Effect of Alpha-S1-Casein Tryptic Hydrolysate and L-Theanine on Poor Sleep Quality: A Double Blind, Randomized Placebo-Controlled Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Investigational Product and Reagents
2.2. Study Design
2.3. Psychological Measurement using PSQI
2.4. Blood Pressure and Heart Rate Measurements
2.5. Salivary Cortisol Analysis
2.5.1. Sample Collection
2.5.2. Solid Phase Extraction
2.5.3. High-Performance Liquid Chromatography (HPLC) Analysis
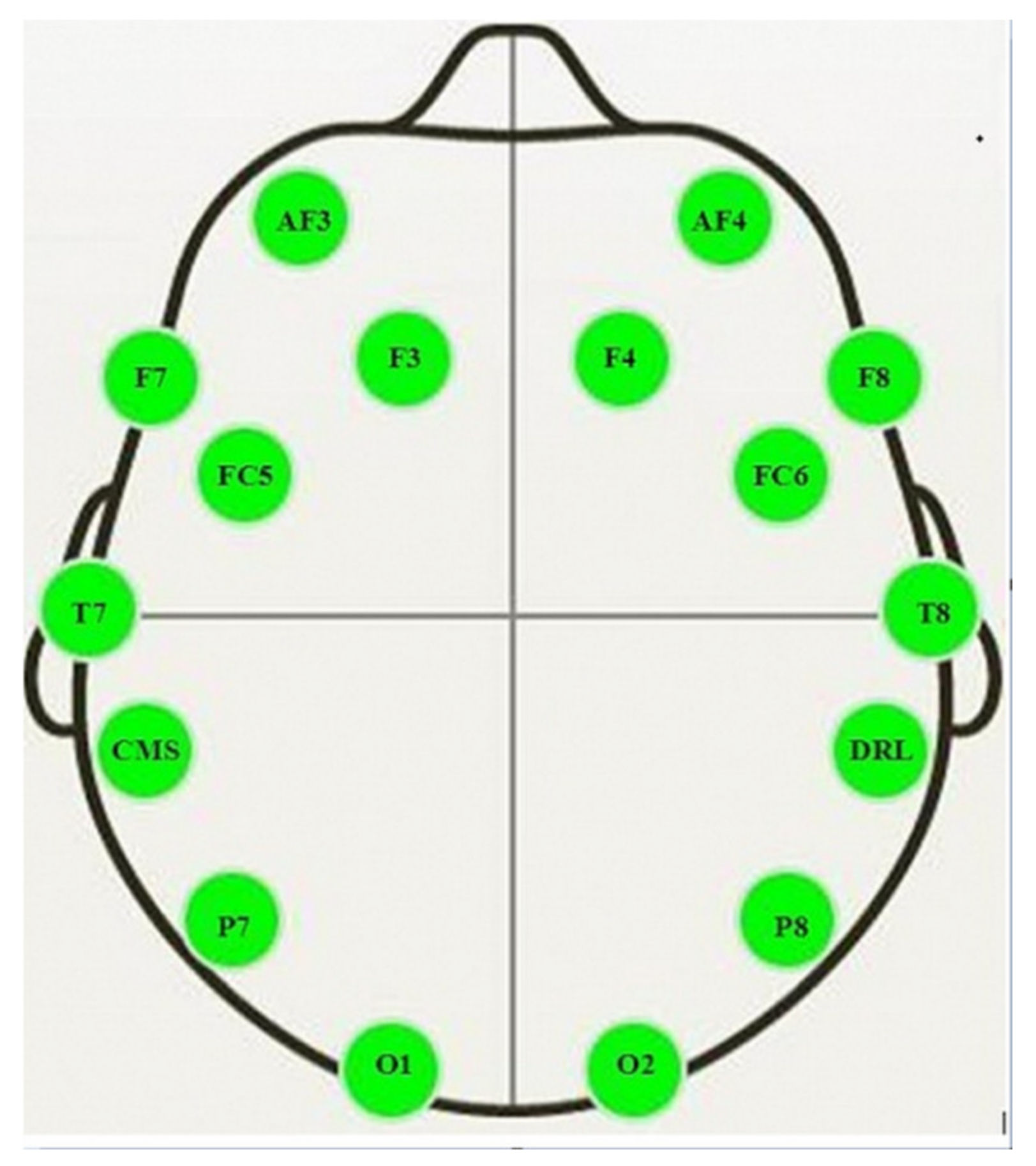
2.6. Electroencephalography (EEG) Analysis
2.7. Statistical Analysis
3. Results
Study Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pilcher, J.J.; Ginter, D.R.; Sadowsky, B. Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well-being and sleepiness in college students. J. Psychosom. Res. 1997, 42, 583–596. [Google Scholar] [CrossRef]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012, 16, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Edinger, J.D.; Bonnet, M.H.; Bootzin, R.R.; Doghramji, K.; Dorsey, C.M.; Espie, C.A.; Jamieson, A.O.; McCall, W.V.; Morin, C.M.; Stepanski, E.J. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep 2004, 27, 1567–1596. [Google Scholar] [CrossRef] [PubMed]
- Zailinawati, A.H.; Mazza, D.; Teng, C.L. Prevalence of insomnia and its impact on daily function amongst Malaysian primary care patients. Asia Pac. Fam. Med. 2012, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Roth, T. Insomnia: Definition, Prevalence, Etiology, and Consequences. J. Clin. Sleep Med. 2007, 3 (Suppl. S5), S7–S10. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, V.; Zakaria, R.; Othaman, Z.; Brzezinski, A.; Prasad, A.; Brown, G.M. Melatonergic drugs for therapeutic use in insomnia and sleep disturbances of mood disorders. CNS Neurol. Disord. Drug Targets 2012, 11, 180–189. [Google Scholar] [CrossRef]
- Bond, A.; Lader, M. Anxiolytics and Sedatives. In Drug Abuse and Addiction in Medical Illness; Verster, J., Brady, K., Galanter, M., Conrod, P., Eds.; Springer: New York, NY, USA, 2012; pp. 231–239. [Google Scholar]
- Procyshyn, R.M.; Bezchlibnyk-Butler, K.Z.; Jeffries, J.J. Clinical Handbook of Psychotropic Drugs; Hogrefe Publishing: Boston, MA, USA, 2021. [Google Scholar]
- Vincent, N.; Lionberg, C. Treatment preference and patient satisfaction in chronic insomnia. Sleep 2001, 24, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, M.; Lefranc–Millot, C.; Desor, D.; Demagny, B.; Bourdon, L. Effects of a tryptic hydrolysate from bovine milk α S1–Casein on hemodynamic responses in healthy human volunteers facing successive mental and physical stress situations. Eur. J. Nutr. 2004, 44, 128–132. [Google Scholar] [CrossRef]
- Luppi, P.H.; Peyron, C.; Fort, P. Not a single but multiple populations of GABAergic neurons control sleep. Sleep Med. Rev. 2017, 32, 85–94. [Google Scholar] [CrossRef]
- Masiulis, S.; Desai, R.; Uchański, T.; Martin, I.S.; Laverty, D.; Karia, D.; Malinauskas, T.; Zivanov, J.; Pardon, E.; Kotecha, A.; et al. GABA A receptor signalling mechanisms revealed by structural pharmacology. Nature 2019, 565, 454–459. [Google Scholar] [CrossRef]
- Yayeh, T.; Leem, Y.H.; Kim, K.M.; Jung, J.C.; Schwarz, J.; Oh, K.W.; Oh, S. Administration of alphas1-casein hydrolysate increases sleep and modulates GABAA receptor subunit expression. Biomol. Ther. 2018, 26, 268–273. [Google Scholar] [CrossRef]
- Giuseppe, C.; Sonia, C.; Lavinia, R.; Dell’Osso, B.; Liliana, D. Short-term anxiolytic and pro-hypnotic actvity of a tryptic hydrolysate of bovine αs1-casein in patients with anxiety spectrum disorders. Nutr. Food Sci. 2017, 2, 1–5. [Google Scholar]
- Kim, H.J.; Kim, J.; Lee, S.; Kim, B.; Kwon, E.; Lee, J.E.; Chun, M.Y.; Lee, C.Y.; Boulier, A.; Oh, S.; et al. A double-blind, randomized, placebo-controlled crossover clinical study of the effects of alpha-s1 casein hydrolysate on sleep disturbance. Nutrients 2019, 11, 1466. [Google Scholar] [CrossRef] [Green Version]
- Juneja, L.R.; Chu, D.C.; Okubo, T.; Nagato, Y.; Yokogoshi, H. L-theanine—A unique amino acid of green tea and its relaxation effect in humans. Trends. Food Sci. Technol. 1999, 10, 199–204. [Google Scholar] [CrossRef]
- Yoto, A.; Motoki, M.; Murao, S.; Yokogoshi, H. Effects of L-theanine or caffeine intake on changes in blood pressure under physical and psychological stresses. J. Physiol. Anthropol. 2012, 31, 28. [Google Scholar] [CrossRef] [Green Version]
- Zukhurova, M.; Prosvirnina, M.; Daineko, A.; Simanenkova, A.; Petrishchev, N.; Sonin, D.; Galagudza, M.; Shamtsyan, M.; Juneja, L.R.; Vlasov, T. L-theanine administration results in neuroprotection and prevents glutamate receptor agonist-mediated injury in the rat model of cerebral ischemia-reperfusion. Phytother. Res. 2013, 27, 1282–1287. [Google Scholar] [CrossRef]
- Adhikary, R.; Mandal, V. L-theanine: A potential multifaceted natural bioactive amide as health supplement. Asian Pac. J. Trop. Biomed. 2017, 7, 842–848. [Google Scholar] [CrossRef]
- Mason, R. 200 mg of Zen: L-theanine boosts alpha waves, promotes alert relaxation. Altern. Complement. Ther. 2001, 7, 91–95. [Google Scholar] [CrossRef]
- Kimura, K.; Ozeki, M.; Juneja, L.R.; Ohira, H. L-Theanine reduces psychological and physiological stress responses. Biol. Psychol. 2007, 74, 39–45. [Google Scholar] [CrossRef]
- Borzelleca, J.F.; Peters, D.; Hall, W. A 13-week dietary toxicity and toxicokinetic study with L-theanine in rats. Food. Chem. Toxicol. 2006, 44, 1158–1166. [Google Scholar] [CrossRef]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Benson, S.; Gibbs, A.; Perry, N.; Sarris, J.; Murray, G. Exploring the effect of Lactium™ and zizyphus complex on sleep quality: A double-blind, randomized placebo-controlled trial. Nutrients 2017, 9, 154. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Elio, F.; Antonelli, G.; Benetazzo, A.; Prearo, M.; Gatti, R. Human saliva cortisone and cortisol simultaneous analysis using reverse phase HPLC technique. Clin. Chim. Acta. 2009, 405, 60–65. [Google Scholar]
- Kawala-Janik, A.; Podpora, M.; Pelc, M.; Piatek, P.; Baranowski, J. Implementation of an inexpensive EEG headset for the pattern recognition purpose. In Proceedings of the 7th IEEE International Conference on Intelligent Data Acquisition and Advanced Computing Systems: Technology and Applications, Berlin, Germany, 12–14 September 2013. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Hilaire, Z.D.; Messaoudi, M.; Desor, D.; Kobayashi, T. Effects of a bovine alpha S1-Casein tryptic hydrolysate (CTH) on sleep disorder in Japanese general population. Open Sleep J. 2009, 2, 26–32. [Google Scholar] [CrossRef]
- Hidese, S.; Ogawa, S.; Ota, M.; Ishida, I.; Yasukawa, Z.; Ozeki, M.; Kunugi, H. Effects of L-theanine administration on stress-related symptoms and cognitive functions in healthy adults: A randomized controlled trial. Nutrients 2019, 11, 2362. [Google Scholar] [CrossRef] [Green Version]
- Evans, M.; McDonald, A.C.; Xiong, L.; Crowley, D.C.; Guthrie, N. A randomized, triple-blind, placebo-controlled, crossover study to investigate the efficacy of a single dose of AlphaWave® l-theanine on stress in a healthy adult population. Neurol. Ther. 2021, 10, 1061–1078. [Google Scholar] [CrossRef]
- Phing, C.H.; Chee, O.Y. Effects of alpha-s1-casein tryptic hydrolysate and l-theanine on sleep disorder and psychological components: A randomized, double-blind, placebo-controlled study. Malays. J. Public Health Med. 2019, 19, 47–55. [Google Scholar] [CrossRef]
- Abell, J.G.; Shipley, M.J.; Ferrie, J.E.; Kivimäki, M.; Kumari, M. Recurrent short sleep, chronic insomnia symptoms and salivary cortisol: A 10-year follow-up in the Whitehall II study. Psychoneuroendocrinology 2016, 68, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Yeganehzad, S.; Pahlevanloo, A.; Kiumarsi, M.; Zayerzadeh, A.; Sadjadi, S.A.; Shahidi, M.; Nadali, N. Effects of sugar-free, αs1-casein–enriched chocolate on stress: Based on salivary cortisol measurement and questionnaire data collection. J. Nutr. Sci. Diet 2018, 4, 10–15. [Google Scholar]
- Pal, P.K.; Thennarasu, K.; Fleming, J.; Schulzer, M.; Brown, T.; Calne, S.M. Nocturnal sleep disturbances and daytime dysfunction in patients with Parkinson’s disease and in their caregivers. Parkinsonism Relat. Disord. 2004, 10, 157–168. [Google Scholar] [CrossRef]
- Laudat, M.H.; Cerdas, S.; Fournier, C.; Guiban, D.; Guilhaume, B.; Luton, J.P. Salivary cortisol measurement: A practical approach to assess pituitary-adrenal function. J. Clin. Endocrinol. Metab. 1988, 66, 343–348. [Google Scholar] [CrossRef]
- Strijkstra, A.M.; Beersma, D.G.; Drayer, B.; Halbesma, N.; Daan, S. Subjective sleepiness correlates negatively with global alpha (8–12 Hz) and positively with central frontal theta (4–8 Hz) frequencies in the human resting awake electroencephalogram. Neurosci. Lett. 2003, 340, 17–20. [Google Scholar] [CrossRef]
- Wong, K.K.; Marshall, N.S.; Grunstein, R.R.; Dodd, M.J.; Rogers, N.L. Comparing the neurocognitive effects of 40 h sustained wakefulness in patients with untreated OSA and healthy controls. J. Sleep Res. 2008, 17, 322–330. [Google Scholar] [CrossRef]
- Fink, A.; Schwab, D.; Papousek, I. Sensitivity of EEG upper alpha activity to cognitive and affective creativity interventions. Int. J. Psychophysiol. 2011, 82, 233–239. [Google Scholar] [CrossRef]
- Qian, J.; Zheng, L.; Su, G.; Huang, M.; Luo, D.; Zhao, M. Identification and Screening of Potential Bioactive Peptides with Sleep-Enhancing Effects in Bovine Milk Casein Hydrolysate. J. Agric. Food Chem. 2021, 69, 11246–11258. [Google Scholar] [CrossRef]

| Variables | Mean ± SD (n = 39) | Range |
|---|---|---|
| Age (years) | 37.3 ± 8.4 | 26–65 |
| Systolic blood pressure (mmHg) | 117.92 ± 13.33 | 91–144 |
| Diastolic blood pressure (mmHg) | 75.82 ± 11.86 | 51–102 |
| Heart rate (beats per min) | 77.54 ± 8.26 | 63–100 |
| Primary Outcomes | Group | Treatments | Pre Mean ± SD | Post Mean ± SD | p-Value |
|---|---|---|---|---|---|
| PSQI Total Score | |||||
| Stage 1 | Group A | Supplement | 9.00 ± 2.27 | 5.83 ± 1.54 | <0.001 *** |
| Group B | Placebo | 7.90 ± 1.37 | 6.52 ± 2.40 | 0.026 * | |
| Stage 2 | Group A | Placebo | 5.56 ± 2.33 | 5.67 ± 2.40 | 0.834 |
| Group B | Supplement | 6.76 ± 2.39 | 4.95 ± 2.48 | <0.001 *** | |
| Total Sleeping Time (h) | |||||
| Stage 1 | Group A | Supplement | 5.28 ± 1.08 | 6.19 ± 0.69 | 0.003 ** |
| Group B | Placebo | 5.71 ± 1.02 | 6.21 ± 1.27 | 0.061 | |
| Stage 2 | Group A | Placebo | 5.97 ± 0.95 | 6.11 ± 0.95 | 0.514 |
| Group B | Supplement | 6.10 ± 1.28 | 6.71 ± 1.35 | 0.002 ** | |
| Component 1 Subjective Sleep Quality | |||||
| Stage 1 | Group A | Supplement | 1.72 ± 0.46 | 1.11 ± 0.58 | 0.004 ** |
| Group B | Placebo | 1.57 ± 0.60 | 1.24 ± 0.70 | 0.049 * | |
| Stage 2 | Group A | Placebo | 1.17 ± 0.62 | 1.06 ± 0.64 | 0.430 |
| Group B | Supplement | 1.38 ± 0.50 | 1.05 ± 0.74 | 0.016 * | |
| Component 2 Sleep Latency | |||||
| Stage 1 | Group A | Supplement | 1.67 ± 0.97 | 1.00 ± 0.77 | 0.004 ** |
| Group B | Placebo | 1.48 ± 0.68 | 1.10 ± 0.83 | 0.029 * | |
| Stage 2 | Group A | Placebo | 0.94 ± 1.06 | 0.94 ±1.00 | 1.000 |
| Group B | Supplement | 1.24 ± 0.94 | 0.90 ± 0.94 | 0.005 ** | |
| Component 3 Sleep Duration | |||||
| Stage 1 | Group A | Supplement | 1.89 ± 0.68 | 1.22 ± 0.43 | 0.001 ** |
| Group B | Placebo | 1.52 ± 0.97 | 1.24 ± 0.89 | 0.137 | |
| Stage 2 | Group A | Placebo | 1.28 ± 0.75 | 1.39 ± 0.70 | 0.495 |
| Group B | Supplement | 1.33 ± 0.97 | 0.95 ± 0.80 | 0.008 ** | |
| Component 4 Sleep Habitual Efficiency | |||||
| Stage 1 | Group A | Supplement | 1.06 ± 1.00 | 0.17 ± 0.38 | 0.003 ** |
| Group B | Placebo | 0.67 ± 0.73 | 0.52 ± 0.87 | 0.576 | |
| Stage 2 | Group A | Placebo | 0.33 ± 0.49 | 0.44 ± 0.62 | 0.542 |
| Group B | Supplement | 0.62 ± 0.86 | 0.33 ± 0.66 | 0.030 * | |
| Component 5 Sleep Disturbances | |||||
| Stage 1 | Group A | Supplement | 1.56 ± 0.51 | 1.39 ± 0.70 | 0.269 |
| Group B | Placebo | 1.48 ± 0.51 | 1.33 ± 0.48 | 0.329 | |
| Stage 2 | Group A | Placebo | 1.17 ± 0.62 | 1.06 ± 0.54 | 0.495 |
| Group B | Supplement | 1.10 ± 0.30 | 1.05 ± 0.50 | 0.666 | |
| Component 7 Daytime Dysfunction | |||||
| Stage 1 | Group A | Supplement | 1.11 ± 0.58 | 1.00 ± 0.49 | 0.331 |
| Group B | Placebo | 1.14 ± 0.48 | 1.10 ± 0.77 | 0.789 | |
| Stage 2 | Group A | Placebo | 0.72 ± 0.57 | 0.78 ± 0.73 | 0.668 |
| Group B | Supplement | 1.14 ± 0.73 | 0.67 ± 0.58 | <0.001 *** | |
| Systolic Blood Pressure (mmHg) | |||||
| Stage 1 | Group A | Supplement | 118.61 ± 12.50 | 121.56 ± 12.24 | 0.330 |
| Group B | Placebo | 117.33 ± 14.30 | 112.52 ± 19.07 | 0.117 | |
| Stage 2 | Group A | Placebo | 123.11 ± 14.44 | 119.17 ± 11.95 | 0.077 |
| Group B | Supplement | 116.62 ± 12.99 | 115.24 ± 13.51 | 0.366 | |
| Diastolic Blood Pressure (mmHg) | |||||
| Stage 1 | Group A | Supplement | 75.78 ± 10.23 | 75.61 ± 7.89 | 0.933 |
| Group B | Placebo | 75.86 ± 13.35 | 74.05 ± 11.23 | 0.289 | |
| Stage 2 | Group A | Placebo | 77.39 ± 10.39 | 75.28 ±7.37 | 0.172 |
| Group B | Supplement | 74.90 ± 12.63 | 73.90 ± 10.93 | 0.492 | |
| Heart Rate (beats per min) | |||||
| Stage 1 | Group A | Supplement | 76.39 ± 7.37 | 75.06 ± 7.63 | 0.451 |
| Group B | Placebo | 78.52 ± 9.60 | 81.00 ± 8.68 | 0.210 | |
| Stage 2 | Group A | Placebo | 76.72 ± 9.14 | 75.33 ± 7.84 | 0.503 |
| Group B | Supplement | 78.90 ± 11.56 | 78.86 ± 11.93 | 0.985 | |
| Cortisol Concentration (nmol/L) | |||||
| Stage 1 | Group A | Supplement | 33.85 ± 14.42 | 32.85 ± 18.35 | 0.871 |
| Group B | Placebo | 27.70 ± 10.41 | 27.33 ± 14.46 | 0.893 | |
| Stage 2 | Group A | Placebo | 31.25 ± 21.70 | 31.82 ± 21.96 | 0.927 |
| Group B | Supplement | 21.57 ± 12.92 | 14.11 ± 9.44 | 0.007 ** | |
| Total Alpha Power (uV2/Hz) | |||||
| Stage 1 | Group A | Supplement | 4.83 ± 5.31 | 14.95 ± 21.50 | 0.031 * |
| Group B | Placebo | 7.79 ± 11.48 | 8.75 ± 11.86 | 0.831 | |
| Stage 2 | Group A | Placebo | 10.22 ± 14.74 | 9.04 ± 12.63 | 0.785 |
| Group B | Supplement | 8.75 ± 8.73 | 11.86 ± 12.69 | 0.337 | |
| Frontal Alpha Power (uV2/Hz) | |||||
| Stage 1 | Group A | Supplement | 3.53 ± 4.63 | 20.05 ± 34.28 | 0.037 * |
| Group B | Placebo | 6.02 ± 6.33 | 7.82 ± 9.73 | 0.381 | |
| Stage 2 | Group A | Placebo | 11.40 ± 15.06 | 10.67 ± 15.77 | 0.866 |
| Group B | Supplement | 8.53 ± 9.87 | 14.43 ± 15.96 | 0.088 | |
| Variables | Pre Mean ± SD | Post Mean ± SD | Paired t-Test p-Value | Pre Mean ± SD | Post Mean ± SD | Paired t-Test p-Value | Value Changes ± SD | Student t-Test p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Supplement | Placebo | Supplement | Placebo | ||||||
| PSQI Total Score | 7.79 ± 2.57 | 5.40 ± 2.12 | <0.001 *** | 6.82 ± 2.20 | 6.13 ± 2.41 | 0.096 | 2.44 ± 1.94 | 0.69 ± 2.54 | 0.001 ** |
| Total Sleeping Hours | 5.72 ± 1.25 | 6.47 ± 1.11 | <0.001 *** | 5.83 ± 0.98 | 6.16 ± 1.12 | 0.053 | −0.76 ± 0.96 | −0.33 ± 1.03 | 0.063 |
| C1 (Subjective Sleep Quality) | 1.54 ± 0.51 | 1.08 ± 0.66 | <0.001 *** | 1.38 ± 0.63 | 1.15 ± 0.67 | 0.037 * | 0.46 ± 0.68 | 0.23 ± 0.67 | 0.135 |
| C2 (Sleep Latency) | 1.43 ± 0.97 | 0.948 ± 0.86 | <0.001 *** | 1.23 ± 0.90 | 1.03 ± 0.90 | 0.088 | 0.49 ± 0.68 | 0.21 ± 0.73 | 0.083 |
| C3 (Sleep Duration) | 1.59 ± 0.88 | 1.08 ± 0.66 | <0.001 *** | 1.41 ± 0.79 | 1.331 ± 0.80 | 0.421 | 0.51 ± 0.64 | 0.10 ± 0.79 | 0.014 * |
| C4 (Habitual Sleep Efficiency) | 0.82 ± 0.94 | 0.26 ± 0.55 | <0.001 *** | 0.51 ± 0.64 | 0.49 ± 0.76 | 0.872 | 0.56 ± 0.88 | 0.03 ± 0.99 | 0.013 * |
| C5 (Sleep Disturbances) | 1.31 ± 0.47 | 1.21 ± 0.61 | 0.253 | 1.33 ± 0.58 | 1.21 ± 0.52 | 0.230 | 0.10 ± 0.55 | 0.13 ± 0.66 | 0.852 |
| C7 (Daytime Dysfunction) | 1.13 ± 0.66 | 0.82 ± 0.56 | 0.001 ** | 0.95 ± 0.56 | 0.95 ± 0.76 | 1.000 | 0.31 ± 0.52 | 0.00 ± 0.69 | 0.029 * |
| Systolic Blood Pressure (mmHg) | 117.54 ± 12.64 | 118.15 ± 13.17 | 0.701 | 120.00 ± 14.47 | 115.59 ± 16.33 | 0.021 * | −0.62 ± 9.94 | 4.41 ± 11.44 | 0.042 * |
| Diastolic Blood Pressure (mmHg) | 75.31 ± 11.45 | 74.69 ± 9.56 | 0.601 | 76.56 ± 11.95 | 74.61 ± 9.54 | 0.088 | 0.62 ± 7.28 | 1.95 ± 6.94 | 0.410 |
| Heart Rate (beats per min) | 77.74 ± 9.51 | 77.10 ± 10.23 | 0.687 | 77.69 ± 9.31 | 78.38 ± 8.68 | 0.626 | 0.64 ± 9.87 | −0.69 ± 8.80 | 0.531 |
| Cortisol (nmol/L) | 27.24 ± 14.81 | 22.76 ± 16.94 | 0.158 | 29.34 ± 16.46 | 29.40 ± 18.19 | 0.984 | 4.48 ± 19.43 | −0.62 ± 19.56 | 0.307 |
| Total Alpha Power (uV2/Hz) | 6.95 ± 7.53 | 13.28 ± 17.11 | 0.022 * | 8.91 ±12.95 | 8.68 ±11.25 | 0.920 | −6.33 ± 16.03 | 0.23 ± 14.11 | 0.066 |
| Frontal Alpha Power (uV2/Hz) | 6.23 ± 8.21 | 17.01 ± 25.78 | 0.008 ** | 8.49 ± 11.37 | 9.13 ± 12.75 | 0.777 | −10.78 ± 23.25 | −0.63 ± 13.49 | 0.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiagarajah, K.; Chee, H.P.; Sit, N.W. Effect of Alpha-S1-Casein Tryptic Hydrolysate and L-Theanine on Poor Sleep Quality: A Double Blind, Randomized Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 652. https://doi.org/10.3390/nu14030652
Thiagarajah K, Chee HP, Sit NW. Effect of Alpha-S1-Casein Tryptic Hydrolysate and L-Theanine on Poor Sleep Quality: A Double Blind, Randomized Placebo-Controlled Crossover Trial. Nutrients. 2022; 14(3):652. https://doi.org/10.3390/nu14030652
Chicago/Turabian StyleThiagarajah, Kokila, Huei Phing Chee, and Nam Weng Sit. 2022. "Effect of Alpha-S1-Casein Tryptic Hydrolysate and L-Theanine on Poor Sleep Quality: A Double Blind, Randomized Placebo-Controlled Crossover Trial" Nutrients 14, no. 3: 652. https://doi.org/10.3390/nu14030652






