Protective Effects of Spirulina maxima against Blue Light-Induced Retinal Damages in A2E-Laden ARPE-19 Cells and Balb/c Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of S. maxima and P-Phycocyanin
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Pre and Post-Treatment of S. maxima with BL Exposure
2.5. A2E Treatment and Blue Light Exposure
2.6. Estimation of Cellular Reactive Oxygen Species (ROS)
2.7. Animals and Experiment Design
2.8. Sample Administration and BL Exposure
2.9. Histological Analysis
2.10. Quantitative Real Time PCR (qRT-PCR)
2.11. Western Immunoblotting
2.12. Statistical Analysis
3. Results
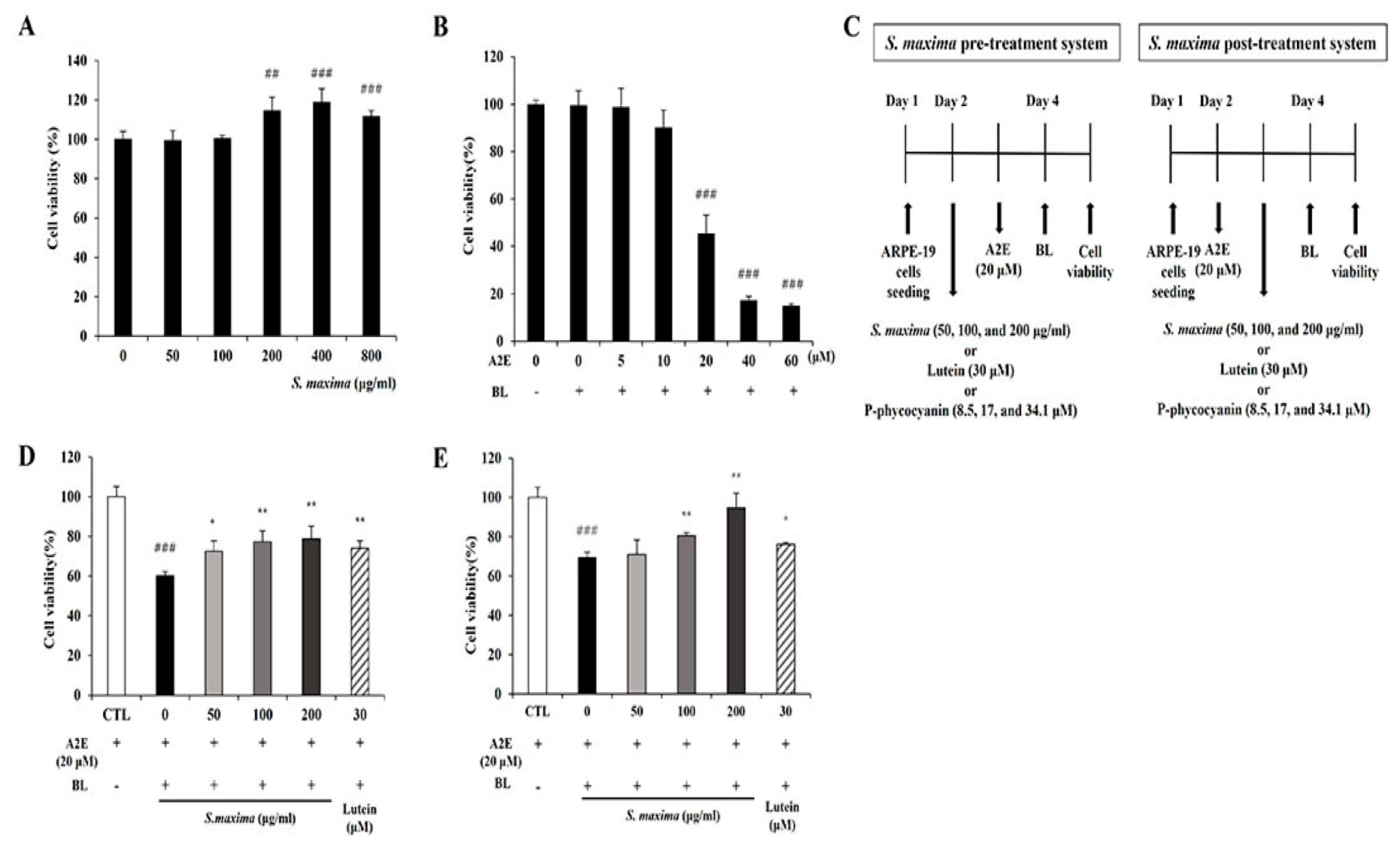
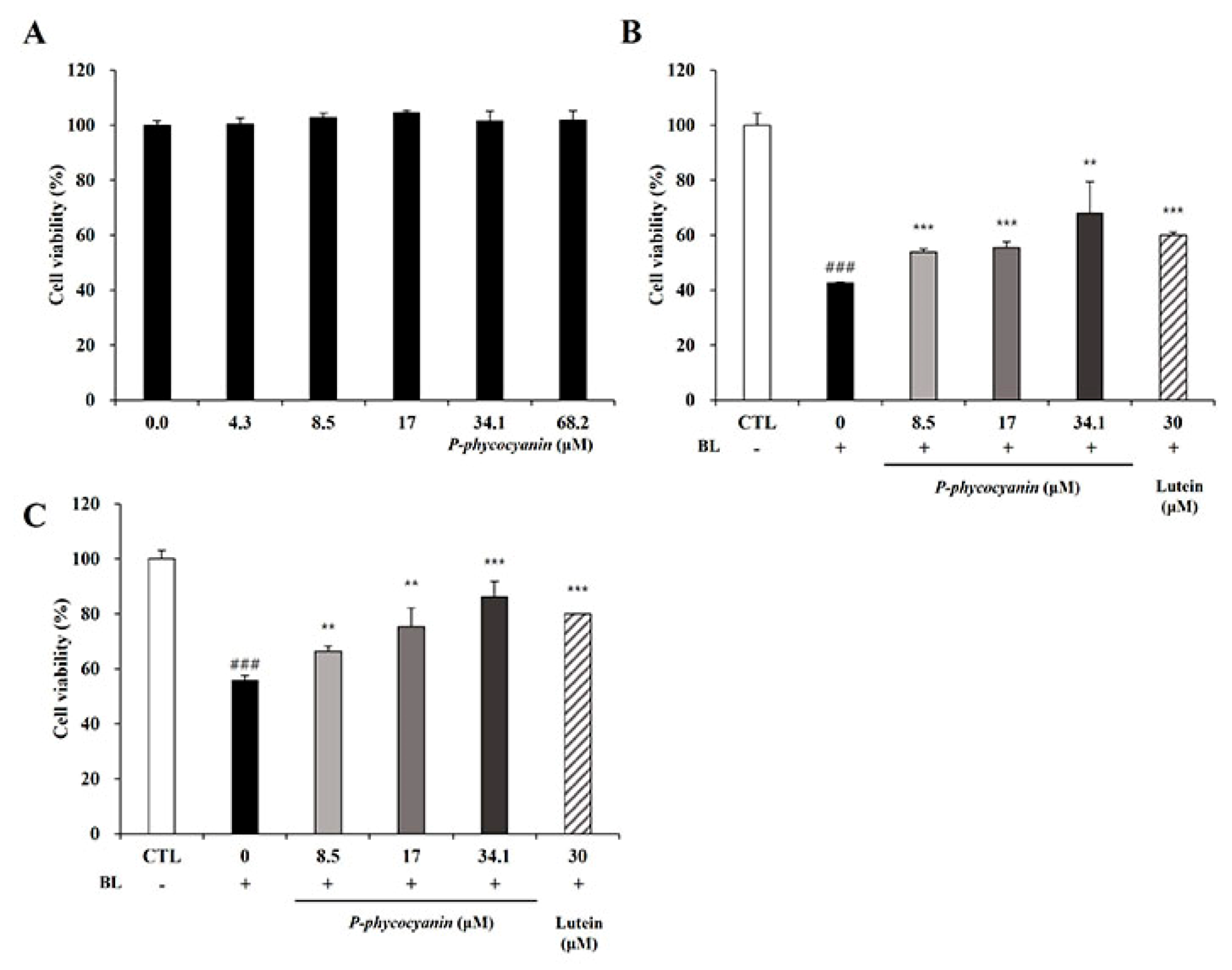
3.1. S. maxima Inhibited Cell Death Caused by A2E Treatment and BL Exposure
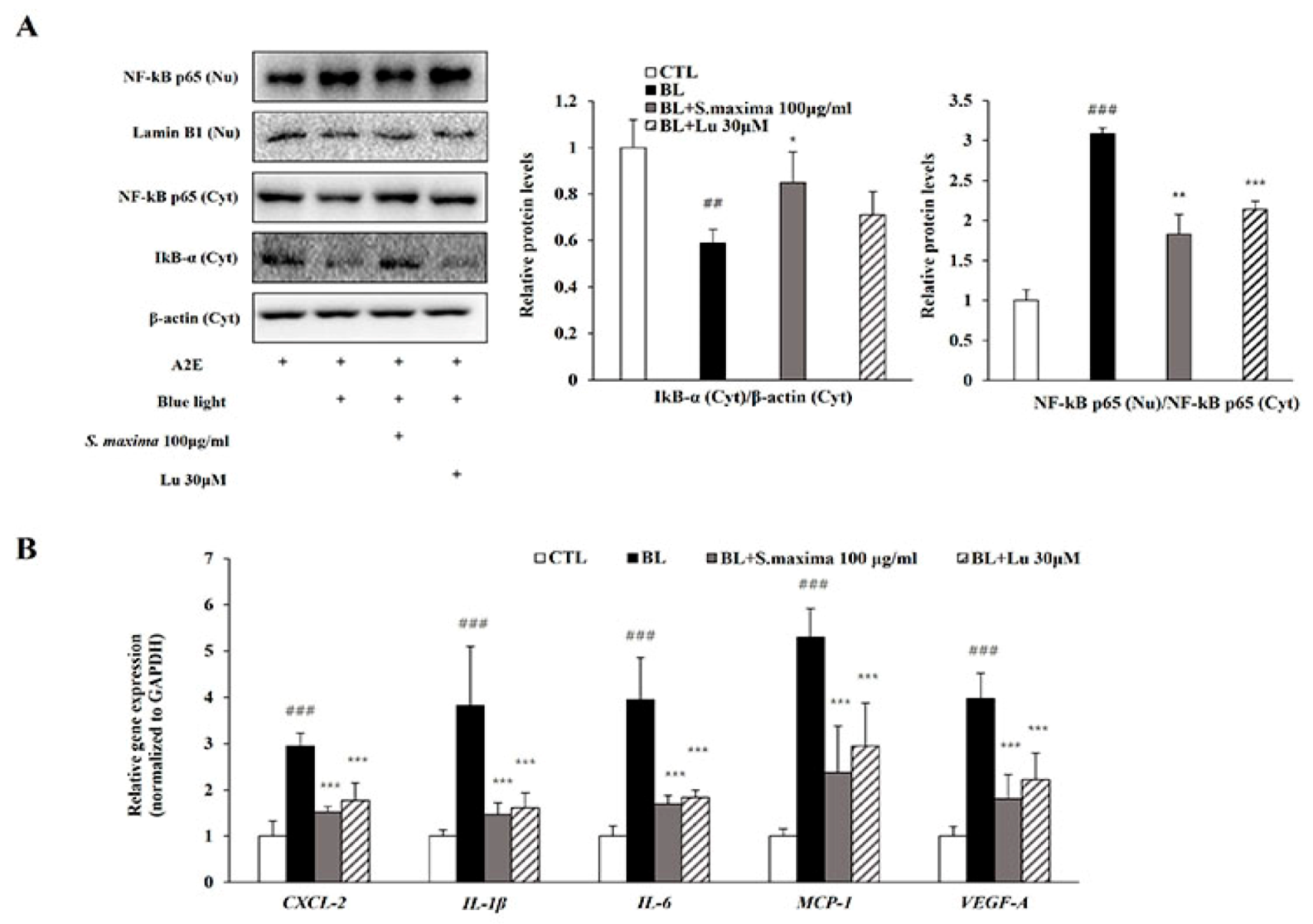
3.2. S. maxima Regulated the Inflammatory Response Caused by BL in A2E-Laden ARPE-19 Cells
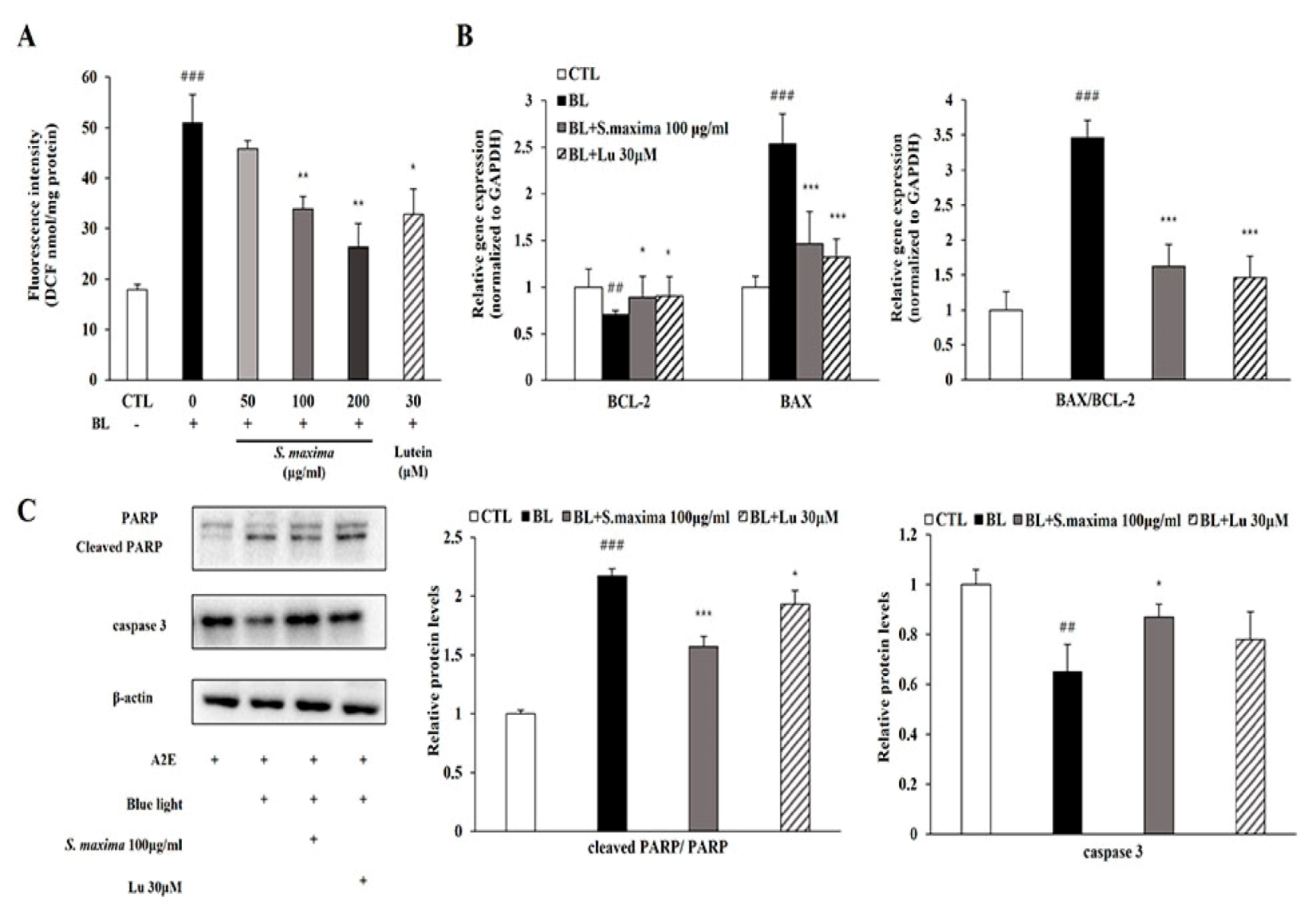
3.3. S. maxima Regulated the Apoptosis Caused by BL in A2E-Laden ARPE-19 Cells
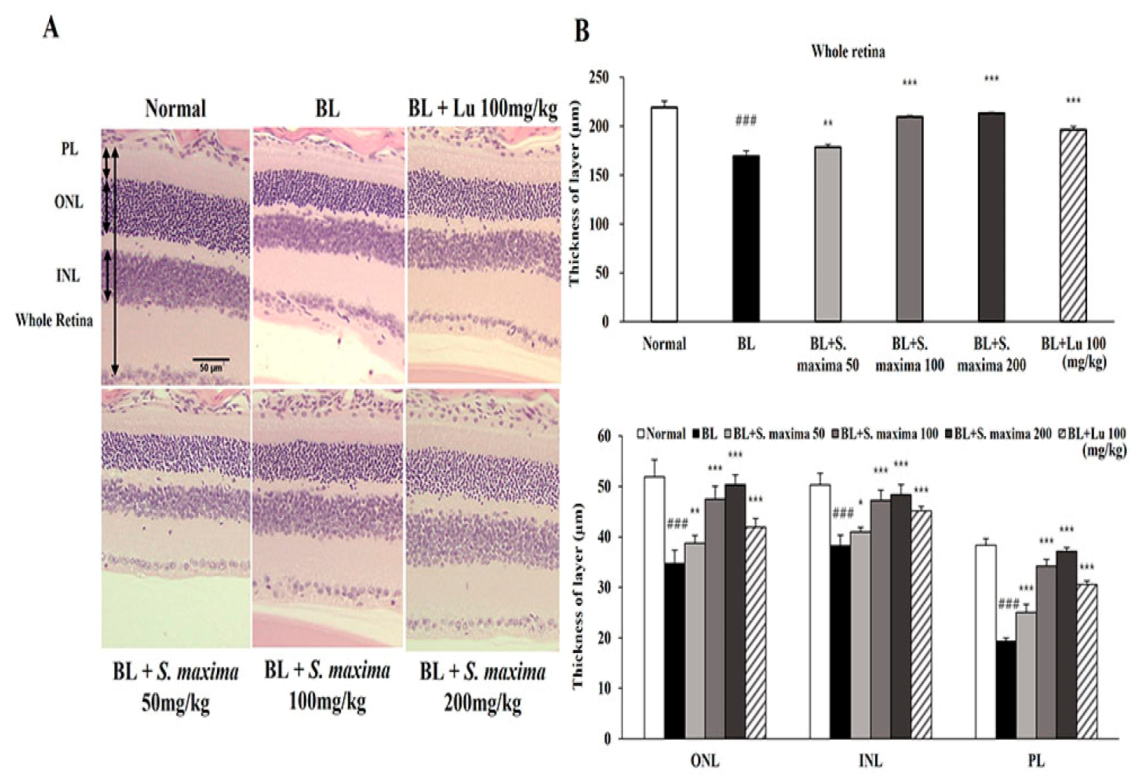
3.4. S. maxima Protected Photoreceptor Degeneration Caused by BL in Retina
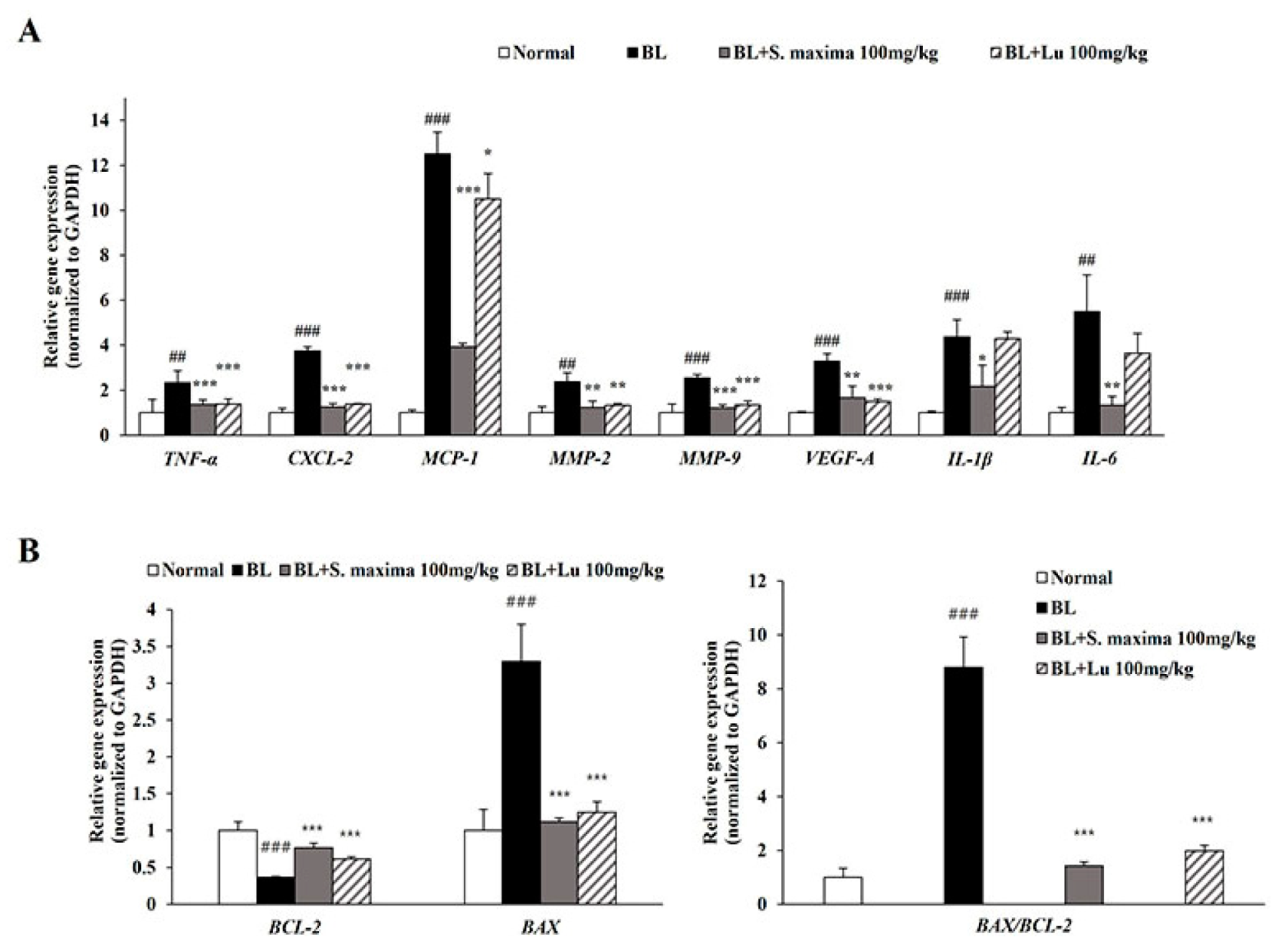
3.5. S. maxima Regulated Inflammation and Apoptosis Caused by BL in Retina
3.6. P-Phycocyanin Was a Major Active Component of S. maxima on Retinal Degeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blehm, C.; Vishnu, S.; Khattak, A.; Mitra, S.; Yee, R.W. Computer vision syndrome: A review. Surv. Ophthalmol. 2005, 50, 253–262. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Fishkin, N.; Zhou, J.; Cai, B.; Jang, Y.P.; Krane, S.; Itagaki, Y.; Nakanishi, K. A2E, a byproduct of the visual cycle. Vis. Res. 2003, 43, 2983–2990. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical damage of the retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Zhang, X.; Honeycutt, A.A.; Lesesne, S.B.; Saaddine, J.; Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: The potential impact of new treatments. Arch. Ophthalmol. 2009, 127, 533–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, R.; Song, L.D.; Choi, J.A.; Jee, D. The cost-effectiveness of systematic screening for age-related macular degeneration in South Korea. PLoS ONE 2018, 13, e0206690. [Google Scholar] [CrossRef] [PubMed]
- Buschini, E.; Piras, A.; Nuzzi, R.; Vercelli, A. Age related macular degeneration and drusen: Neuroinflammation in the retina. Prog. Neurobiol. 2011, 95, 14–25. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Boulton, M. RPE lipofuscin and its role in retinal pathobiology. Exp. Eye Res. 2005, 80, 595–606. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Kovach, J.L.; Schwartz, S.G.; Flynn, H.W.; Scott, I.U. Anti-VEGF treatment strategies for wet AMD. J. Ophthalmol. 2012, 2012, 786870. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Klein, B.E.; Knudtson, M.D.; Meuer, S.M.; Swift, M.; Gangnon, R.E. Fifteen-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology 2007, 114, 253–262. [Google Scholar] [CrossRef]
- Jang, Y.P.; Matsuda, H.; Itagaki, Y.; Nakanishi, K.; Sparrow, J.R. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J. Biol. Chem. 2005, 280, 39732–39739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-Y.; Bao, X.-L.; Cong, Y.-Y.; Fan, B.; Li, G.-Y. Autophagy in age-related macular degeneration: A regulatory mechanism of oxidative stress. Oxid. Med. Cell. Longev. 2020, 2020, 2896036. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Zhou, J.; Ben-Shabat, S.; Vollmer, H.; Itagaki, Y.; Nakanishi, K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1222–1227. [Google Scholar]
- Sparrow, J.R.; Nakanishi, K.; Parish, C.A. The lipofuscin fluorophore A2E mediates blue light–induced damage to retinal pigmented epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1981–1989. [Google Scholar]
- Jin, H.L.; Choung, S.-Y.; Jeong, K.W. Protective mechanisms of polyphenol-enriched fraction of Vaccinium uliginosum L. Against blue light-induced cell death of human retinal pigmented epithelial cells. J. Funct. Foods 2017, 39, 28–36. [Google Scholar] [CrossRef]
- Lee, B.-L.; Kang, J.-H.; Kim, H.-M.; Jeong, S.-H.; Jang, D.-S.; Jang, Y.-P.; Choung, S.-Y. Polyphenol-enriched Vaccinium uliginosum L. fractions reduce retinal damage induced by blue light in A2E-laden ARPE19 cell cultures and mice. Nutr. Res. 2016, 36, 1402–1414. [Google Scholar] [CrossRef]
- Kim, J.; Jin, H.L.; Jang, D.S.; Jeong, K.W.; Choung, S.-Y. Quercetin-3-O-α-l-arabinopyranoside protects against retinal cell death via blue light-induced damage in human RPE cells and Balb-c mice. Food Funct. 2018, 9, 2171–2183. [Google Scholar] [CrossRef]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D. Dietary nutrient intake and progression to late age-related macular degeneration in the age-related eye disease studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef]
- Li, L.H.; Lee, J.C.-Y.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-D.; Kim, J.; Choung, S.-Y. Protective effects of quercetin-3-O-α-L-arabinopyranoside against UVA induced apoptosis via regulating inflammatory pathways in ARPE-19 cells and Balb/c mice. J. Funct. Foods 2019, 62, 103541. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Rebolledo, G.A.; Galar-Martínez, M.; García-Rodríguez, R.V.; Chamorro-Cevallos, G.A.; Hernández-Reyes, A.G.; Martínez-Galero, E. Antioxidant effect of Spirulina (Arthrospira) maxima on chronic inflammation induced by Freund's complete adjuvant in rats. J. Med. Food 2015, 18, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Ponce-Canchihuamán, J.C.; Pérez-Méndez, O.; Hernández-Muñoz, R.; Torres-Durán, P.V.; Juárez-Oropeza, M.A. Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis. 2010, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Koh, E.-J.; Seo, Y.-J.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Kim, K.-J.; Lee, B.-Y. Spirulina maxima extract prevents neurotoxicity via promoting activation of BDNF/CREB signaling pathways in neuronal cells and mice. Molecules 2017, 22, 1363. [Google Scholar] [CrossRef] [PubMed]
- Korea Food and Drug Administration. Food Code; Korea Food and Drug Administration: Seoul, Korea, 2002; pp. 3–4. [Google Scholar]
- Kang, M.S.; Moon, J.-H.; Park, S.C.; Jang, Y.P.; Choung, S.Y. Spirulina maxima reduces inflammation and alveolar bone loss in Porphyromonas gingivalis-induced periodontitis. Phytomedicine 2021, 81, 153420. [Google Scholar] [CrossRef] [PubMed]
- Marie, M.; Gondouin, P.; Pagan, D.; Barrau, C.; Villette, T.; Sahel, J.; Picaud, S. Blue-violet light decreases VEGFa production in an in vitro model of AMD. PLoS ONE 2019, 14, e0223839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruotti, J.; Biggs, R.; Katti, S.; Lauder, S.; Onteniente, B. Development of a high-throughput assay for dry AMD based on chronic exposure of hiPSC-RPE to A2E and blue light. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4151. [Google Scholar]
- Beatty, S.; Koh, H.-H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [Green Version]
- Wessler, S.; Muenzner, P.; Meyer, T.F.; Naumann, M. The Anti-Inflammatory Compound Curcumin Inhibits Neisseria Gonorrhoeae-Induced NF-κB Signaling, Release of Pro-Inflammatory Cytokines/Chemokines and Attenuates Adhesion in Late Infection; De Gruyter: Berlin, German, 2005. [Google Scholar]
- Ha, J.; Choi, H.-S.; Lee, Y.; Kwon, H.-J.; Song, Y.W.; Kim, H.-H. CXC chemokine ligand 2 induced by receptor activator of NF-κB ligand enhances osteoclastogenesis. J. Immunol. 2010, 184, 4717–4724. [Google Scholar] [CrossRef]
- Higgins, G.T.; Wang, J.H.; Dockery, P.; Cleary, P.E.; Redmond, H.P. Induction of angiogenic cytokine expression in cultured RPE by ingestion of oxidized photoreceptor outer segments. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Su, T.; Qiu, X.; Mao, P.; Xu, Y.; Hu, Z.; Zhang, Y.; Zheng, X.; Xie, P.; Liu, Q. Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death. Sci. Rep. 2016, 6, 21018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shen, D.; Wang, V.M.; Yu, C.-R.; Wang, R.-X.; Tuo, J.; Chan, C.-C. Enhanced apoptosis in retinal pigment epithelium under inflammatory stimuli and oxidative stress. Apoptosis 2012, 17, 1144–1155. [Google Scholar] [CrossRef] [Green Version]
- Jürgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Li, W.; Wang, G.; Guo, L.; Jiang, Y.; Kang, Y.J. Hyperglycemia-induced apoptosis in mouse myocardium: Mitochondrial cytochrome C–mediated caspase-3 activation pathway. Diabetes 2002, 51, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, J.G.; Yang, L.; Bula, D.; Chen, D.F. Photoreceptor apoptosis in human retinal detachment. Am. J. Ophthalmol. 2005, 139, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, S.; Haeseleer, F.; Hendrickson, A. A comparison of immunocytochemical markers to identify bipolar cell types in human and monkey retina. Vis. Neurosci. 2003, 20, 589–600. [Google Scholar] [CrossRef]
- Willermain, F.; Libert, S.; Motulsky, E.; Salik, D.; Caspers, L.; Perret, J.; Delporte, C. Origins and consequences of hyperosmolar stress in retinal pigmented epithelial cells. Front. Physiol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorani, L.; Passacantando, M.; Santucci, S.; Di Marco, S.; Bisti, S.; Maccarone, R. Cerium oxide nanoparticles reduce microglial activation and neurodegenerative events in light damaged retina. PLoS ONE 2015, 10, e0140387. [Google Scholar] [CrossRef] [Green Version]
- Guillonneau, X.; Eandi, C.M.; Paques, M.; Sahel, J.-A.; Sapieha, P.; Sennlaub, F. On phagocytes and macular degeneration. Prog. Retin. Eye Res. 2017, 61, 98–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in retinal degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; McGuire, P.G.; Eriqat, C.; Ober, R.R.; DeJuan, E.; Williams, G.A.; McLamore, A.; Biswas, J.; Johnson, D.W. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Investig. Ophthalmol. Vis. Sci. 1999, 40, 809–813. [Google Scholar]
- Abd El-Baky, H.H.; El Baz, F.K.; El-Baroty, G.S. Characterization of nutraceutical compounds in blue green alga Spirulina maxima. J. Med. Plants Res. 2008, 2, 292–300. [Google Scholar]
- Heocha, C. Biliproteins of algae. Annu. Rev. Plant Physiol. 1965, 16, 415–434. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; Garcia, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef]
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| Bcl-2 | ATGTGTGTGGAGAGCGTCAA | ACAGTTCCACAAAGGCATCC |
| Bax | GGGGACGAACTGGACAGTAA | CAGTTGAAGTTGCCGTCAGA |
| IL-1β | GGACAAGCTGAGGAAGATGC | TCGTTATCCCATGTGTCGAA |
| IL-6 | CACAGACAGCCACTCACCTC | TTTTCTGCCAGTGCCTCTTT |
| CXCL-2 | GGGCAGAAGCTTGTCTCAA | AGCTTCCTCCTTCCTTCTGG |
| MCP-1 | ATGAAAGTCTCTGCCGCCCTCA | GAGATCTGTGCTGACCCCAA |
| VEGF-A | TTGCCTTGCTGCTCTACCTC | AAATGCTTTCTCCGCTCTGA |
| GAPDH | CGAGATCCCTCCAAAATCAA | TTCACACCCATGACGAACAT |
| Bcl-2 | CTCGTCGCTACCGTCGTGACTTCG | CAGATGCCGGTTCAGGTACTCAGTC |
| Bax | AAGCTGAGCGAGTGTCTCCGGCG | GCCACAAAGATGGTCACTGTCTGCC |
| IL-1β | TCGCAGCAGCACATCAACAAG | TCCACGGGAAAGACACAGGTAG |
| IL-6 | TGTGCAATGGCAATTCTGAT | GGTACTCCAGAAGACCAGAGGA |
| CXCL-2 | CGCTGTCAATGCCTGAAGAC | ACACTCAAGCTCTGGATGTTCTTG |
| TNF-α | CACAAGATGCTGGGACAGTGA | TCCTTGATGGTGGTGCATGA |
| MCP-1 | TTAAGGCATCACAGTCCGAG | TGAATGTGAAGTTGACCCGT |
| MMP-2 | TGGCAAGGTGTGGTGTGCGAC | TCGGGGCCATCAGAGCTCCAG |
| MMP-9 | GGTGTGCCCTGGAACTCACACG | AGGGCACTGCAGGAGGTCGT |
| VEGF-A | CCTGGTGGACATCTTCCAGGAGTACC | GAAGCTCATCTCTCCTATGTGCTGGC |
| GAPDH | CGGCCGCATCTTCTTGTG | CCGACCTTCACCATTTTGTCTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-M.; Jo, Y.-D.; Choung, S.-Y. Protective Effects of Spirulina maxima against Blue Light-Induced Retinal Damages in A2E-Laden ARPE-19 Cells and Balb/c Mice. Nutrients 2022, 14, 401. https://doi.org/10.3390/nu14030401
Cho H-M, Jo Y-D, Choung S-Y. Protective Effects of Spirulina maxima against Blue Light-Induced Retinal Damages in A2E-Laden ARPE-19 Cells and Balb/c Mice. Nutrients. 2022; 14(3):401. https://doi.org/10.3390/nu14030401
Chicago/Turabian StyleCho, Hye-Mi, Ye-Dam Jo, and Se-Young Choung. 2022. "Protective Effects of Spirulina maxima against Blue Light-Induced Retinal Damages in A2E-Laden ARPE-19 Cells and Balb/c Mice" Nutrients 14, no. 3: 401. https://doi.org/10.3390/nu14030401
APA StyleCho, H.-M., Jo, Y.-D., & Choung, S.-Y. (2022). Protective Effects of Spirulina maxima against Blue Light-Induced Retinal Damages in A2E-Laden ARPE-19 Cells and Balb/c Mice. Nutrients, 14(3), 401. https://doi.org/10.3390/nu14030401





