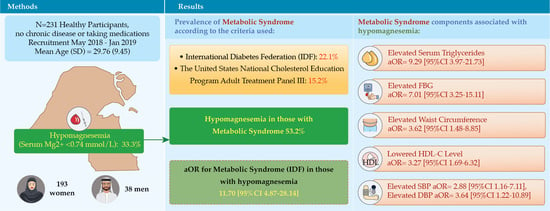
Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Recruitment, and Participant Selection
2.2. MetS Diagnostic Criteria
2.3. Anthropometric and Blood Pressure Measurements
2.4. Biochemical Measurements
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Prevalence of MetS
3.3. Cardiometabolic Risk Factors by Sex
3.4. Hypomagnesemia and MetS
3.5. Correlations of Serum Magnesium Level with Cardiometabolic Risk Factors
3.6. Correlations of Hypomagnesemia with MetS and Its Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 4186. Available online: www.efsa.europa.eu/efsajournal (accessed on 12 June 2020). [CrossRef]
- Song, Y.; Ridker, P.; Manson, J.; Cook, N.; Buring, J.; Liu, S. Magnesium Intake, C-Reactive Protein, and the Prevalence of Metabolic Syndrome in Middle-Aged and Older U.S. Women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Busardo, A.; Paolisso, G. Role of Magnesium in Insulin Action, Diabetes and Cardio-Metabolic Syndrome X. Mol. Asp. Med. 2003, 24, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Akyüz, O.; Gücün, M.; Demirci, R.; Celik, M. Relationship between Serum Magnesium Level and Insulin Resistance in Turkey Non-obese Adult Population. Biol. Trace Elem. Res. 2022, 200, 3070–3077. [Google Scholar] [CrossRef]
- Pelczyńska, M.; Moszak, M.; Bogdański, P. The Role of Magnesium in the Pathogenesis of Metabolic Disorders. Nutrients 2022, 14, 1714. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Jaquez-Chairez, F.O.; Rodríguez-Morán, M. Magnesium in metabolic syndrome: A review based on randomized, double-blind clinical trials. Magnes. Res. 2016, 29, 146–153. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Ridker, P.; Buring, J.; Cook, N.; Rifai, N. C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events. An 8-year Follow-Up of 14,719 Initially Healthy American Women. Circulation 2003, 12, 391–397. [Google Scholar] [CrossRef]
- Micke, O.; Vormann, J.; Kraus, A.; Kisters, K. Serum magnesium: Time for a standardized and evidence-based reference range. Magnes. Res. 2021, 34, 84–89. [Google Scholar] [CrossRef]
- Guembe, M.J.; Fernandez-Lazaro, C.I.; Sayon-Orea, C.; Toledo, E.; Morena-Iribas, C. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Al Zenki, S.; Al Omirah, H.; Al Hooti, S.; Al Hamad, N.; Jackson, R.; Rao, A.; Al Jahmah, N.; Al Obaid, I.; Al Ghanim, J.; Al Somaie, M.; et al. High Prevalence of Metabolic Syndrome among Kuwaiti Adults —A Wake-Up Call for Public Health Intervention. Int. J. Env. Res. Public Health 2012, 9, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Sorkhou, E.; Al-Qallaf, B.; Al-Namash, H.; Ben-Nakhi, A.; Al-Batish, M.; Habiba, S. Prevalence of Metabolic Syndrome among Hypertensive Patients Attending a Primary Care Clinic in Kuwait. Med. Princ. Pract. 2004, 13, 39–42. [Google Scholar] [CrossRef]
- Badr, H.; Al Orifan, F.; Amasha, M.; Khadadah, K.; Younis, H.; Se’adah, M. Prevalence of Metabolic Syndrome among Healthy Kuwaiti Adults: Primary Health Care Centers Based Study. Middle East J. Fam. Med. 2008, 5, 8–11. [Google Scholar]
- Al-Isa, A. Prevalence of Metabolic Syndrome (MetS) among Male Kuwaiti Adolescents Aged 10–19 Years. Health 2013, 5, 938–942. [Google Scholar] [CrossRef]
- Al-Isa, A.; Akanji, A.; Thalib, L. Prevalence of the Metabolic Syndrome among Female Kuwaiti Adolescents Using Two Different Criteria. Br. J. Nutr. 2009, 103, 77–81. [Google Scholar] [CrossRef]
- Pourhoseingholi, M.A.; Vahedi, M.; Rahimzadeh, M. Sample size calculation in medical studies. Gastroenterol. Hepatol. Bed Bench 2013, 6, 14–17. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4017493/ (accessed on 19 June 2020).
- Bahijri, M.S.; Al Raddadi, R.M.; Jambi, H.; Alaama, M.A.; Ferns, G. The Prevalence of Metabolic Syndrome in an Apparently Healthy, Normotensive and Non-Diabetic Population in Saudi Arabia by Two Definitions: Implications for Local Practice. Open J. Endocri. Metab. Dis. 2013, 3, 18–24. [Google Scholar] [CrossRef]
- Alberti, K.; Zimmet, P.; Shaw, J. Metabolic Syndrome-a New Worldwide Definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical Magnesium Deficiency: A Principal Driver of Cardiovascular Disease and a Public Health Crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Alawi, A.; Majoni, A.M.; Falhammar, S.W. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, E.; Ghoreishy, S.; Hemmati, S.M.; Mohammadi, A. Association between Magnesium Concentrations and Prediabetes: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 24388. [Google Scholar] [CrossRef] [PubMed]
- Assaad-Khalil, S.H.; Mikhail, M.M.; Aati, T.A.; Zaki, A.; Helmy, M.A.; Megallaa, M.H.; Hassanien, R.; Rohoma, K.H. Optimal waist circumference cutoff points for the determination of abdominal obesity and detection of cardiovascular risk factors among adult Egyptian population. Indian J. Endocrinol. Metab. 2015, 19, 804–810. [Google Scholar] [CrossRef]
- Alzahrani, A.; Karawagh, A.; Alshahrani, F.; Naser, T.; Ahmed, A.; Alsharef, E. Prevalence and Predictors of Metabolic Syndrome among Healthy Saudi Adults. Br. J. Diabetes Vasc. Dis. 2012, 12, 78–80. [Google Scholar] [CrossRef]
- Al-Lawati, J.; Mohammed, A.; Al-Hinai, H.; Jousilahti, P. Prevalence of the Metabolic Syndrome among Omani Adults. Diabetes Care 2003, 26, 1781–1785. [Google Scholar] [CrossRef]
- Alnohair, S. Obesity in Gulf Countries. Int. J. Health Sci. 2014, 8, 79–83. [Google Scholar] [CrossRef]
- Malik, M.; Razig, S. The Prevalence of the Metabolic Syndrome among the Multiethnic Population of the United Arab Emirates: A Report of a National Survey. Metab. Syndr. Relat. Disord. 2008, 6, 177–186. [Google Scholar] [CrossRef]
- Han, T.; Lean, M. A Clinical Perspective of Obesity, Metabolic Syndrome and Cardiovascular Disease. JRSM Cardiovasc. Dis. 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Oguoma, V.M.; Coffee, N.T.; Alsharrah, S. Prevalence of overweight and obesity, and associations with socio-demographic factors in Kuwait. BMC Public Health 2021, 21, 667. [Google Scholar] [CrossRef]
- Jung, U.; Choi, M. Obesity and its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Shamnani, G.; Rukadikar, C.; Gupta, V.; Singh, S.; Tiwari, S.; Bhartiy, S.; Sharma, P. Serum Magnesium in Relation with Obesity. Natl. J. Physiol. Pharm. Pharm. 2018, 8, 1074–1077. [Google Scholar] [CrossRef]
- Kew, S.; Ye, C.; Hanley, A.J.; Connelly, W.P.; Sermer, M.; Zinman, B.; Retnakaran, R. Cardiometabolic Implications of Postpartum Weight Changes in the First Year After Delivery. Diabetes Care 2014, 37, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shen, S.; Sun, L.; Yang, H.; Jin, B.; Cao, X. Metabolic Syndrome Risk after Gestational Diabetes: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87863. [Google Scholar] [CrossRef] [PubMed]
- Garovic, V.; August, P. Preeclampsia and the Future Risk of Hypertension: The Pregnant Evidence. Curr. Hypertens. Rep. 2013, 15, 114–121. [Google Scholar] [CrossRef]
- Bartels, Ä.; O’Donoghue, K. Cholesterol in Pregnancy: A Review of Knowns and Unknowns. Obs. Med. 2011, 4, 147–151. [Google Scholar] [CrossRef]
- Bentley-Lewis, R.; Koruda, K.; Seely, E. The Metabolic Syndrome in Women. Nat. Clin. Pr. Endocrinol. Metab. 2007, 3, 696–704. [Google Scholar] [CrossRef]
- Tiwari, J.; Naagar, J.K. Changes in Serum Lipid Profile in Postmenopausal Women with Reference to Body Mass Index (BMI). Int. J. Med. Res. Rev. 2015, 3, 456–460. [Google Scholar] [CrossRef][Green Version]
- Ligia, J.D.; Mario, B. The biology of the metabolic syndrome and aging. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 5–11. [Google Scholar] [CrossRef]
- Kuk, J.L.; Ardern, C.I. Age and sex differences in the clustering of metabolic syndrome factors: Association with mortality risk. Diabetes Care 2010, 33, 2457–2461. [Google Scholar] [CrossRef]
- Ge, H.; Yang, Z.; Li, X.; Liu, D.; Li, Y.; Pan, Y.; Luo, D.; Wu, X. The prevalence and associated factors of metabolic syndrome in Chinese aging population. Sci. Rep. 2020, 10, 20034. [Google Scholar] [CrossRef] [PubMed]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.A.M.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the Inflammatory Response: Potential Physiopathological Implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef]
- Obeidat, A.; Ahmad, M.; Haddad, F.; Azzeh, F. Alarming High Prevalence of Metabolic Syndrome among Jordanian Adults. Pak. J. Med. Sci. 2015, 31, 1377–1382. [Google Scholar] [CrossRef]
| Characteristics | Overall | Women | Men | |
|---|---|---|---|---|
| n = 231 | n = 193 (83.5%) | n = 38 (16.5%) | ||
| Age (years) | Mean ± SD | 29.76 ± 9.45 | 29.41 ± 9.48 | 31.53 ± 9.19 |
| n (%) | ||||
| Nationality | Kuwaiti | 201 (87) | 168 (87) | 33 (86.8) |
| Non-Kuwaitis | 30 (13) | 25 (13) | 5 (13.2) | |
| Marital status | Single | 112 (48.5) | 98 (50.8) | 14 (36.8) |
| Married | 109 (47.2) | 85 (44) | 24 (63.2) | |
| Divorced | 10 (4.3) | 10 (5.2) | -- | |
| Educational level | High school or lower | 57 (24.7) | 44 (22.8) | 13 (34.2) |
| College degree or higher | 174 (75.3) | 149 (77.2) | 25 (65.8) | |
| Employment | Yes | 136 (58.9) | 106 (54.9) | 30 (78.9) |
| No | 95 (41.1) | 87 (45.1) | 8 (21.1) | |
| Annual family income | <5000 KD | 44 (19) | 40 (20.7) | 4 (10.5) |
| 5000 KD or more | 98 (42.4) | 70 (36.3) | 28 (73.7) | |
| Did not disclose | 89 (38.5) | 83 (43) | 6 (15.8) | |
| Smoking | Yes, current | 7 (3) | -- | 7 (18.4) |
| No, never | 222 (96.1) | 193 (100) | 29 (76.3) | |
| No, but used to | 2 (0.9) | -- | 2 (5.3) | |
| Regular periods | Yes | -- | 152 (78.8) | -- |
| No | -- | 41 (21.2) | -- | |
| Menopause | Yes | -- | 11 (5.7) | -- |
| No | -- | 182 (94.3) | -- | |
| Hormones/BC | Yes | -- | 15 (7.8) | -- |
| No | -- | 178 (92.2) | -- | |
| Family History | ||||
| Diabetes | Yes | 160 (69.3) | 130 (67.4) | 30 (78.9) |
| HTN | Yes | 132 (57.1) | 119 (61.7) | 13 (34.2) |
| Heart disease | Yes | 39 (16.9) | 36 (18.7) | 3 (7.9) |
| Weight status | Underweight | 8 (3.5) | 7 (3.6) | 1 (2.6) |
| Normal weight | 110 (47.6) | 95 (49.2) | 15 (39.5) | |
| Overweight | 82 (35.5) | 67 (34.7) | 15 (39.5) | |
| With obesity | 31 (13.4) | 24 (12.4) | 7 (18.4) | |
| Characteristics | IDF Criteria | ATP III Criteria | |||||
|---|---|---|---|---|---|---|---|
| With MetS | Without MetS | Sig * | With MetS | Without MetS | Sig * | ||
| Overall | 51 (22.1) | 180 (77.9) | 35 (15.2) | 196 (84.8) | |||
| Age-adjusted | (27.4) | (72.6) | (19.1) | (80.9) | |||
| Sex | Female | 45 (23.3) | 148 (76.7) | 0.307 | 32 (16.6) | 161 (83.4) | 0.172 |
| Male | 6 (15.8) | 32 (84.2) | 3 (7.9) | 35 (92.1) | |||
| Age-adjusted | Female | (28.3) | (71.75) | <0.001 | (20.6) | (79.4) | <0.0001 |
| Male | (23.0) | (77.0) | (11.9) | (88.1) | |||
| Age group | 18–29 years | 15 (11.7) | 113 (88.3) | <0.001 | |||
| 30–49 years | 24 (26.7) | 66 (73.3) | |||||
| 50–65 years | 12 (92.3) | 1 (7.7) | |||||
| Weight status | Underweight | -- | 8 (100) | <0.001 | |||
| Normal | 4 (3.6) | 106 (96.4) | |||||
| Overweight | 31 (37.8) | 51 (62.2) | |||||
| Obesity | 16 (51.6) | 15 (48.8) | |||||
| Irregular menstrual period | Yes | 26 (54.2) | 22 (45.8) | <0.001 | |||
| No | 19 (13.1) | 126 (86.9) | |||||
| Menopause | Yes | 7 (63.6) | 4 (36.4) | <0.001 | |||
| No | 38 (20.9) | 144 (79.1) | |||||
| Family annual income | <5000 KD | 10 (22.7) | 34 (77.3) | 0.630 | |||
| ≥5000 KD | 26 (26.5) | 72 (73.5) | |||||
| Educational level | High school or lower | 12 (21.1) | 45 (78.9) | 0.830 | |||
| College degree or higher | 39 (22.4) | 135 (77.6) | |||||
| Marital status | Single | 20 (17.9) | 92 (82.1) | 0.289 | |||
| Married | 29 (26.6) | 80 (73.4) | |||||
| Divorced | 2 (20.0) | 8 (80.0) | |||||
| Nationality | Kuwaiti | 46 (22.9) | 155 (77.1) | 0.444 | |||
| Non-Kuwaiti | 5 (16.7) | 25 (83.3) | |||||
| Serum magnesium level | <0.74 mmol/L | 41 (53.2) | 36 (46.8) | <0.001 | |||
| >0.74 mmol/L | 10 (6.5) | 144 (93.5) | |||||
| Cardiometabolic Risk Factors | Normal Reference Ranges ** | Cutoff Point * for At-Risk Category | n (%) | ||
|---|---|---|---|---|---|
| Overall, n = 231 | Female, n = 193 | Male, n = 38 | |||
| WC (cm), IDF | Obesogenic: Men ≥ 94, Women ≥ 80 | 127 (55) | 117 (60.6) | 10 (26.3) | |
| Normal | 104 (45) | 76 (39.4) | 28 (73.7) | ||
| WC (cm), ATP III | Obesogenic: Men > 102, Women > 88 | 51 (22.1) | 48 (24.9) | 3 (7.9) | |
| Normal | 180 (77.9) | 145 (75.1) | 35 (92.1) | ||
| TG level (mmol/L) | 0.56–1.31 | Elevated ≥ 1.69 | 44 (19) | 38 (19.7) | 6 (15.8) |
| Normal | 187 (81) | 155 (80.3) | 32 (84.2) | ||
| FBG (mmol/L) | 3.9–6.1 | Elevated ≥ 5.6 | 51 (22.1) | 42 (21.8) | 9 (23.7) |
| Normal | 180 (77.9) | 151 (78.2) | 29 (76.3) | ||
| SBP (mmHg) | 90–120 | Elevated ≥ 130 | 37 (16) | 26 (13.5) | 11 (28.9) |
| Normal | 194 (84) | 167 (86.5) | 27 (71.1) | ||
| DBP (mmHg) | 60–80 | Elevated ≥ 85 | 24 (10.4) | 18 (9.3) | 6 (15.8) |
| Normal | 207 (89.6) | 175 (90.7) | 32 (84.2) | ||
| LDL-C level (mmol/L) | 2.1–4.10 | Elevated ≥ 4.12 | 13 (5.6) | 12 (6.2) | 1 (2.6) |
| Normal | 218 (94.4) | 181 (93.8) | 37 (97.4) | ||
| HDL-C level (mmol/L) | 1.04–1.89 | Low HDL-C Men < 1.03, Women < 1.29 | 77 (33.3) | 72 (37.3) | 5 (13.2) |
| Normal | 154 (66.7) | 121 (62.7) | 33 (86.8) | ||
| Serum magnesium (mmol/L) | 0.74–0.99 | Low Status < 0.74 | 77 (33.3) | 62 (32.1) | 15 (39.5) |
| Normal | 154 (66.7) | 131 (67.9) | 23 (60.5) | ||
| Cardiometabolic Risk Factors | Overall Sample, n = 231 | Without MetS, n = 180 | With MetS n = 51 | Sig a |
|---|---|---|---|---|
| Median [Interquartile Range] | ||||
| Age, years | 28.00 [22.00–35.00] | 27.00 [22.00–33.00] | 35.00 [29.00–47.00] | 0.000 |
| BMI, kg/m2 | 24.90 [22.50–27.68] | 24.06 [22.15–25.71] | 28.12 [26.34–31.14] | 0.000 |
| WC, cm | 85.00 [78.00–90.00] | 81.00 [78.00–88.00] | 89.50 [86.00–96.00] | 0.000 |
| SBP, mmHg | 120.00 [118.00–123.00] | 120.00 [117.25–122.00] | 122.00 [119.00–134.00] | 0.000 |
| DBP, mmHg | 80.00 [78.00–82.00] | 79.50 [77.00–81.00] | 82.00 [79.00–85.00] | 0.000 |
| FBG, mmol/L | 5.00 [4.62–5.51] | 4.85 [4.56–5.15] | 5.80 [5.50–6.49] | 0.000 |
| TG, mmol/L | 0.99 [0.71–1.51] | 0.87 [0.64–1.19] | 1.84 [1.26–2.60] | 0.000 |
| HDL-C, mmol/L | 1.35 [1.12–1.56] | 1.42 [1.22–1.60] | 1.03 [0.89–1.24] | 0.000 |
| LDL-C, mmol/L | 2.49 [2.01–2.94] | 2.40 [1.94–2.85] | 2.77 [2.17–3.31] | 0.008 |
| Total cholesterol, mmol/L | 4.43 [3.78–5.12] | 4.28 [3.72–5.05] | 4.76 [4.22–5.62] | 0.003 |
| Serum magnesium level, mmol/L | 0.79 [0.73–0.86] | 0.81 [0.76–0.90] | 0.70 [0.63–0.74] | 0.000 |
| Correlation Coefficients, r-Value | |
|---|---|
| BMI, kg/m2 | −0.423 ** |
| WC, cm | −0.412 ** |
| SBP, mmHg | −0.194 ** |
| DBP, mmHg | −0.188 ** |
| FBG, mmol/L | −0.517 ** |
| TG, mmol/L | −0.586 ** |
| HDL-C, mmol/L | 0.368 ** |
| LDL-C, mmol/L | −0.265 ** |
| Total cholesterol, mmol/L | −0.280 ** |
| Model Type | Sig | Serum Magnesium Categories 1 = Serum Magnesium < 0.74 mmol/L | ||||
|---|---|---|---|---|---|---|
| Exp(B) | 95% CI | Nagelkerke R Square | ||||
| Lower | Upper | |||||
| IDF Criteria | ||||||
| Overall sample n = 51/231 | Unadjusted | 0.000 | 16.40 | 7.50 | 35.84 | 0.334 |
| Adjusted a | 0.000 | 11.70 | 4.87 | 28.14 | 0.364 | |
| Men only n = 6/38 | Unadjusted | 0.039 | 11.00 | 1.13 | 106.84 | 0.192 |
| Adjusted b | 0.575 | -- | -- | -- | -- | |
| Women only n = 45/193 | Unadjusted | 0.000 | 18.77 | 8.07 | 43.66 | 0.192 |
| Adjusted c | 0.000 | 13.36 | 5.27 | 33.83 | 0.385 | |
| ATP III criteria | ||||||
| Overall sample n = 35/231 | Unadjusted | 0.000 | 9.86 | 4.21 | 23.10 | 0.189 |
| Adjusted a | 0.001 | 5.44 | 2.10 | 14.10 | 0.260 | |
| Men only n = 3/38 | Unadjusted | 0.999 | -- | -- | -- | -- |
| Adjusted b | 0.999 | -- | -- | -- | -- | |
| Women only n= 32/193 | Unadjusted | 0.000 | 9.71 | 4.03 | 23.39 | 0.204 |
| Adjusted c | 0.001 | 5.27 | 1.99 | 13.99 | 0.252 | |
| Dependent Variable a | Model Type | Serum Magnesium Categories, 1 = Serum Magnesium Level < 0.74 mmol/L | ||||
|---|---|---|---|---|---|---|
| Sig | Exp(B) | 95% CI | Nagelkerke R Square | |||
| Lower | Upper | |||||
| IDF Obese waist | Unadjusted | 0.000 | 5.08 | 2.96 | 9.61 | 0.163 |
| IDF Obese waist | Adjusted b | 0.005 | 3.62 | 1.48 | 8.85 | 0.236 |
| ATP III Obese waist | Unadjusted | 0.001 | 2.93 | 1.54 | 5.54 | 0.064 |
| ATPIII Obese waist | Adjusted b | 0.572 | -- | -- | -- | -- |
| Elevated TG level | Unadjusted | 0.000 | 12.74 | 5.67 | 28.63 | 0.259 |
| Elevated TG level | Adjusted b | 0.000 | 9.29 | 3.97 | 21.73 | 0.342 |
| Elevated FBG | Unadjusted | 0.000 | 10.57 | 5.13 | 21.77 | 0.261 |
| Elevated FBG | Adjusted b | 0.000 | 7.01 | 3.25 | 15.11 | 0.323 |
| Elevated Systolic BP | Unadjusted | 0.000 | 5.69 | 2.67 | 12.14 | 0.126 |
| Elevated Systolic BP | Adjusted b | 0.022 | 2.88 | 1.16 | 7.11 | 0.221 |
| Elevated Diastolic BP | Unadjusted | 0.000 | 7.53 | 2.85 | 19.89 | 0.113 |
| Elevated Diastolic BP | Adjusted b | 0.021 | 3.64 | 1.22 | 10.89 | 0.223 |
| Low HDL level | Unadjusted | 0.000 | 3.13 | 1.76 | 5.58 | 0.089 |
| Low HDL | Adjusted b | 0.000 | 3.27 | 1.70 | 6.32 | 0.259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkazemi, D.; Alsouri, N.; Zafar, T.; Kubow, S. Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study. Nutrients 2022, 14, 5257. https://doi.org/10.3390/nu14245257
Alkazemi D, Alsouri N, Zafar T, Kubow S. Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study. Nutrients. 2022; 14(24):5257. https://doi.org/10.3390/nu14245257
Chicago/Turabian StyleAlkazemi, Dalal, Noora Alsouri, Tasleem Zafar, and Stan Kubow. 2022. "Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study" Nutrients 14, no. 24: 5257. https://doi.org/10.3390/nu14245257
APA StyleAlkazemi, D., Alsouri, N., Zafar, T., & Kubow, S. (2022). Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study. Nutrients, 14(24), 5257. https://doi.org/10.3390/nu14245257