.; Dhibi, S.; Ncib, S.; ElFeki, A.; Hfaiedh, N. Therapeutic Effect of Apple Pectin in Obese Rats. Biomed. Pharmacother. 2016, 83, 1233–1238. [Google Scholar] [CrossRef]
- McKie, A.T. The Role of Dcytb in Iron Metabolism: An Update. Biochem. Soc. Trans. 2008, 36, 1239–1241. [Google Scholar] [CrossRef] [Green Version]
- Lane, D.; Bae, D.-H.; Merlot, A.; Sahni, S.; Richardson, D. Duodenal Cytochrome b (Dcytb) in Iron Metabolism: An Update on Function and Regulation. Nutrients 2015, 7, 2274–2296. [Google Scholar] [CrossRef] [Green Version]
- Warkentin, T.; Kolba, N.; Tako, E. Low Phytate Peas (Pisum sativum L.) Improve Iron Status, Gut Microbiome, and Brush Border Membrane Functionality in Vivo (Gallus Gallus). Nutrients 2020, 12, 2563. [Google Scholar] [CrossRef]
- Shah, M.; Griffin, I.J.; Lifschitz, C.H.; Abrams, S.A. Effect of Orange and Apple Juices on Iron Absorption in Children. Arch. Pediatr. Adolesc. Med. 2003, 157, 1232–1236. [Google Scholar] [CrossRef] [Green Version]
- Ravn-Haren, G.; Krath, B.N.; Markowski, J.; Poulsen, M.; Hansen, M.; Kolodziejczyk, K.; Kosmala, M.; Dragsted, L.O. Apple Pomace Improves Gut Health in Fisher Rats Independent of Seed Content. Food. Funct. 2018, 9, 2931–2941. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T. Regulation of Intestinal Epithelial Permeability by Tight Junctions. Cell Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J. Cell Iology 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [Green Version]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A Novel Integral Membrane Protein Localizing at Tight Junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sun, Z.; Chen, C.; Zhang, L.; Zhu, S. Simultaneous Separation and Determination of Fructose, Sorbitol, Glucose and Sucrose in Fruits by Hplc-Elsd. Food. Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of Physiological Characteristics, Soluble Sugars, Organic Acids and Volatile Compounds in “Orin” Apples (Malus domestica) at Different Ripening Stages. Molecules 2021, 26, 807. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Kaden-Volynets, V.; Filipe Rosa, L.; Guseva, D.; Seethaler, B. Regulation of the Gut Barrier by Carbohydrates from Diet–Underlying Mechanisms and Possible Clinical Implications. Int. J. Med. Microbiol. 2021, 311, 151499. [Google Scholar] [CrossRef] [PubMed]
- Volynets, V.; Louis, S.; Pretz, D.; Lang, L.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal Barrier Function and the Gut Microbiome Are Differentially Affected in Mice Fed a Western-Style Diet or Drinking Water Supplemented with Fructose. J. Nutr. 2017, 147, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agarwal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A High-Sugar Diet Rapidly Enhances Susceptibility to Colitis via Depletion of Luminal Short-Chain Fatty Acids in Mice. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.; Morris, W.E.; Loidl, C.F.; Tironi-Farinatti, C.; McClane, B.A.; Uzal, F.A.; Fernandez Miyakawa, M.E. Clostridium Perfringens Epsilon Toxin Increases the Small Intestinal Permeability in Mice and Rats. PLoS ONE 2009, 4, e7065. [Google Scholar] [CrossRef] [Green Version]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading Players in the Maintenance of Gut Homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Moore, R.; Pothoulakis, C.; LaMont, J.T.; Carlson, S.; Madara, J.L.C. Difficile Toxin A Increases Intestinal Permeability and Induces Cl- Secretion. Am. J. Physiol. 1990, 259, G165–G172. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella Pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Bang, S.-J.; Kim, G.; Lim, M.Y.; Song, E.-J.; Jung, D.-H.; Kum, J.-S.; Nam, Y.-D.; Park, C.-S.; Seo, D.-H. The Influence of in Vitro Pectin Fermentation on the Human Fecal Microbiome. AMB Express 2018, 8, 10–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, N.; Bussolo de Souza, C.; Krych, L.; Barbosa Cahu, T.; Wiese, M.; Kot, W.; Meyer Hansen, K.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium Species as Probiotics: Potentials and Challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Licht, T.R.; Hansen, M.; Bergström, A.; Poulsen, M.; Krath, B.N.; Markowski, J.; Dragsted, L.O.; Wilcks, A. Effects of Apples and Specific Apple Components on the Cecal Environment of Conventional Rats: Role of Apple Pectin. BMC Microbiol. 2010, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufourny, S.; Antoine, N.; Pitchugina, E.; Delcenserie, V.; Godbout, S.; Douny, C.; Scippo, M.-L.; Froidmont, E.; Rondia, P.; Wavreille, J.; et al. Apple Pomace and Performance, Intestinal Morphology and Microbiota of Weaned Piglets—A Weaning Strategy for Gut Health? Microorganisms 2021, 9, 572. [Google Scholar] [CrossRef]
- Laudadio, V.; Passantino, L.; Perillo, A.; Lopresti, G.; Passantino, A.; Khan, R.U.; Tufarelli, V. Productive Performance and Histological Features of Intestinal Mucosa of Broiler Chickens Fed Different Dietary Protein Levels. Poult. Sci. 2012, 91, 265–270. [Google Scholar] [CrossRef]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of Villus Height and Crypt Depth, and Enhancement of Disaccharide Digestion and Monosaccharide Absorption, in Piglets Fed on Cows’ Whole Milk after Weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.C.; Rodrigues, E.A.; Marques, R.H.; Gravena, R.A.; Guandolini, G.C.; Moraes, V.M.B. Performance and Morphology of Intestinal Mucosa of Broilers Fed Mannan-Oligosaccharides and Enzymes [Desempenho e Morfologia Da Mucosa Intestinal de Frangos de Corte Alimentados Com Mananoligossacarídeos e Enzimas]. Arq. Bras. Med. Vet. Zootec. 2008, 60, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Birchenough, G.M.H.; Johansson, M.E.V.; Gustafsson, J.K.; Bergström, J.H.; Hansson, G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal. Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Li, Q.; Zang, Y.; Zhao, Y.; Liu, N.; Wang, Y.; Xu, X.; Liu, L.; Mei, Q. Apple Polysaccharide Inhibits Microbial Dysbiosis and Chronic Inflammation and Modulates Gut Permeability in HFD-Fed Rats. Int. J. Biol. Macromol. 2017, 99, 282–292. [Google Scholar] [CrossRef]
- Lueschow, S.R.; McElroy, S.J. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front. Immunol. 2020, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzman, N.H.; Bevins, C.L. Dysbiosis-A Consequence of Paneth Cell Dysfunction. Semin. Immunol. 2013, 25, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.; Salzman, N. Paneth Cells, Antimicrobial Peptides and Maintenance of Intestinal Homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Beukema, M.; Faas, M.; de Vos, P. The Effects of Different Dietary Fiber Pectin Structures on the Gastrointestinal Immune Barrier: Impact via Gut Microbiota and Direct Effects on Immune Cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
| Analyte | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Base Pair | GI Identifier |
|---|---|---|---|---|
| Iron Metabolism | ||||
| DcytB | CATGTGCATTCTCTTCCAAAGTC | CTCCTTGGTGACCGCATTAT | 103 | 20380692 |
| DMT1 | TTGATTCAGAGCCTCCCATTAG | GCGAGGAGTAGGCTTGTATTT | 101 | 206597489 |
| Ferroportin | CTCAGCAATCACTGGCATCA | ACTGGGCAACTCCAGAAATAAG | 98 | 423984 |
| Hepcidin | AGACGACAATGCAGACTAACC | CTGCAGCAATCCCACATTTC | 132 | SAMN08056490 |
| Zinc Metabolism | ||||
| ZnT1 | GGTAACAGAGCTGCCTTAACT | GGTAACAGAGCTGCCTTAACT | 105 | 54109718 |
| ZnT7 | GGAAGATGTCAGGATGGTTCA | CGAAGGACAAATTGAGGCAAAG | 87 | 56555152 |
| ZIP4 | TCTCCTTAGCAGACAATTGAG | GTGACAAACAAGTAGGCGAAAC | 95 | 107050877 |
| ZIP1 | TGCCTCAGTTTCCCTCAC | GGCTCTTAAGGGCACTTCT | 144 | 121112053 |
| Vitamin A Metabolism | ||||
| CRBP2 | GGCTACATGGTTGCACTAGACA | AACCACCCGGTTATCGAGTC | 195 | NM_001277417.1 |
| LRAT | GATTTTGCCTATGGCGGCAG | TTGTCGGTCTGGAAGCTGAC | 197 | XM_420371.7 |
| RBP4 | TGCCACCAACACAGAACTCTC | CTTTGAAGCTGCTCACACGG | 149 | NM_205238.2 |
| STRA6 | GTGCGCTGAACTTTGTCTGC | TTCTTCCTGCTCCCGACCT | 116 | NM_001293202.2 |
| Inflammatory Response | ||||
| NF-κB | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG | 100 | 2130627 |
| TNF-α | GACAGCCTATGCCAACAAGTA | TTACAGGAAGGGCAACTCATC | 109 | 53854909 |
| IL6 | ACCTCATCCTCCGAGACTTTA | GCACTGAAACTCCTGGTCTT | 105 | 302315692 |
| Brush Border Membrane Functionality | ||||
| VDAC2 | CAGCACTCGCTTTGGAATTG | GTGTAACCCACTCCAACTAGAC | 99 | 395498 |
| OCLN | GTCTGTGGGTTCCTCATCGT | GTTCTTCACCCACTCCTCCA | 124 | 396026 |
| SI | CCAGCAATGCCAGCATATTG | CGGTTTCTCCTTACCACTTCTT | 95 | 2246388 |
| MUC6 | CCAACTTGCAGTGTTCCAAAG | CTGACAGTGTAGAGCAAGTACAG | 106 | XM_015286750.1 |
| 18S rRNA | GCAAGACGAACTAAAGCGAAAG | TCGGAACTACGACGGTATCT | 100 | 7262899 |
| Sample | TPC (mg/g GAE) | ADF (%/DM) | NDF (%/DM) | NFC (%/DM) |
|---|---|---|---|---|
| Pomace | 0.834 ± 0.059 b | 22.6 | 25.6 | 62 |
| Juice | 0.300 ± 0.029 c | NA | NA | NA |
| Pulp | 1.57 ± 0.074 a | 6.7 | 7.9 | 85.4 |
| Treatment Group | Body Weight (g) | Blood Glucose (mg/dL) | Glycogen (mg/g) |
|---|---|---|---|
| NI | 40.83 ± 1.24 a | 254.11 ± 23.83 a | 0.396 ± 0.101 a |
| H2O | 38.29 ± 4.31 a | 234.4 ± 11.16 a | 0.294 ± 0.093 a |
| AJ | 40 ± 0.99 a | 225.5 ± 11.43 a | 0.431 ± 0.092 a |
| APo | 35.7 ± 0.67 a | 230.56 ± 21.28 a | 0.271 ± 0.054 a |
| APu | 36.44 ± 0.85 a | 205.5 ± 32.05 a | 0.442 ± 0.077 a |
| Treatment Group | Villi Surface Area (µm2) | Crypt Depth (µm) |
|---|---|---|
| NI | 16,458.04 ± 771.84 ᵇ | 22.1 ± 0.81 ᵃ |
| H2O | 16,101.54 ± 383.07 ᵇ | 21.93 ± 0.72 ᵃ |
| AJ | 17,470.91 ± 444.08 ᵇ | 14.45 ± 0.54 ᶜ |
| APo | 23,116.65 ± 509.84 ᵃ | 16.85 ± 0.79 ᵇ |
| APu | 23,520.69 ± 739.04 ᵃ | 17.38 ± 0.71 ᵇ |
| Treatment Group | Villi Goblet Diameter (µm) | Crypt Goblet Diameter (µm) | Crypt Goblet Cell Number | Crypt Goblet Cell Number | ||
|---|---|---|---|---|---|---|
| Acidic | Neutral | Mixed | ||||
| NI | 3.48 ± 0.07 a | 3.01 ± 0.05 a | 7.01 ± 0.24 c | 5.79 ± 0.2 c | 0.02 ± 0.02 b | 1.21 ± 0.13 c |
| H2O | 3.17 ± 0.06 b | 2.89 ± 0.05 a | 8.55 ± 0.32 b | 6.92 ± 0.27 b | 0.13 ± 0.03 a | 1.51 ± 0.12 bc |
| AJ | 3.55 ± 0.07 a | 2.88 ± 0.05 a | 7.51 ± 0.26 c | 5.81 ± 0.22 c | 0.02 ± 0.01 b | 1.69 ± 0.11 ab |
| APo | 3.17 ± 0.06 b | 2.71 ± 0.06 b | 8.36 ± 0.27 b | 6.75 ± 0.23 b | 0.01 ± 0.01 b | 1.60 ± 0.10 b |
| APu | 3.08 ± 0.08 b | 2.84 ± 0.05 a | 9.14 ± 0.31 a | 7.35 ± 0.26 a | 0.00 ± 0.00 b | 1.79 ± 0.11 a |
| Treatment Group | Crypt Paneth Cell Number | Paneth Cell Diameter (µm) |
|---|---|---|
| NI | 1.22 ± 0.03 c | 1.37 ± 0.02 c |
| H2O | 1.04 ± 0.01 ᵈ | 1.5 ± 0.02 ᵃ |
| AJ | 1.31 ± 0.04 bc | 1.45 ± 0.02 ᵃᵇ |
| APo | 1.44 ± 0.04 ᵃ | 1.43 ± 0.02 b |
| APu | 1.38 ± 0.04 b | 1.45 ± 0.02 ᵃᵇ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, C.; Shukla, V.; Kolba, N.; Agarwal, N.; Padilla-Zakour, O.I.; Tako, E. Empire Apple (Malus domestica) Juice, Pomace, and Pulp Modulate Intestinal Functionality, Morphology, and Bacterial Populations In Vivo (Gallus gallus). Nutrients 2022, 14, 4955. https://doi.org/10.3390/nu14234955
Jackson C, Shukla V, Kolba N, Agarwal N, Padilla-Zakour OI, Tako E. Empire Apple (Malus domestica) Juice, Pomace, and Pulp Modulate Intestinal Functionality, Morphology, and Bacterial Populations In Vivo (Gallus gallus). Nutrients. 2022; 14(23):4955. https://doi.org/10.3390/nu14234955
Chicago/Turabian StyleJackson, Cydney, Viral Shukla, Nikolai Kolba, Nikita Agarwal, Olga I. Padilla-Zakour, and Elad Tako. 2022. "Empire Apple (Malus domestica) Juice, Pomace, and Pulp Modulate Intestinal Functionality, Morphology, and Bacterial Populations In Vivo (Gallus gallus)" Nutrients 14, no. 23: 4955. https://doi.org/10.3390/nu14234955
APA StyleJackson, C., Shukla, V., Kolba, N., Agarwal, N., Padilla-Zakour, O. I., & Tako, E. (2022). Empire Apple (Malus domestica) Juice, Pomace, and Pulp Modulate Intestinal Functionality, Morphology, and Bacterial Populations In Vivo (Gallus gallus). Nutrients, 14(23), 4955. https://doi.org/10.3390/nu14234955