Efficacy and Safety of Ferrous Bisglycinate and Folinic Acid in the Control of Iron Deficiency in Pregnant Women: A Randomized, Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
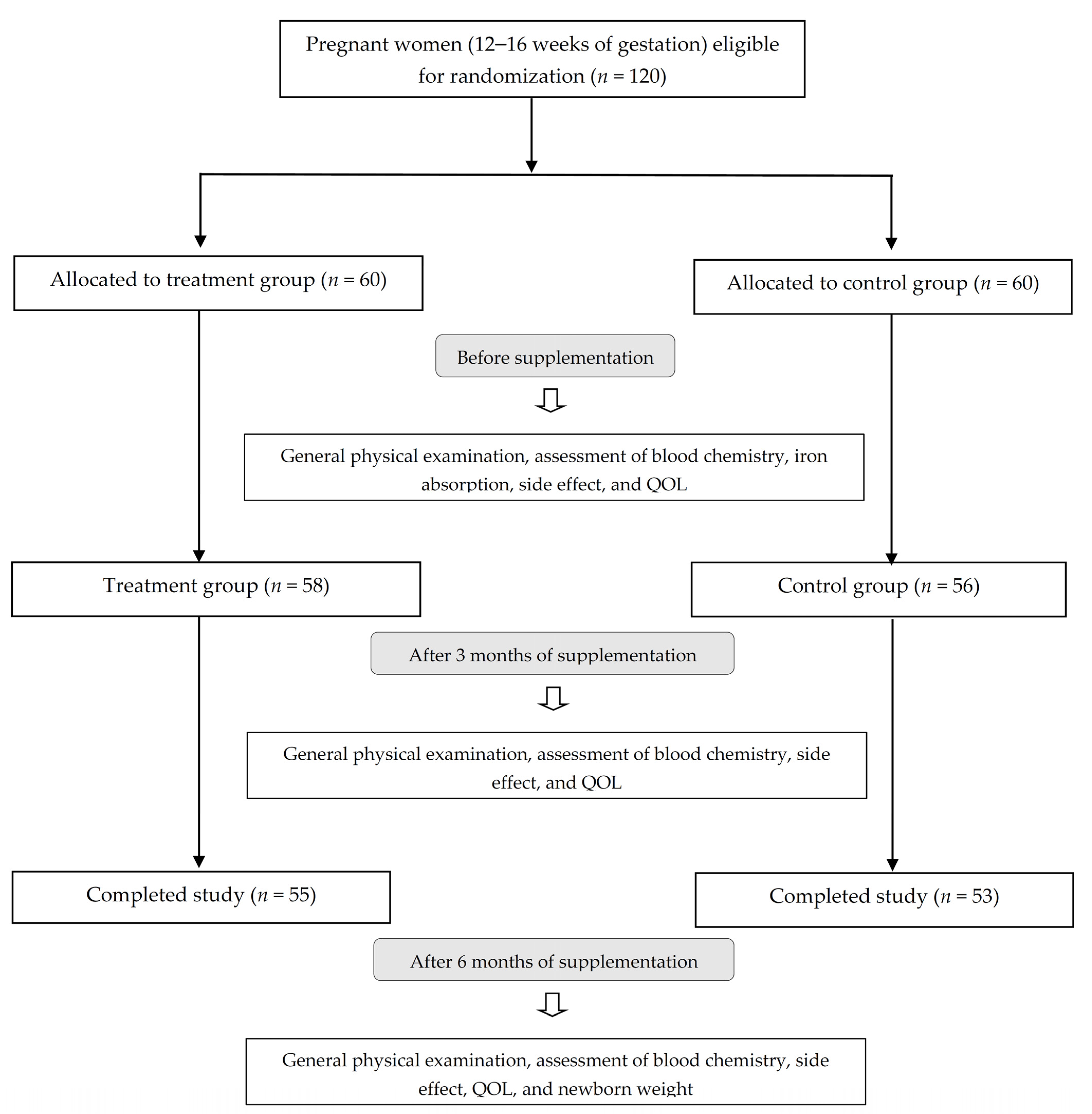
2.1. Subjects and Study Design
2.2. Biochemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Subjects
3.2. Iron Absorption
3.3. Effects of Supplements on Biomarkers of Hematological and Iron Status
3.4. Maternal Side Effects, Quality of Life, and Birth Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholl, T.O. Iron status during pregnancy: Setting the stage for mother and infant. Am. J. Clin. Nutr. 2005, 81, 1218S–1222S. [Google Scholar] [CrossRef] [PubMed]
- Jeha, D.; Usta, I.; Ghulmiyyah, L.; Nassar, A. A review of the risks and consequences of adolescent pregnancy. J. Neonatal. Perinatal. Med. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Milman, N. Iron and pregnancy-a delicate balance. Ann. Hematol. 2006, 85, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Bergholt, T.; Byg, K.E.; Eriksen, L.; Graudal, N. Iron status and iron balance during pregnancy. A critical reappraisal of iron supplementation. Acta. Obstet. Gynecol. Scand. 1999, 78, 749–757. [Google Scholar]
- Stoffel, N.U.; Zeder, C.; Brittenham, G.M.; Moretti, D.; Zimmermann, M.B. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica 2020, 105, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda, O.; Ashmead, H.D. Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate. Nutrition 2001, 17, 381–384. [Google Scholar] [CrossRef]
- Szarfarc, S.C.; de Cassana, L.M.; Fujimori, E.; Guerra-Shinohara, E.M.; de Oliveira, I.M. Relative effectiveness of iron bis-glycinate chelate (Ferrochel) and ferrous sulfate in the control of iron deficiency in pregnant women. Arch. Latinoam. Nutr. 2001, 51, 42–47. [Google Scholar]
- Juarez-Vazquez, J.; Bonizzoni, E.; Scotti, A. Iron plus folate is more effective than iron alone in the treatment of iron deficiency anaemia in pregnancy: A randomised, double blind clinical trial. BJOG 2002, 109, 1009–1014. [Google Scholar] [CrossRef]
- Youssef, A.M.; Shata, A.F.; Kamal, H.M.; El-Saied, Y.; Ali, O.F. A comparative study of efficacy, tolerability, and compliance of oral iron preparations for iron deficiency anemia in pregnant women. Am. J. Med. Med. Sci. 2014, 4, 244–249. [Google Scholar]
- Jantacumma, N.; Powwattana, A.; Lagampan, S.; Chansatitporn, N. Predictive model of quality of life among Thai pregnant teenagers. Pacific. Rim. Int. J. Nurs. Res. 2018, 22, 30–42. [Google Scholar]
- Iqbal, T.; Stein, J.; Sharma, N.; Kulnigg-Dabsch, S.; Vel, S.; Gasche, C. Clinical significance of C-reactive protein levels in predicting responsiveness to iron therapy in patients with inflammatory bowel disease and iron deficiency anemia. Dig. Dis. Sci. 2015, 60, 1375–1381. [Google Scholar] [CrossRef] [Green Version]
- Layrisse, M.; García-Casal, M.N.; Solano, L.; Barón, M.A.; Arguello, F.; Llovera, D.; Ramírez, J.; Leets, I.; Tropper, E. Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J. Nutr. 2000, 130, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, L.; Talarico, V.; Mazza, G.A.; Marrazzo, S.; Gangemi, P.; Miniero, R.; Bertini, M. Feralgine™ a new approach for iron deficiency anemia in celiac patients. Nutrients 2019, 11, 887. [Google Scholar] [CrossRef] [Green Version]
- Milman, N.; Jønsson, L.; Dyre, P.; Pedersen, P.L.; Larsen, L.G. Ferrous bisglycinate 25 mg iron is as effective as ferrous sulfate 50 mg iron in the prophylaxis of iron deficiency and anemia during pregnancy in a randomized trial. J. Perinat. Med. 2014, 42, 197–206. [Google Scholar] [CrossRef]
- Latham, M.C.; Ash, D.M.; Makola, D.; Tatala, S.R.; Ndossi, G.D.; Mehansho, H. Efficacy trials of a micronutrient dietary supplement in schoolchildren and pregnant women in Tanzania. Food. Nutr. Bull. 2003, 24, S120–S128. [Google Scholar] [CrossRef]
- Pineda, O.; Ashmead, H.D.; Perez, J.M.; Lemus, C.P. Effectiveness of iron amino acid chelate on the treatment of iron deficiency anemia in adolescents. J. Appl. Nutr. 1994, 46, 2–13. [Google Scholar]
- Duque, X.; Martinez, H.; Vilchis-Gil, J.; Mendoza, E.; Flores-Hernández, S.; Morán, S.; Navarro, F.; Roque-Evangelista, V.; Serrano, A.; Mera, R.M. Effect of supplementation with ferrous sulfate or iron bis-glycinate chelate on ferritin concentration in Mexican schoolchildren: A randomized controlled trial. Nutr. J. 2014, 13, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.M.; Abdelbadee, S.A.; Alanwar, A.; Mostafa, S. Efficacy of ferrous bis-glycinate versus ferrous glycine sulfate in the treatment of iron deficiency anemia with pregnancy: A randomized double-blind clinical trial. J. Matern. Fetal. Neonatal. Med. 2019, 32, 4139–4145. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, V.M.; Freedman, M.H.; Rivard, G.E.; Townsend, S.R. Response to folinic acid in B 12-deficiency anaemia. Lancet 1971, 2, 552–554. [Google Scholar] [CrossRef]
- Davidson, L.S.; Girdwood, R.H. Treatment of the megaloblastic anaemias with citrovorum factor. Lancet 1951, 2, 1193–1195. [Google Scholar] [CrossRef]
- Board, B.E. Folic acid and combined iron and folic acid preparations. Br. Med. J. 1968, 4, 102–103. [Google Scholar]
- Horn, E. Iron and folate supplements during pregnancy: Supplementing everyone treats those at risk and is cost effective. BMJ 1988, 297, 1325–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Haider, B.A.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane. Database. Syst. Rev. 2015, 11, CD004905. [Google Scholar]
- Sharma, D.C.; Mathur, R. Correction of anemia and iron deficiency in vegetarians by administration of ascorbic acid. Indian J. Physiol. Pharmacol. 1995, 39, 403–406. [Google Scholar]
- Chiamchanya, N. Rapid recovery time of hemoglobin level in female regular blood donors with ferrous fumarate and high dose of ascorbic acid supplement. J. Med. Assoc. Thai. 2013, 96, 165–171. [Google Scholar]
- Cepeda-Lopez, A.C.; Melse-Boonstra, A.; Zimmermann, M.B.; Herter-Aeberli, I. In overweight and obese women, dietary iron absorption is reduced and the enhancement of iron absorption by ascorbic acid is one-half that in normal-weight women. Am. J. Clin. Nutr. 2015, 102, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Andraos, S.; Jones, B.; Wall, C.; Thorstensen, E.; Kussmann, M.; Cameron-Smith, D.; Lange, K.; Clifford, S.; Saffery, R.; Burgner, D.; et al. Plasma B vitamers: Population epidemiology and parent-child concordance in children and adults. Nutrients 2021, 13, 821. [Google Scholar] [CrossRef]
- Mei, Z.; Serdula, M.K.; Liu, J.M.; Flores-Ayala, R.C.; Wang, L.; Ye, R.; Grummer-Strawn, L.M. Iron-containing micronutrient supplementation of Chinese women with no or mild anemia during pregnancy improved iron status but did not affect perinatal anemia. J. Nutr. 2014, 144, 943–948. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.M.; Mei, Z.; Ye, R.; Serdula, M.K.; Ren, A.; Cogswell, M.E. Micronutrient supplementation and pregnancy outcomes: Double-blind randomized controlled trial in China. JAMA Intern. Med. 2013, 173, 276–782. [Google Scholar] [CrossRef]
| Ferrous Bisglycinate and Folinic Acid | Control | ||
|---|---|---|---|
| Ferrous bisglycinate | 120 mg | Ferrous fumarate | 200 mg |
| Equiv. iron | 24 mg | Equiv. iron | 66 mg |
| Folic acid (vitamin B9) | 400 mcg | Folic acid (vitamin B9) | 400 mcg |
| Calcium folinate | 127 mcg | Calcium folinate | - |
| Equiv. folinic acid | 100 mcg | Equiv. folinic acid | - |
| Ascorbic acid (vitamin C) | 50 mg | Ascorbic acid (vitamin C) | 50 mg |
| Thiamine nitrate (vitamin B1) | 5 mg | Thiamine nitrate (vitamin B1) | 5 mg |
| Riboflavine (vitamin B2) | 5 mg | Riboflavine (vitamin B2) | 5 mg |
| Pyridoxine (vitamin B6) | 5 mg | Pyridoxine (vitamin B6) | 5 mg |
| Cyanocobalamin (vitamin B12) | 10 mcg | Cyanocobalamin (vitamin B12) | 10 mcg |
| General Characteristics | Ferrous Bisglycinate Plus Folinic Acid | Control | P1 | P2 | P3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | Baseline | 3 Months | 6 Months | ||||
| Age (year) | 27.80 ± 3.66 | - | - | 29.11 ± 3.41 | - | - | 0.057 | - | - |
| Weight (kg) | 51.74 ± 4.77 | 57.08 ± 5.07 | 64.43 ± 5.44 | 49.04 ± 5.15 | 55.63 ± 5.57 | 61.90 ± 6.01 | 0.060 | 0.160 | 0.052 |
| Body mass index (kg/m2) | 20.81 ± 1.62 | 22.96 ± 1.74 | 25.92 ± 1.94 | 19.65 ± 1.49 | 22.29 ± 1.59 | 24.81 ± 1.64 | 0.054 | 0.059 | 0.057 |
| Blood pressure (mmHg) | |||||||||
| Systolic | 116.36 ± 7.28 | 118.04 ± 6.00 | 119.02 ± 6.14 | 114.43 ± 7.40 | 115.11 ± 6.15 | 117.13 ± 5.42 | 0.175 | 0.074 | 0.094 |
| Diastolic | 72.84 ± 6.48 | 73.65 ± 5.65 | 73.62 ± 5.73 | 73.96 ± 6.22 | 74.94 ± 5.48 | 75.64 ± 5.08 | 0.360 | 0.232 | 0.055 |
| Pulse rate (bpm) | 90.763 ± 4.80 | 92.05 ± 3.76 | 93.27 ± 2.85 | 92.19 ± 4.59 | 93.04 ± 4.37 | 92.87 ± 2.69 | 0.118 | 0.212 | 0.450 |
| hs-CRP (mg/L) | 0.91 ± 0.33 | 0.88 ± 0.28 | 0.87 ± 0.23 | 0.80 ± 0.28 | 0.85 ± 0.24 | 0.81 ± 0.22 | 0.054 | 0.583 | 0.208 |
| Time | Serum Iron (µg/dL) | P | |
|---|---|---|---|
| Ferrous Bisglycinate Plus Folinic Acid | Control | ||
| Fasting | 35.13 ± 5.35 | 34.85 ± 4.33 | 0.762 |
| 1 h | 104.63 ± 11.86 | 88.16 ± 6.64 | <0.001 |
| 2 h | 142.07 ± 8.78 | 120.76 ± 9.70 | <0.001 |
| Iron Status | Baseline | 3 Months after Supplementation | 6 Months after Supplementation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ferrous Bisglycinate Plus Folinic Acid | Control | P | Ferrous Bisglycinate Plus Folinic Acid | Control | P | Ferrous Bisglycinate Plus Folinic Acid | Control | P | |
| Hb (g/dL) | 10.04 ± 0.83 | 10.17 ± 0.77 | 0.415 | 12.40 ± 0.68 | 11.78 ± 0.72 | <0.001 | 12.82 ± 0.66 | 12.09 ± 0.60 | <0.001 |
| Mean Change | 2.356 ± 0.69 | 1.61 ± 0.838 | <0.001 | 2.78 ± 0.822 | 1.92 ± 0.89 | <0.001 | |||
| Erythrocytes (×1012/L) | 2.23 ± 0.42 | 2.11 ± 0.30 | 0.066 | 3.93 ± 0.437 | 3.23 ± 0.36 | <0.001 | 4.22 ± 0.30 | 3.41 ± 0.317 | <0.001 |
| Mean Change | 1.69 ± 0.581 | 1.12 ± 0.33 | <0.001 | 1.98 ± 0.49 | 1.30 ± 0.35 | <0.001 | |||
| Reticulocytes (×109/L) | 45.98 ± 4.53 | 43.15 ± 4.15 | 0.051 | 63.71 ± 6.69 | 55.77 ± 3.88 | <0.001 | 69.91 ± 6.32 | 57.70 ± 4.48 | <0.001 |
| Mean Change | 17.73 ± 7.99 | 12.62 ± 4.03 | <0.001 | 23.93 ± 8.12 | 14.55 ± 5.02 | <0.001 | |||
| MCV (fL) | 72.76 ± 3.77 | 70.38 ± 3.67 | 0.061 | 79.55 ± 3.70 | 75.81 ± 3.01 | <0.001 | 81.75 ± 3.37 | 78.91 ± 3.38 | <0.001 |
| Mean Change | 6.78 ± 4.45 | 5.43 ± 3.00 | 0.067 | 8.98 ± 4.72 | 8.53 ± 4.24 | 0.601 | |||
| MCH (pg) | 26.84 ± 3.19 | 25.59 ± 2.54 | 0.059 | 33.73 ± 1.83 | 30.06 ± 2.04 | <0.001 | 34.64 ± 1.52 | 31.35 ± 2.00 | <0.001 |
| Mean Change | 4.8 ± 2.489 | 3.905 ± 1.757 | 0.033 | 5.909 ± 2.619 | 4.981 ± 2.240 | 0.051 | |||
| MCHC (g/dL) | 26.84 ± 3.19 | 25.59 ± 2.54 | 0.059 | 33.73 ± 1.83 | 30.06 ± 2.04 | <0.001 | 34.64 ± 1.52 | 31.35 ± 2.00 | <0.001 |
| Mean Change | 6.89 ± 3.51 | 4.46 ± 1.86 | <0.001 | 7.81 ± 3.49 | 5.76 ± 2.98 | 0.002 | |||
| Transferrin Saturation (%) | 19.90 ± 3.62 | 20.68 ± 2.88 | 0.1598 | 34.78 ± 3.92 | 28.96 ± 1.92 | <0.001 | 36.47 ± 3.59 | 30.08 ± 2.11 | <0.001 |
| Mean Change | 14.88 ± 3.62 | 8.28 ± 3.457 | <0.001 | 16.58 ± 3.76 | 9.40 ± 3.43 | <0.001 | |||
| Ferritin (µg/L) | 25.63 ± 3.128 | 23.90 ± 3.31 | 0.060 | 38.70 ± 4.04 | 30.12 ± 2.91 | <0.001 | 40.45 ± 3.13 | 31.02 ± 1.70 | <0.001 |
| Mean Change | 13.077 ± 2.886 | 6.218 ± 1.86 | <0.001 | 14.78 ± 3.305 | 7.119 ± 3.18 | <0.001 | |||
| Side Effects | Ferrous Bisglycinate Plus Folinic Acid n (%) | Control n (%) | p |
|---|---|---|---|
| Nausea | |||
| - No | 51 (92.73) | 28 (52.83) | <0.001 |
| - Yes | 4 (7.27) | 25 (47.17) | |
| Vomiting | |||
| - No | 54 (98.18) | 47 (88.68) | 0.058 |
| - Yes | 1 (1.82) | 6 (11.32) | |
| Abdominal pain | |||
| - No | 53 (96.36) | 32 (60.38) | <0.001 |
| - Yes | 2 (3.64) | 21 (39.62) | |
| Bloating | |||
| - No | 53 (96.36) | 34 (64.15) | <0.001 |
| - Yes | 2 (3.64) | 19 (35.85) | |
| Constipation | |||
| - No | 51 (92.73) | 17 (32.08) | <0.001 |
| - Yes | 4 (7.27) | 36 (67.92) | |
| Diarrhea | |||
| - No | 54 (98.18) | 50 (94.34) | 0.359 |
| - Yes | 1 (1.82) | 3 (5.66) | |
| Metallic taste | |||
| - No | 53 (96.36) | 29 (54.72) | <0.001 |
| - Yes | 2 (3.64) | 24 (45.28) |
| Quality of Life | Ferrous Bisglycinate Plus Folinic Acid | Control | P1 | P2 | P3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | Baseline | 3 Months | 6 Months | ||||
| QOL-score | 3.13 ± 0.45 a | 3.03 ± 0.44 b | 2.93 ± 0.47 c | 2.96 ± 0.42 a | 2.83 ± 0.38 b | 2.69 ± 0.38 c | 0.039 | 0.013 | 0.004 |
| Iron Supplementation | P | ||
|---|---|---|---|
| Ferrous Bisglycinate Plus Folinic Acid | Control | ||
| Newborn weight (g) | 3103.82 ± 270.85 | 2992.26 ± 254.86 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumrungpert, A.; Pavadhgul, P.; Piromsawasdi, T.; Mozafari, M.R. Efficacy and Safety of Ferrous Bisglycinate and Folinic Acid in the Control of Iron Deficiency in Pregnant Women: A Randomized, Controlled Trial. Nutrients 2022, 14, 452. https://doi.org/10.3390/nu14030452
Bumrungpert A, Pavadhgul P, Piromsawasdi T, Mozafari MR. Efficacy and Safety of Ferrous Bisglycinate and Folinic Acid in the Control of Iron Deficiency in Pregnant Women: A Randomized, Controlled Trial. Nutrients. 2022; 14(3):452. https://doi.org/10.3390/nu14030452
Chicago/Turabian StyleBumrungpert, Akkarach, Patcharanee Pavadhgul, Theera Piromsawasdi, and M. R. Mozafari. 2022. "Efficacy and Safety of Ferrous Bisglycinate and Folinic Acid in the Control of Iron Deficiency in Pregnant Women: A Randomized, Controlled Trial" Nutrients 14, no. 3: 452. https://doi.org/10.3390/nu14030452
APA StyleBumrungpert, A., Pavadhgul, P., Piromsawasdi, T., & Mozafari, M. R. (2022). Efficacy and Safety of Ferrous Bisglycinate and Folinic Acid in the Control of Iron Deficiency in Pregnant Women: A Randomized, Controlled Trial. Nutrients, 14(3), 452. https://doi.org/10.3390/nu14030452








