Proposal for a Standard Protocol to Assess the Rheological Behavior of Thickening Products for Oropharyngeal Dysphagia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thickening Product (TP)
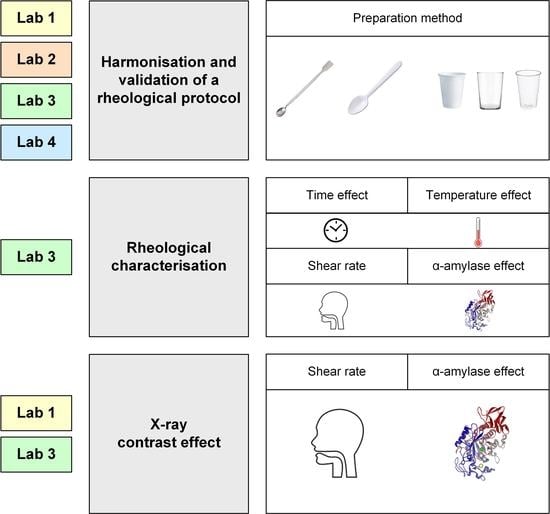
2.2. Laboratories and Equipment
2.3. Study Design
2.4. Methods
2.4.1. Rheological Protocol
- (1)
- Harmonization of the preparation protocol. In order to standardize the preparation method and analysis for the identical rheological protocol to be applied in the four laboratories, the reference laboratory (Lab1) previously assessed the factors that differed, which included: (a) Stirring conditions: rotations per second, stirring speed and time. (a) Stirrer (metallic spatula, 160 mm length plastic spoon and 100 mm length plastic spoon) for all viscosity levels; (b) Container (glass beaker, white plastic cup, clear plastic cup) and; (c) Standing time before measurement (immediately, 10 and 30 min) for 100, 400 and 1600 mPa·s.
- (2)
- Laboratory Measurements Variability. Four different facilities: All the laboratories (1, 2, 3 and 4) validated the common rheological protocol to analyze the shear viscosity of the selected TP at different doses (Figure 1). The following harmonized protocol was established: (a) weigh the dissolvent in a clear plastic cup; (b) weigh the TP; (c) add it to the dissolvent over 5 s while stirring at 4 rps with a metallic spatula; (d) continue stirring for 30 s at the same velocity; (e) rest for 10 min; (f) analyze viscosity by increasing the shear rate from 0 to 1000 s−1 in a 10 min test at 25 °C. Viscosity measurements were performed in triplicate on three samples for each viscosity level. Daily condition (DC) doses (TP with mineral water) have been used for this test presented in Table 2. Doses have been selected to determine different viscosity levels ranging between 100 and 1600 mPa·s to validate the protocol in a wide range of shear viscosities according to previous studies [5]. These levels allow viscosity behavior to be observed at very low (100–200 mPa·s), medium (400–800 mPa·s) and high (1600 mPa·s) viscosity values. In addition, the therapeutic effect of these viscosity levels for this specific TP is being analyzed in a clinical trial NCT04565587.
2.4.2. Rheological Characterization of TP
2.4.3. Effect of X-ray Contrast on the ShV of TP
2.5. Participants
2.6. Measurements and Data Analysis
3. Results
3.1. Design and Validation of a Common Protocol to Standardize Rheological Measurements
3.2. Rheological Characterization
3.3. Effect of the X-ray Contrast Omnipaque on the Rheological Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31, 232–249. [Google Scholar] [CrossRef] [Green Version]
- Sworn, G. Rheology Modifiers for the Management of Dysphagia. In Rheology of Biological Soft Matter; Kaneda, I., Ed.; Springer: Paris, France, 2017; pp. 233–263. [Google Scholar]
- Rofes, L.; Arreola, V.; Mukherjee, R.; Swanson, J.; Clavé, P. The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Aliment Pharmacol. Ther. 2014, 39, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Clavé, P.; De Kraa, M.; Arreola, V.; Girvent, M.; Farré, R.; Palomera, E.; Serra-Prat, M. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment. Pharmacol. Ther. 2006, 24, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Bolivar-Prados, M.; Rofes, L.; Arreola, V.; Guida, S.; Nascimento, W.V.; Martin, A.; Vilardell, N.; Fernández, O.O.; Ripken, D.; Lansink, M.; et al. Effect of a gum-based thickener on the safety of swallowing in patients with poststroke oropharyngeal dysphagia. Neurogastroenterol. Motil. 2019, 31, e13695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, O.; Bolívar-Prados, M.; Arreola, V.; Nascimento, W.; Tomsen, N.; Gallegos, C.; La Fuente, E.B.-D.; Clavé, P. Therapeutic Effect, Rheological Properties and Xanthan Gum Thickener on Four Different. Nutrients 2020, 12, 1873. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Counciol of the European Union. Regulation (UE) No 609/2013 on Food Intended for Infants and Young Children, Food for Special Medical Purposes, and Total Diet Replacement for Weight Control and Repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. Off. J. Eur. Union 2013, 35–56. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. JFST 2010, 47, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Vilardell, N.; Rofes, L.; Arreola, V.; Speyer, R.; Clavé, P. A Comparative Study between modified starch and xanthan gum thickeners in Post-Stroke Oropharyngeal Dysphagia. Dysphagia 2016, 31, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Hadde, E.; Nicholson, T.; Cichero, J. Evaluation of thickened fluids used in dysphagia amangement using extensional rheology. Dysphagia 2020, 35, 242–252. [Google Scholar] [CrossRef]
- Gallegos, C.; Brito-de la Funete, E.; Clavé, P.; Costa, A.; Assegehegn, G. Nutritional Aspects of Dysphagia Management. Adv. Food Nutr. Res. 2017, 81, 271–318. [Google Scholar] [PubMed]
- European Parliament and Council of the European Union. Directive 71/354/EU, on the apporixmation of the laws of the Member States relating to units of measurement and on the repeal of Directive. Off. J. Eur. Union 1979, 39, 40–50. [Google Scholar]
- Hadde, E. Understanding the Rheological Parameters of Thickened Fluids for Dysphagia Sufferers. Ph.D. Thesis, University of Queensland, School of Chemical Engineering, St Lucia, QLD, Australia, 2016. [Google Scholar]
- Barnes, H. A Handbook of Elementary Rheology, 1st ed.; University of Wales Institute of Non-Newtonian Fluid Mechanics, Department of Mathematics, University of Wales Aberystwyth: Aberystwyth, UK, 2000. [Google Scholar]
- Brito-de la Fuente, E.; Turcanu, M.; Ekberg, O.; Gallegos, C. Rheological Aspects of Swallowing and Dysphagia: Shear and Elongational Flows. In Dysphagia; Ekberg, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 687–716. [Google Scholar]
- American Dietetic Association. National Dysphagia Diet Standarization for Optimal Care; American Dietetic Association: Chicago, Illinois, USA, 2002. [Google Scholar]
- Rofes, L.; Arreola, V.; Romea, M.; Palomera, E.; Almirall, J.; Cabré, M.; Serra-Prat, M.; Clavé, P. Pathophysiology of oropharyngeal dysphagia in the frail elderly. J. Neurogastroenterol. Motil. 2010, 22, 851-e230. [Google Scholar] [CrossRef] [PubMed]
- Vallons, K.; Helmens, H.; Oudhuis, A. Effect of Human saliva on the consistency of thickened drinks for individuals with dysphagia. Int. J. Lang Commun. Disord. 2015, 50, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.; O’Leary, M.; Smith, C. The effect of saliva on the viscosity of thickened drinks. Dysphagia 2012, 27, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Hwa-Young, L.; Seung-Ro, Y.; Whachun, Y.; Byoungseung, Y. Effect of salivary reaction time on flow properties of commercial food thickeners used for dysphagic patients. Clin. Nutr. Res. 2016, 5, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Bolivar-Prados, M.; Tomsen, N.; Arenas, C.; Clave, P. A bit thick: Hidden risks in thickening products’ lablelling for dysphagia treatment. Food Hydrocoll. 2021, 123, 106960. [Google Scholar] [CrossRef]
- Chinyoka, T. Comparative response of Newtonian and Non-Newtonian fluids subjected to exothermic reactions in shear flow. Int. J. Appl. Comput. Math. 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, B.; Rachal, C. Comparison of Comparison of the Rheological Properties of Ready-to-Serve and Powdered Instant Food–Thickened Beverages at Different Temperatures for Dysphagic Patients. J. Am. Diet. Assoc. 2007, 107, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Popa Nita, S.; Murith, M.; Chisholm, H.; Engmann, J. Matching the Rheological Properties of Videofluoroscopic Contrast Agents and Thickened Liquid Prescriptions. Dysphagia 2013, 28, 245–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Dysphagia Diet Standardisation Initiative (IDDSI). Complete IDDSI Framework Detailed Definitions. 2019. Available online: https://iddsi.org/iddsi/media/images/complete_iddsi_framework_final_31july2019.pdf (accessed on 1 November 2022).
- Côté, C.; Giroux, A.; Villeneuve-Rhéaume, A.; Gagnon, C.; Germain, I. Is IDDSI an Evidence-Based Framework? A Relevant Question for the Frail Older Population. Geriatrics 2020, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Wood, F. Psychophysical Studies on the Consistency of Liquid Foods. In Rheology and Texture of Foodstuffs; SCI: London, UK, 1968; pp. 40–49. [Google Scholar]
- Muhammad, W. Exploring the Role of Rheology in Bolus Flow using an In Vitro Approach. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2019. [Google Scholar]




| Ingredients | Dextrin, xanthan gum, calcium lactate, trisodium citrate | |
| Nutritional Composition | Energy | 270 kcal |
| Protein | 0.5 g | |
| Lipid | 0 g | |
| Carbohydrates | 88.9 g | |
| Fiber | 21.9 g | |
| Sodium | 960 mg | |
| Potassium | 980 mg | |
| Phosphorus | 30 mg | |
| Ash | 4.5 g | |
| Water | 6.1 g | |
| DC Doses (g/mL) | ||
|---|---|---|
| Tsururinko Quickly (g) | Final Volume (mL) | Solvent |
| 1.25 | 100 | Mineral water |
| 2 | 100 | Mineral water |
| 3.2 | 100 | Mineral water |
| 5.8 | 100 | Mineral water |
| 10.5 | 100 | Mineral water |
| Stirrer | ||||||
|---|---|---|---|---|---|---|
| Targeted Viscosity at 50 s−1 (mPa·s) | Metallic Spatula 180 mm | Plastic Spoon 160 mm | Plastic Spoon 100 mm | |||
| Viscosity at 50 s−1 (mPa·s) | Variability (%) | Viscosity at 50 s−1 (mPa·s) | Variability (%) | Viscosity at 50 s−1 (mPa·s) | Variability (%) | |
| 100 | 97.6 ± 3.3 | 1.2–5.8 | 107.0 ± 10.8 | 2.4–18.3 | 109.1 ± 9.0 | 3.9–14.6 |
| 200 | 203.8 ± 6.0 | 212.0 ± 5.4 | 202.3 ± 9.1 | |||
| 400 | 400.4 ± 4.2 | 402.2 ± 8.6 | 388.2 ± 8.0 | |||
| 800 | 802.8 ± 7.1 | 767.8 ± 31.1 | 811.6 ± 21.6 | |||
| 1600 | 1602.4 ± 10.1 | 1572.4 ± 19.6 | 1595.5 ± 31.7 | |||
| Container | ||||||
| Targeted Viscosity at 50 s−1 (mPa·s) | Glass Beaker | White Plastic Cup | Clear Plastic Cup | |||
| Viscosity at 50 s−1 (mPa·s) | Variability (%) | Viscosity at 50 s−1 (mPa·s) | Variability (%) | Viscosity at 50 s−1 (mPa·s) | Variability (%) | |
| 100 | 98.1 ± 3.5 | 1.8–6.3 | 107.6 ± 4.7 | 5.2–12.0 | 101.9 ± 3.3 | 1.9–5.7 |
| 400 | 398.4 ± 3.90 | 401.0 ± 25.6 | 407.4 ± 5.0 | |||
| 1600 | 1597.0 ± 18.1 | 1555.3 ± 42.1 | 1573.1 ± 16.0 | |||
| Standing Time–Glass Beaker | ||||||
| Targeted Viscosity at 50 s−1 (mPa·s) | 0 min | 10 min | 30 min | |||
| Viscosity at 50 s−1 (mPa·s) | Variability (%) | Viscosity at 50 s−1 (mPa·s) | Variability (%) | Viscosity at 50 s−1 (mPa·s) | Variability (%) | |
| 100 | 100.9 ± 6.2 | 2.9–12.8 | 100.0 ± 3.0 | 1.4–5.6 | 98.4 ± 3.0 | 1.3–5.4 |
| 400 | 394.3 ± 29.6 | 403.7 ± 6.8 | 398.5 ± 4.1 | |||
| 1600 | 1620.3 ± 24.7 | 1588.3 ± 13.0 | 1595.1 ± 12.0 | |||
| Standing Time–Clear Plastic Cup | ||||||
| Targeted Viscosity at 50 s−1 (mPa·s) | 0 min | 10 min | 30 min | |||
| Viscosity at 50 s−1 (mPa·s) | Variability (%) | Viscosity at 50 s−1 (mPa·s) | Variability (%) | Viscosity at 50 s−1 (mPa·s) | Variability (%) | |
| 100 | 102.2 ± 6.4 | 3.1–12.9 | 100.8 ± 3.2 | 1.3–6.0 | 99.4 ± 3.4 | 1.4–6.6 |
| 400 | 415.1 ± 29.6 | 401.4 ± 7.8 | 407.4 ± 5.0 | |||
| 1600 | 1670.1 ± 27.4 | 1644.3 ± 11.1 | 1605.4 ± 12.5 | |||
| Dosage TP (g/100 mL) | Viscosity (mPa·s) at 50 s−1 | Mean Interlaboratory Variability (%) | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lab1 | Lab2 | Lab3 | Lab4 | |||||||
| Mean (mPa·s) | ±CV (%) | Mean (mPa·s) | ±CV (%) | Mean (mPa·s) | ±CV (%) | Mean (mPa·s) | ±CV (%) | |||
| 1.25 | 101 | ±2.6 | 105 | ±4.9 | 81 | ±5.2 | 101 | ±4.3 | 22.9 | * |
| 2 | 205 | ±0.09 | 199 | ±4.5 | 190 | ±1.3 | 197 | ±2.3 | 7.3 | 0.08 |
| 3.2 | 403 | ±0.85 | 396 | ±4.1 | 386 | ±0.97 | 381 | ±3.2 | 5.5 | 0.13 |
| 5.8 | 805 | ±0.38 | 818 | ±3.0 | 768 | ±2.6 | 797 | ±3.4 | 6.1 | 0.11 |
| 10.5 | 1602 | ±0.35 | 1601 | ±2.8 | 1552 | ±3.5 | 1632 | ±1.3 | 4.9 | 0.16 |
| Healthy Volunteers (n = 8) | |||
|---|---|---|---|
| Targeted Viscosity (mPa·s) at 50 s−1 | Viscosity (mPa·s) at 50 s−1 Mean ± SD | Amylase Effect (%) | p-Value |
| 100 | 93.1 ± 6.5 | 5.9 | 0.99 |
| 200 | 160.0 ± 7.3 | 15.6 | 0.75 |
| 400 | 355.5 ± 23.7 | 7.1 | 0.81 |
| 800 | 771.8 ± 42.0 | −0.37 | >0.99 |
| 1600 | 1449.0 ± 72.8 | 6.7 | *** |
| Targeted Viscosity (mPa·s) at 50 s−1 | Viscosity at 300 s−1 Post-Oral Incubation (mean ± SD) | Shear Rate + Amylase Effect (%) | p-Value |
| 100 | 25.2 ± 1.7 | 74.5 | **** |
| 200 | 39.1 ± 1.8 | 79.4 | **** |
| 400 | 78.8 ± 4.1 | 79.4 | **** |
| 800 | 168.1 ± 9.0 | 78.1 | **** |
| 1600 | 329.3 ± 20.0 | 78.8 | **** |
| VFS Doses (g/mL) | |||
|---|---|---|---|
| Targeted Viscosity (mPa·s) at 50 s−1 | Tsururinko Quickly (g) | Final Volume (mL) | Dissolvent (mL) |
| 100 | 0.58 | 50 | 1:1 (water:Omnipaque) |
| 200 | 1 | 50 | 1:1 (water:Omnipaque) |
| 400 | 1.45 | 50 | 1:1 (water:Omnipaque) |
| 800 | 2.45 | 50 | 1:1 (water:Omnipaque) |
| 1600 | 4.3 | 50 | 1:1 (water:Omnipaque) |
| Targeted Viscosity at 50 s−1 (mPa·s) | Dosage (g/50 mL) | Average Viscosity at 50 s−1 (mPa·s), n = 3 | Variations Within Facilities (%, n = 3) | Variations between Facilities (%) | Lab1-Targeted Viscosity (%) | Lab3-Targeted Viscosity (%) | ||
|---|---|---|---|---|---|---|---|---|
| Lab1 | Lab3 | Lab1 | Lab3 | |||||
| 100 | 0.58 | 114 | 94 | 1.0 | 7.2 | 17.5 | 14 | 6 |
| 200 | 1 | 239 | 233 | 0.54 | 6.7 | 2.5 | 19.5 | 16.5 |
| 400 | 1.45 | 446 | 443 | 0.68 | 12.0 | 0.7 | 11.5 | 10.8 |
| 800 | 2.45 | 833 | 852 | 1.0 | 8.4 | 2.3 | 4.1 | 6.5 |
| 1600 | 4.3 | 1598 | 1672 | 0.6 | 3.7 | 4.6 | 0.13 | 4.5 |
| Targeted Viscosity at 50 s−1 (mPa·s) | Dosage (g/50 mL) | Average Viscosity at 300 s−1 (mPa·s, n = 3) | Variations Within Facilities (%, n = 3) | Variations Between Facilities (%) | ||||
| Lab1 | Lab3 | Lab1 | Lab3 | |||||
| 100 | 0.58 | 32 | 28 | 0.77 | 1.9 | 12.5 | ||
| 200 | 1 | 58 | 57 | 0.52 | 4.23 | 1.7 | ||
| 400 | 1.45 | 99 | 100 | 0.5 | 13.4 | 1.0 | ||
| 800 | 2.45 | 178 | 187 | 0.82 | 9.2 | 5.1 | ||
| 1600 | 4.3 | 349 | 384 | 0.63 | 5.6 | 10.0 | ||
| Healthy Volunteers (n = 8) | |||
| Targeted Viscosity (mPa·s) at 50 s−1 | Viscosity (mPa·s) at 50 s−1 Mean ± SD | Amylase Effect (%) | p-Value |
| 100 | 99.0 ± 11.6 | −4.47 (increment) | >0.99 |
| 200 | 187.0 ± 21.4 | 19.7 | 0.98 |
| 400 | 355.5 ± 23.7 | 19.8 | 0.75 |
| 800 | 731.5 ± 33.1 | 14.1 | ** |
| 1600 | 1476.3 ± 115.9 | 11.7 | **** |
| Targeted Viscosity (mPa·s) at 50 s−1 | Viscosity at 300 s−1 Post-Oral Incubation (Mean ± SD) | Shear Rate + Amylase Effect (%) | p-Value |
| 100 | 28.9 ± 3.1 | 69.5 | **** |
| 200 | 47.5 ± 5.0 | 79.6 | **** |
| 400 | 78.8 ± 4.1 | 83.1 | **** |
| 800 | 161.9 ± 6.3 | 82.2 | **** |
| 1600 | 331.6 ± 29.0 | 80.5 | **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolivar-Prados, M.; Tomsen, N.; Hayakawa, Y.; Kawakami, S.; Miyaji, K.; Kayashita, J.; Clavé, P. Proposal for a Standard Protocol to Assess the Rheological Behavior of Thickening Products for Oropharyngeal Dysphagia. Nutrients 2022, 14, 5028. https://doi.org/10.3390/nu14235028
Bolivar-Prados M, Tomsen N, Hayakawa Y, Kawakami S, Miyaji K, Kayashita J, Clavé P. Proposal for a Standard Protocol to Assess the Rheological Behavior of Thickening Products for Oropharyngeal Dysphagia. Nutrients. 2022; 14(23):5028. https://doi.org/10.3390/nu14235028
Chicago/Turabian StyleBolivar-Prados, Mireia, Noemí Tomsen, Yuki Hayakawa, Satomi Kawakami, Kazuhiro Miyaji, Jun Kayashita, and Pere Clavé. 2022. "Proposal for a Standard Protocol to Assess the Rheological Behavior of Thickening Products for Oropharyngeal Dysphagia" Nutrients 14, no. 23: 5028. https://doi.org/10.3390/nu14235028
APA StyleBolivar-Prados, M., Tomsen, N., Hayakawa, Y., Kawakami, S., Miyaji, K., Kayashita, J., & Clavé, P. (2022). Proposal for a Standard Protocol to Assess the Rheological Behavior of Thickening Products for Oropharyngeal Dysphagia. Nutrients, 14(23), 5028. https://doi.org/10.3390/nu14235028