Applying Zinc Nutrient Reference Values as Proposed by Different Authorities Results in Large Differences in the Estimated Prevalence of Inadequate Zinc Intake by Young Children and Women and in Cameroon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Settings and Design
2.2. Dietary Modeling
2.2.1. Zinc NRVs
2.2.2. Zinc Absorption
2.2.3. Simulation of Effects of Zinc Fortification
2.2.4. Estimation of Usual Intake
2.2.5. Fortification Program Costs and Cost-Effectiveness
3. Results
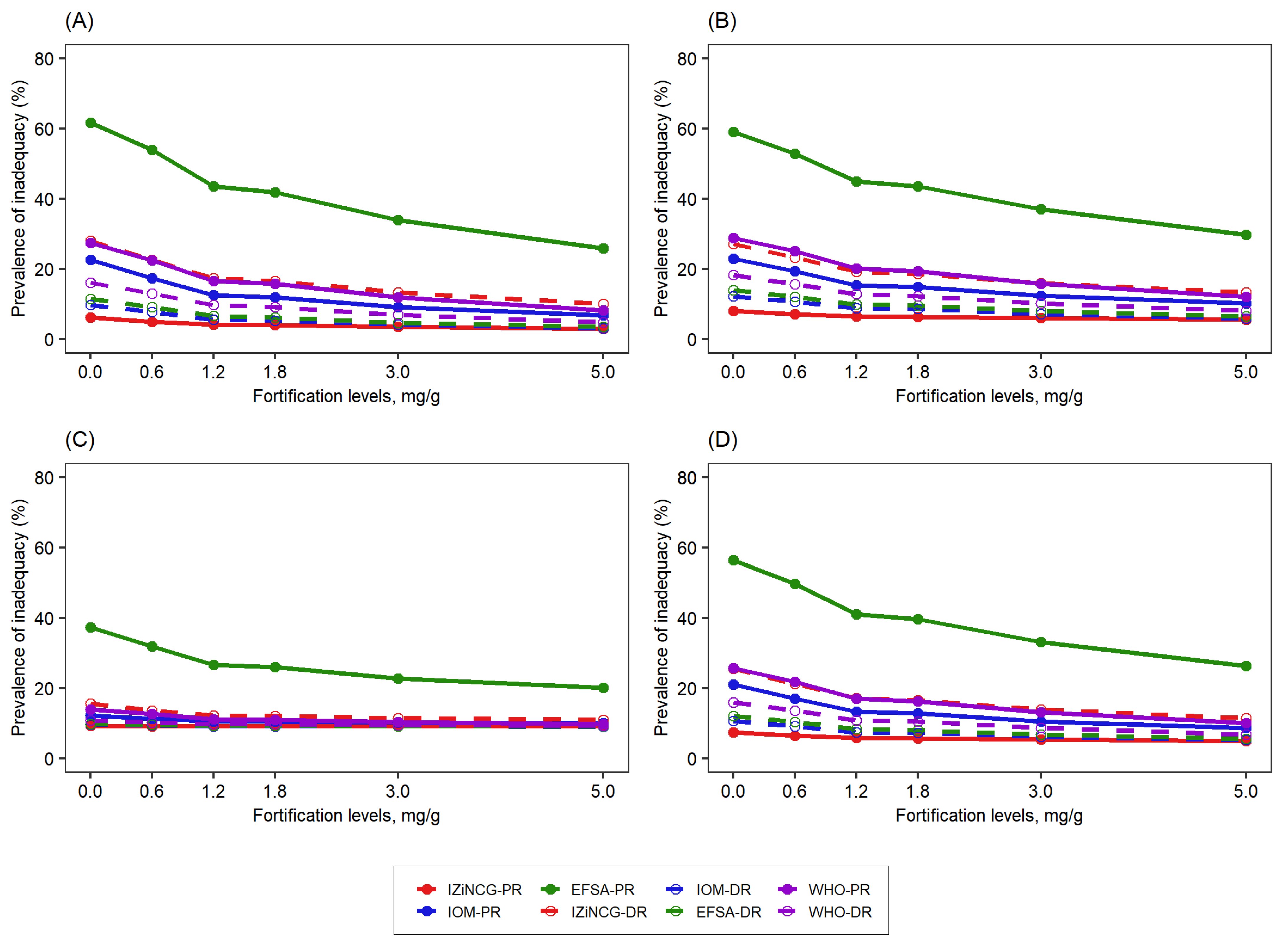
3.1. Effects of Using Alternative Zinc NRVs on Estimated Prevalence of Inadequate Zinc Intake
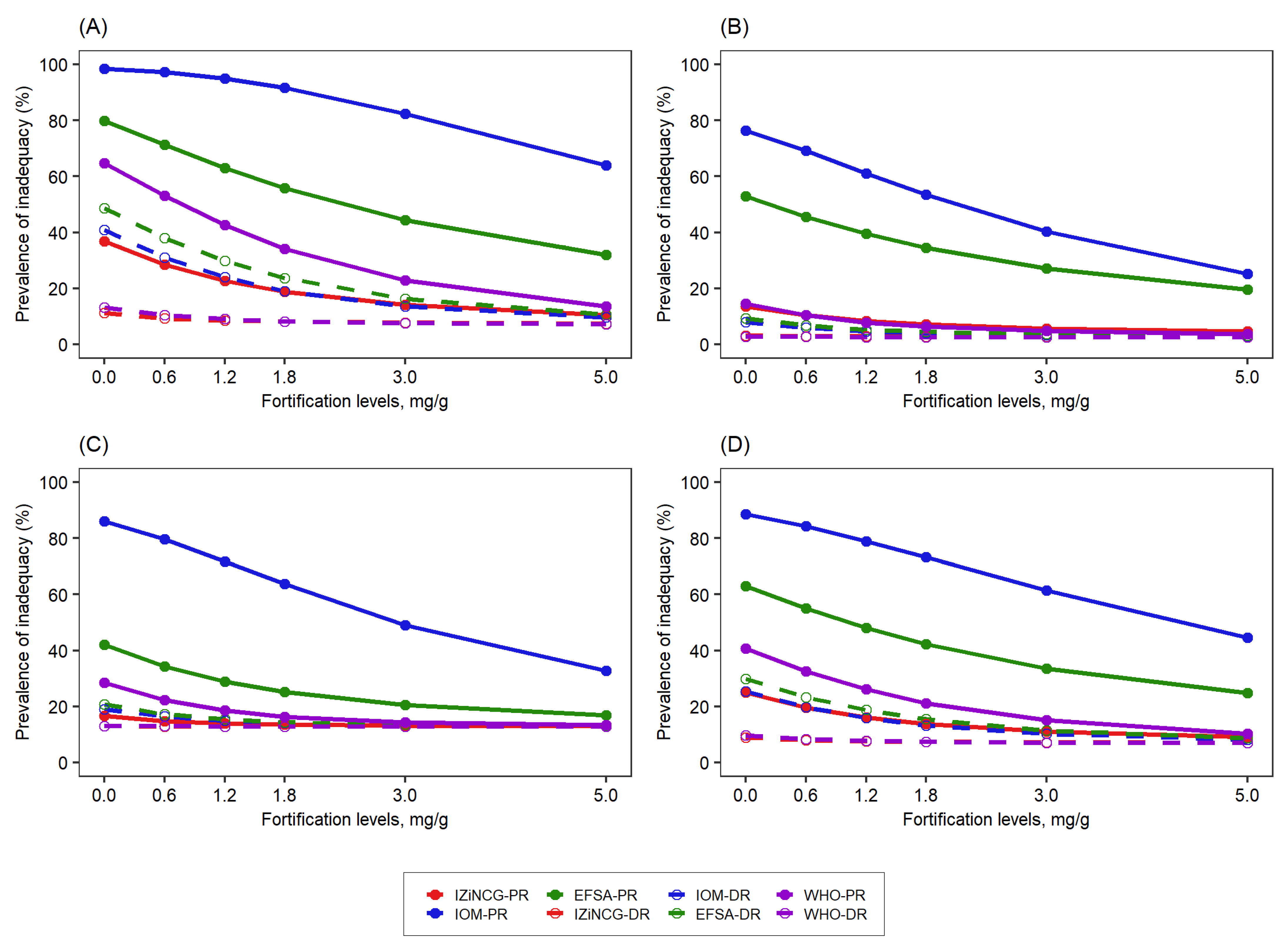
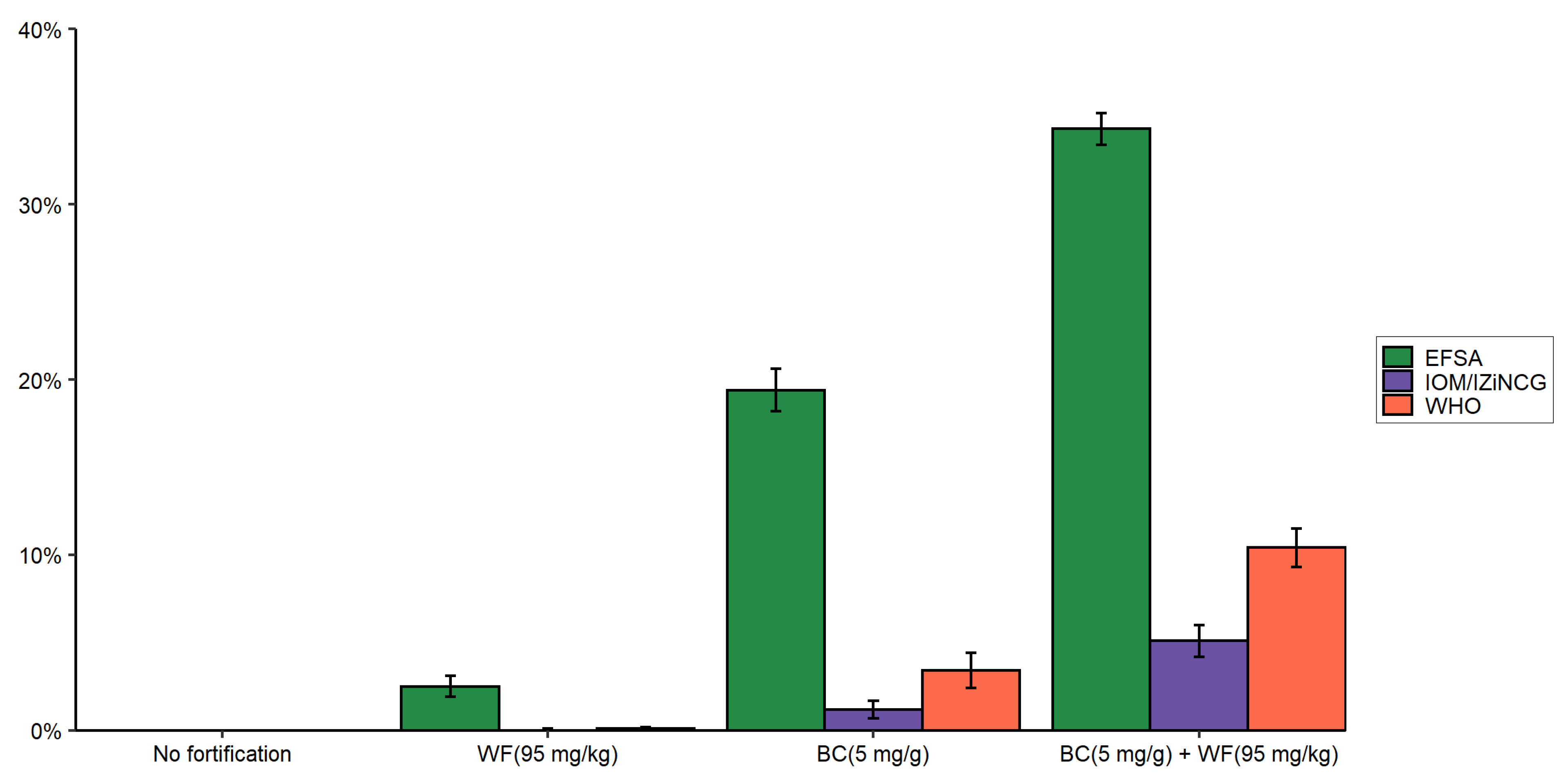
3.2. Effects of Using Alternative Zinc NRVs on the Predicted Reduction in the Prevalence of Zinc Inadequacy Due to the Presence of Zinc Fortification Programs and Simulated Levels of Fortification
3.3. Effects of Using Alternative Zinc NRVs on Cost-Effectiveness of Zinc Fortification Programs
3.4. Effects of Using Alternative Zinc UL Values on Prevalence of Zinc Intake above the UL Due to Zinc Fortification Programs
4. Discussion
5. Conclusions and Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, D.; Sachdev, H.P.S. Effect of gestational zinc deficiency on pregnancy outcomes: Summary of observation studies and zinc supplementation trials. Br. J. Nutr. 2001, 85, S101–S108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rerksuppaphol, S.; Rerksuppaphol, L. Zinc supplementation enhances linear growth in school-aged children: A randomized controlled trial. Pediatr. Rep. 2018, 9, 7294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-G.; Choi, H.-N.; Yang, H.-R.; Yim, J.-E. Effects of zinc supplementation on catch-up growth in children with failure to thrive. Nutr. Res. Pract. 2017, 11, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, E.; Mori, R.; Middleton, P.; Tobe-Gai, R.; Mahomed, K.; Miyazaki, C.; Bhutta, Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2015, 2015, CD000230. [Google Scholar] [CrossRef]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc deficiency in low- and middle-income countries: Prevalence and approaches for mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef]
- Hess, S.Y. National Risk of Zinc Deficiency as Estimated by National Surveys. Food Nutr. Bull. 2017, 38, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Victora, C.G.; Christian, P.; Vidaletti, L.P.; Gatica-Domínguez, G.; Menon, P.; Black, R.E. Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet 2021, 397, 1388–1399. [Google Scholar] [CrossRef]
- Belay, A.; Gashu, D.; Joy, E.J.M.; Lark, R.M.; Chagumaira, C.; Likoswe, B.H.; Zerfu, D.; Ander, E.L.; Young, S.D.; Bailey, E.H.; et al. Zinc deficiency is highly prevalent and spatially dependent over short distances in Ethiopia. Sci. Rep. 2021, 11, 6510. [Google Scholar] [CrossRef]
- Gibson, R.S.; Anderson, V.P. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr. Bull. 2009, 30, S108–S143. [Google Scholar] [CrossRef]
- Hess, S.Y.; Brown, K.H. Impact of zinc fortification on zinc nutrition. Food Nutr. Bull. 2009, 30, S79–S107. [Google Scholar] [CrossRef]
- Shah, D.; Sachdev, H.S.; Gera, T.; De-Regil, L.M.; Peña-Rosas, J.P. Fortification of staple foods with zinc for improving zinc status and other health outcomes in the general population. Cochrane Database Syst. Rev. 2016, 2016, Cd010697. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Kumar, R.; Salam, R.A.; Bhutta, Z.A. Systematic Review of Zinc Fortification Trials. Ann. Nutr. Metab. 2013, 62 (Suppl. S1), 44–56. [Google Scholar] [CrossRef] [PubMed]
- Tsang, B.L.; Holsted, E.; McDonald, C.M.; Brown, K.H.; Black, R.; Mbuya, M.N.N.; Grant, F.; Rowe, L.A.; Manger, M.S. Effects of Foods Fortified with Zinc, Alone or Cofortified with Multiple Micronutrients, on Health and Functional Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1821–1837. [Google Scholar] [CrossRef]
- Keats, E.C.; Das, J.K.; Salam, R.A.; Lassi, Z.S.; Imdad, A.; Black, R.E.; Bhutta, Z.A. Effective interventions to address maternal and child malnutrition: An update of the evidence. Lancet Child Adolesc. Health 2021, 5, 367–384. [Google Scholar] [CrossRef]
- WHO/FAO. Guidelines on Food Fortification with Micronutrients. 2006. Available online: https://www.who.int/publications/i/item/9241594012 (accessed on 12 November 2021).
- Katapodis, N.D.; Zhang, D.; Giabbanelli, P.J.; Li, Y.; Lyford, C.P.; Rajbhandari-Thapa, J. Evaluating the Impact of Improving Access on Consumption of Fruits and Vegetables in a Rural Community in Texas: A Modeling Study. Health Equity 2019, 3, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Dodd, K.W.; Arnold, C.D.; Engle-Stone, R. Introduction to the SIMPLE Macro, a Tool to Increase the Accessibility of 24-Hour Dietary Recall Analysis and Modeling. J. Nutr. 2021, 151, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Coates, J.C.; Colaiezzi, B.A.; Bell, W.; Charrondiere, U.R.; Leclercq, C. Overcoming Dietary Assessment Challenges in Low-Income Countries: Technological Solutions Proposed by the International Dietary Data Expansion (INDDEX) Project. Nutrients 2017, 9, 289. [Google Scholar] [CrossRef] [Green Version]
- Vosti, S.A.; Kagin, J.; Engle-Stone, R.; Luo, H.; Tarini, A.; Clermont, A.; Assiene, J.G.; Nankap, M.; Brown, K.H. Strategies to achieve adequate vitamin A intake for young children: Options for Cameroon. Ann. N. Y. Acad. Sci. 2020, 1465, 161–180. [Google Scholar] [CrossRef]
- Kagin, J.; Vosti, S.A.; Engle-Stone, R.; Rettig, E.; Brown, K.H.; Nankap, M.; Ndjebayi, A. Measuring the Costs of Vitamin A Interventions: Institutional, Spatial, and Temporal Issues in the Context of Cameroon. Food Nutr. Bull. 2015, 36, S172–S192. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.H.; Engle-Stone, R.; Kagin, J.; Rettig, E.; Vosti, S.A. Use of Optimization Modeling for Selecting National Micronutrient Intervention Strategies: An Example Based on Potential Programs for Control of Vitamin A Deficiency in Cameroon. Food Nutr. Bull. 2015, 36, S141–S148. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.S.; King, J.C.; Lowe, N. A Review of Dietary Zinc Recommendations. Food Nutr. Bull. 2016, 37, 443–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.V.; Krebs, N.F.; Hambidge, K.M. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J. Nutr. 2007, 137, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.V.; Hambidge, K.M.; Krebs, N.F. Zinc Absorption Is Not Related to Dietary Phytate Intake in Infants and Young Children Based on Modeling Combined Data from Multiple Studies. J. Nutr. 2015, 145, 1763–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engle-Stone, R.; Nankap, M.; Ndjebayi, A.O.; Erhardt, J.G.; Brown, K.H. Plasma ferritin and soluble transferrin receptor concentrations and body iron stores identify similar risk factors for iron deficiency but result in different estimates of the national prevalence of iron deficiency and iron-deficiency anemia among women and children in Cameroon. J. Nutr. 2013, 143, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Schakel, S.F.; Sievert, Y.A.; Buzzard, I.M. Sources of data for developing and maintaining a nutrient database. J. Am. Diet. Assoc. 1988, 88, 1268–1271. [Google Scholar] [CrossRef]
- Hotz, C.; Abdelrahman, L.; Sison, C.; Moursi, M.; Loechl, C. A Food Composition Table for Central and Eastern Uganda. HarvestPlus Technical Monograph 9. Washington and Cali, Colombia: International Food Policy Research Institute (IFPRI) and International Center for Tropical Agriculture (CIAT). 2012. Available online: https://www.harvestplus.org/sites/default/files/publications/Tech_Mono_9_Web_1_0.pdf (accessed on 14 December 2020).
- Sharma, S.; Mbanya, J.C.; Cruickshank, K.; Cade, J.; Tanya, A.K.N.; Cao, X.; Hurbos, M.; Wong, M.R.K.M. Nutritional composition of commonly consumed composite dishes from the Central Province of Cameroon. Int. J. Food Sci. Nutr. 2007, 58, 475–485. [Google Scholar] [CrossRef]
- Engle-Stone, R.; Nankap, M.; Ndjebayi, A.O.; Brown, K.H. Simulations based on representative 24-h recall data predict region-specific differences in adequacy of vitamin A intake among Cameroonian women and young children following large-scale fortification of vegetable oil and other potential food vehicles. J. Nutr. 2014, 144, 1826–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engle-Stone, R.; Ndjebayi, A.O.; Nankap, M.; Brown, K.H. Consumption of potentially fortifiable foods by women and young children varies by ecological zone and socio-economic status in Cameroon. J. Nutr. 2012, 142, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Hambidge, K.M.; Miller, L.V.; Krebs, N.F. Physiological requirements for zinc. Int. J. Vitam. Nutr. Res. 2011, 81, 72–78. [Google Scholar] [CrossRef] [Green Version]
- WHO/FAO. Vitamin and Mineral Requirement in Human Nutrition, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: http://apps.who.int/iris/bitstream/handle/10665/42716/9241546123.pdf;jsessionid=BF40F86437F026BD28D5FA318F4C8D62?sequence=1 (accessed on 4 April 2021).
- Taylor, C.M.; Bacon, J.R.; Aggett, P.J.; Bremner, I. Homeostatic regulation of zinc absorption and endogenous losses in zinc-deprived men. Am. J. Clin. Nutr. 1991, 53, 755–763. [Google Scholar] [CrossRef]
- Milne, D.B.; Canfield, W.K.; Gallagher, S.K.; Hunt, J.R.; Klevay, L.M. Ethanol metabolism in postmenopausal women fed a diet marginal in zinc. Am. J. Clin. Nutr. 1987, 46, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization, International Atomic Energy Agency & Food and Agriculture Organization of the United Nations. Trace Elements in Human Nutrition and Health. World Health Organization, 1996. Available online: https://apps.who.int/iris/handle/10665/37931 (accessed on 5 April 2021).
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222317 (accessed on 11 December 2020).
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for zinc. EFSA J. 2014, 12, 3844. [Google Scholar] [CrossRef] [Green Version]
- Fischer, P.W.; Giroux, A.; L’Abbé, M.R. Effect of zinc supplementation on copper status in adult man. Am. J. Clin. Nutr. 1984, 40, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Yadrick, M.K.; Kenney, M.A.; Winterfeldt, E.A. Iron, copper, and zinc status: Response to supplementation with zinc or zinc and iron in adult females. Am. J. Clin. Nutr. 1989, 49, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.; Lönnerdal, B.; Stenlund, H.; Ismail, D.; Seswandhana, R.; Ekström, E.-C.; Persson, L.-Å. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: Interactions between iron and zinc. Am. J. Clin. Nutr. 2003, 77, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Tolerable Upper Intake Levels for Vitamins and Minerals. 2006. Available online: https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf (accessed on 11 December 2020).
- Hambidge, K.M.; Miller, L.V.; Westcott, J.E.; Sheng, X.; Krebs, N.F. Zinc bioavailability and homeostasis. Am. J. Clin. Nutr. 2010, 91, 1478s–1483s. [Google Scholar] [CrossRef] [Green Version]
- Engle-Stone, R.; Ndjebayi, A.O.; Nankap, M.; Killilea, D.W.; Brown, K.H. Stunting prevalence, plasma zinc concentrations, and dietary zinc intakes in a nationally representative sample suggest a high risk of zinc deficiency among women and young children in Cameroon. J. Nutr. 2014, 144, 382–391. [Google Scholar] [CrossRef] [Green Version]
- WHO; FAO; UNICEF; GAIN; Micronutrient Initiative (MI) and Food Fortification Initiative (FFI). Recommendations on Wheat and Maize Flour Fortifcation. Meeting Report: Interim Consensus Statement; World Health Organization: Geneva, Switzerland, 2009; Available online: http://www.who.int/nutrition/publications/micronutrients/wheat_maize_fort.pdf (accessed on 12 June 2021).
- Davis, K.A.; Gonzalez, A.; Loukine, L.; Qiao, C.; Sadeghpour, A.; Vigneault, M.; Wang, K.C.; Ibañez, D. Early Experience Analyzing Dietary Intake Data from the Canadian Community Health Survey-Nutrition Using the National Cancer Institute (NCI) Method. Nutrients 2019, 11, 1908. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute. Usual Dietary Intakes: SAS Macros for the NCI Method. Available online: https://epi.grants.cancer.gov/diet/usualintakes/macros.html (accessed on 21 March 2019).
- Brown, K.H.; Engle-Stone, R.; Krebs, N.F.; Peerson, J.M. Dietary intervention strategies to enhance zinc nutrition: Promotion and support of breastfeeding for infants and young children. Food Nutr. Bull. 2009, 30, S144–S171. [Google Scholar] [CrossRef] [Green Version]
- Bailey, R.L.; Dodd, K.W.; Gahche, J.J.; Dwyer, J.T.; Cowan, A.E.; Jun, S.; Eicher-Miller, H.A.; Guenther, P.M.; Bhadra, A.; Thomas, P.R.; et al. Best Practices for Dietary Supplement Assessment and Estimation of Total Usual Nutrient Intakes in Population-Level Research and Monitoring. J. Nutr. 2019, 149, 181–197. [Google Scholar] [CrossRef]
- IZiNCG. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S94–S203. [Google Scholar]
- Horton, S. The economics of food fortification. J. Nutr. 2006, 136, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Sharieff, W.; Horton, S.E.; Zlotkin, S. Economic gains of a home fortification program: Evaluation of "Sprinkles" from the provider’s perspective. Can. J. Public Health 2006, 97, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Zyba, S.J.; Wegmüller, R.; Woodhouse, L.R.; Ceesay, K.; Prentice, A.M.; Brown, K.H.; Wessells, K.R. Effect of exogenous phytase added to small-quantity lipid-based nutrient supplements (SQ-LNS) on the fractional and total absorption of zinc from a millet-based porridge consumed with SQ-LNS in young Gambian children: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1465–1475. [Google Scholar] [CrossRef]
- Brnić, M.; Hurrell, R.F.; Songré-Ouattara, L.T.; Diawara, B.; Kalmogho-Zan, A.; Tapsoba, C.; Zeder, C.; Wegmüller, R. Effect of phytase on zinc absorption from a millet-based porridge fed to young Burkinabe children. Eur. J. Clin. Nutr. 2017, 71, 137–141. [Google Scholar] [CrossRef]
- Ahluwalia, N.; Herrick, K.A.; Rossen, L.M.; Rhodes, D.; Kit, B.; Moshfegh, A.; Dodd, K.W. Usual nutrient intakes of US infants and toddlers generally meet or exceed Dietary Reference Intakes: Findings from NHANES 2009–2012. Am. J. Clin. Nutr. 2016, 104, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, J.E.; Brown, K.H. Zinc intake of US preschool children exceeds new dietary reference intakes. Am. J. Clin. Nutr. 2003, 78, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, J.E.; Yakes, E.A.; Hossain, M.B.; Islam, M.M.; Ahmed, T.; Hotz, C.; Lewis, B.; Rahman, A.S.; Jamil, K.M.; Brown, K.H. The current high prevalence of dietary zinc inadequacy among children and women in rural Bangladesh could be substantially ameliorated by zinc biofortification of rice. J. Nutr. 2010, 140, 1683–1690. [Google Scholar] [CrossRef] [Green Version]
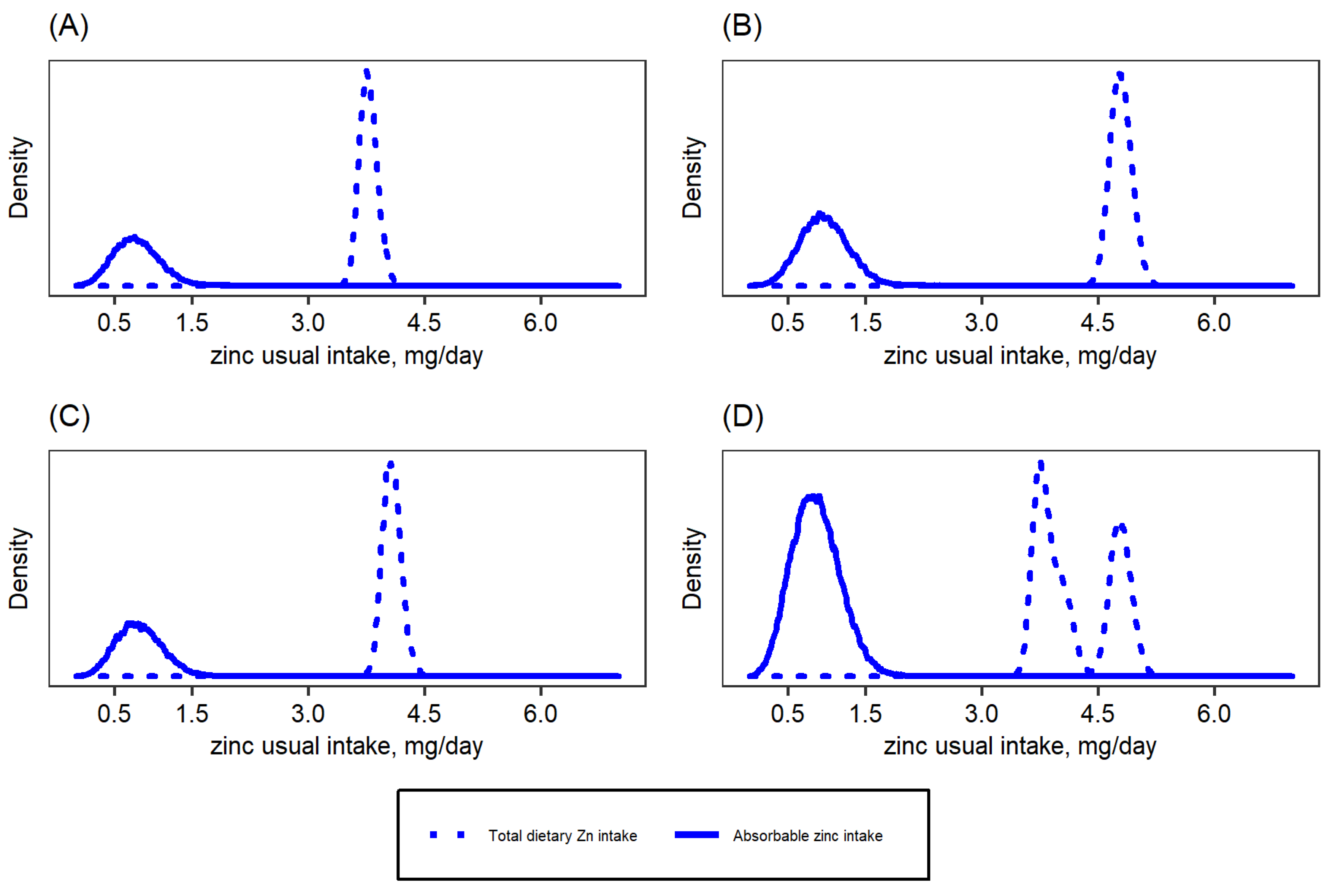
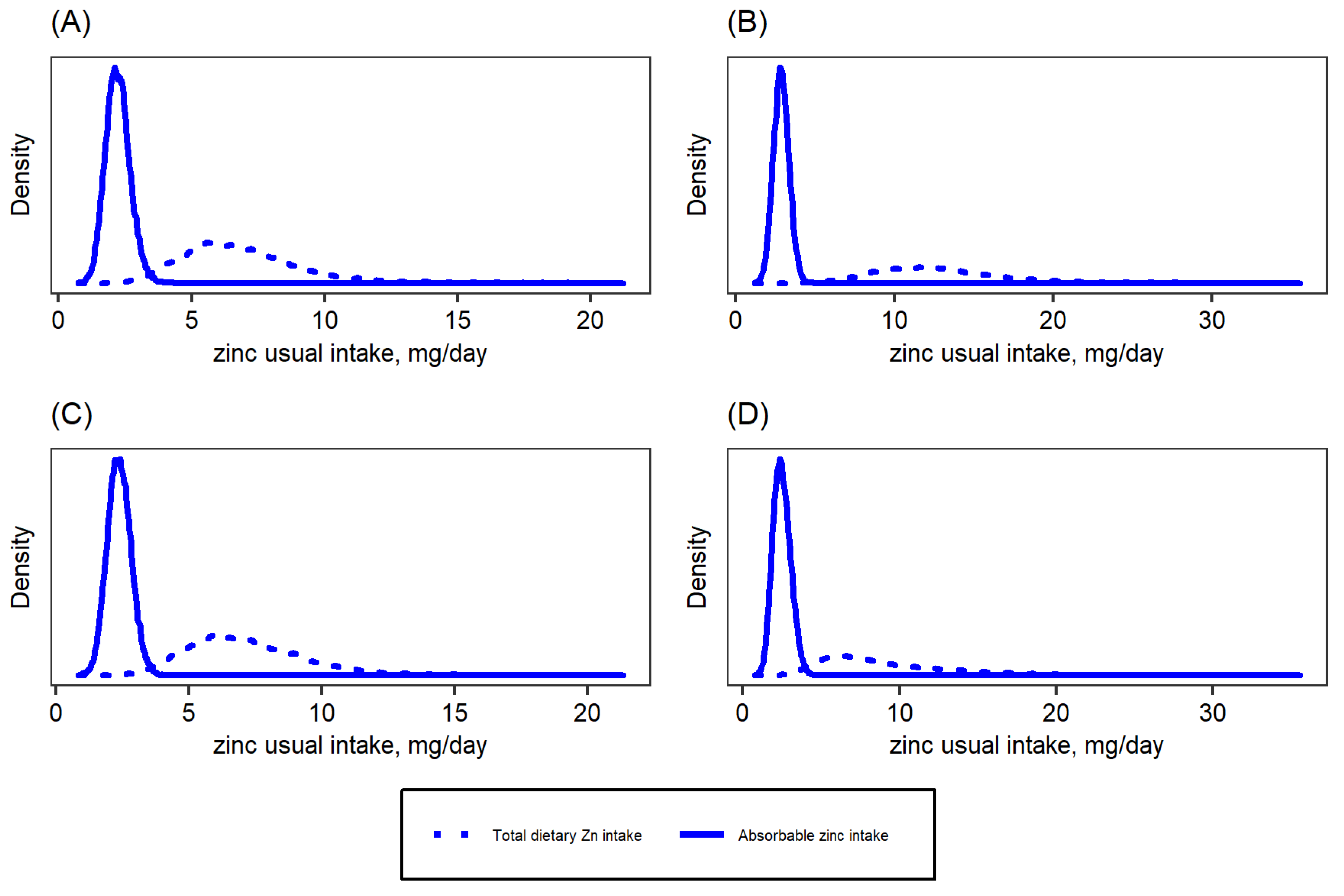
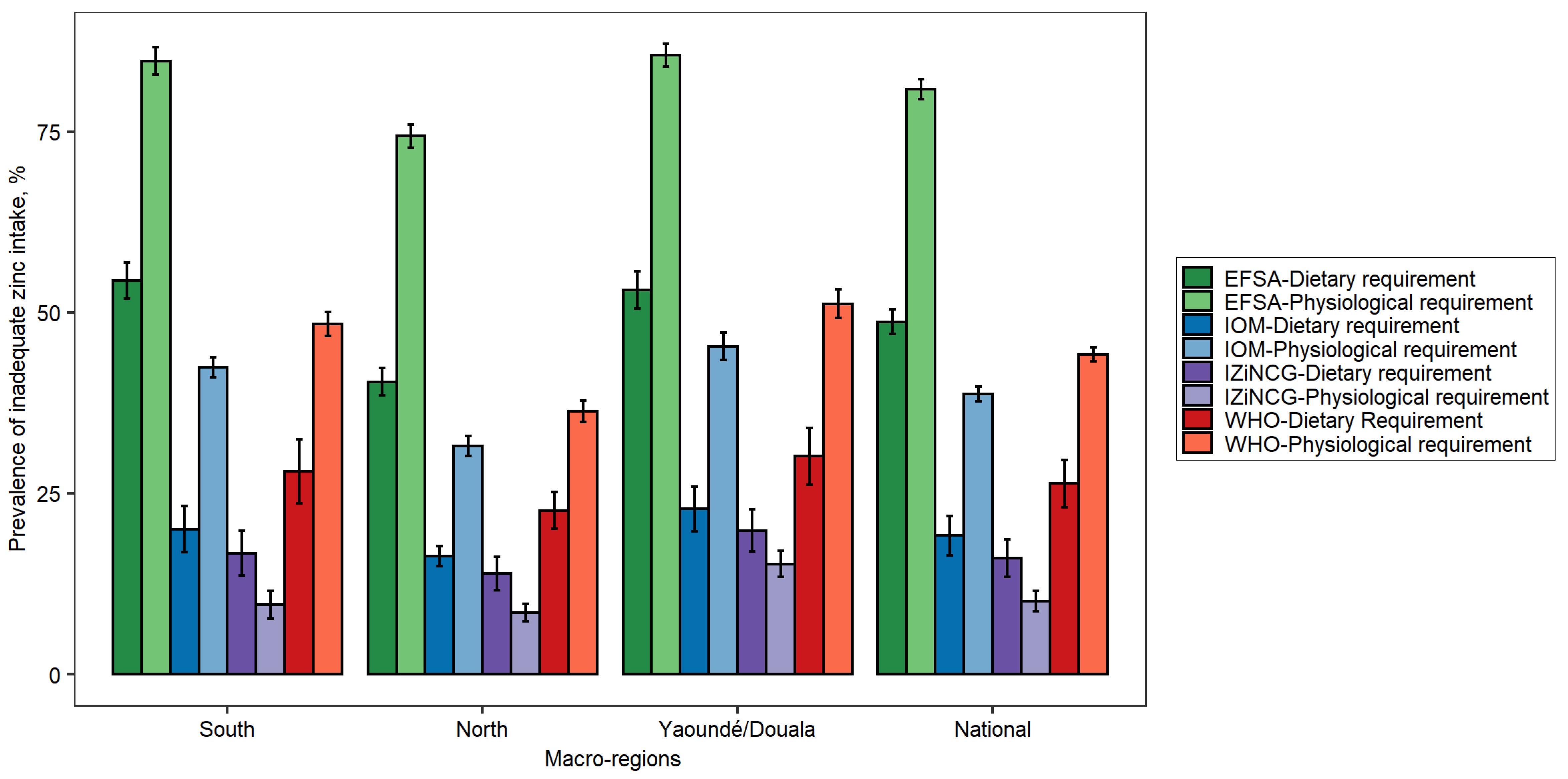
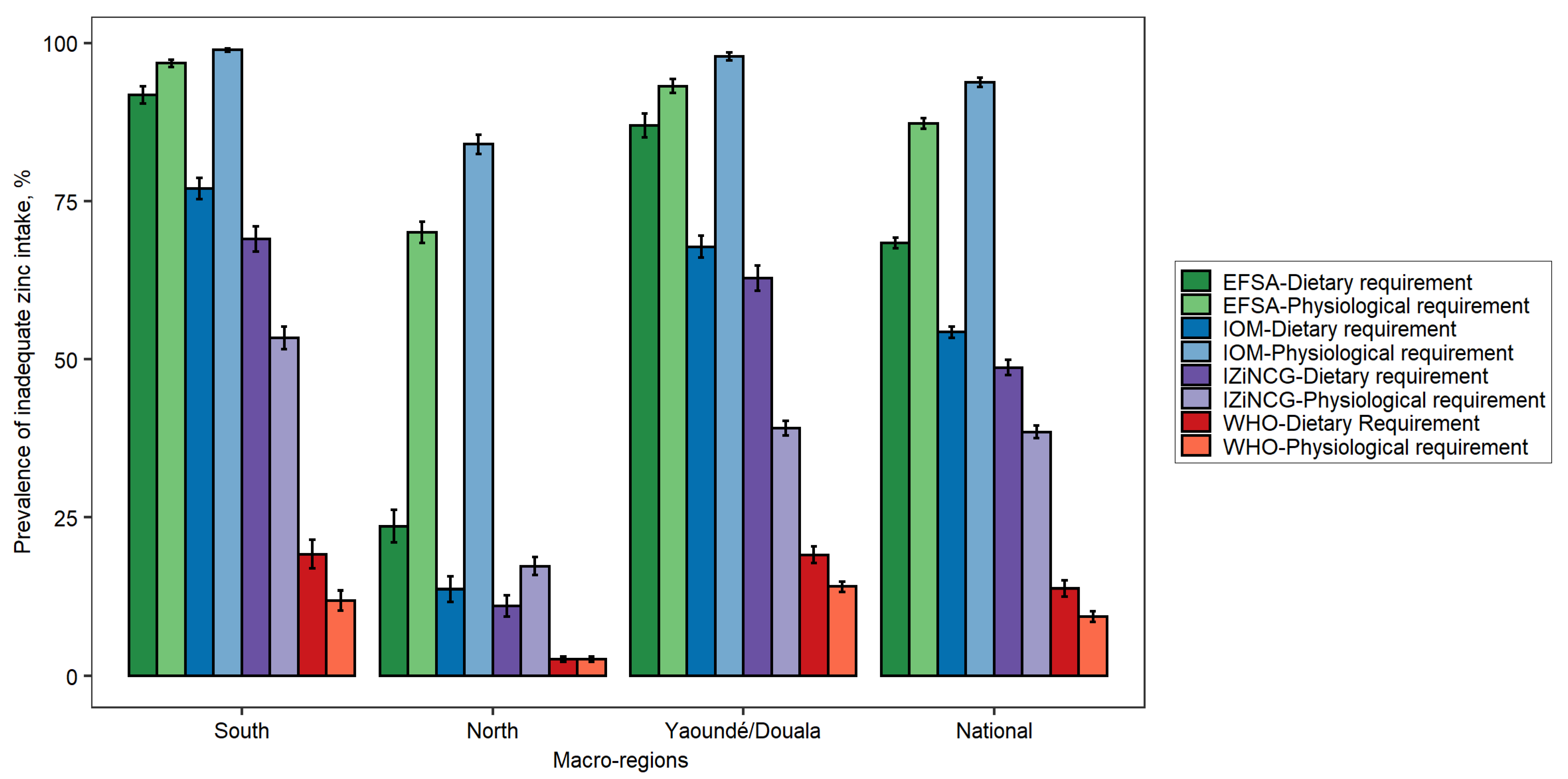

| Target Group | WHO 2 | IOM | IZiNCG 2 | EFSA 3 | Hambidge et al. Corrected Values [31]. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | DR | UL | PR | DR | UL | PR | DR | UL | PR | DR | UL | IOM-PR | IZINCG-PR | ||
| Children | 1–3 years | 0.83 | 2.76 | 23 | 0.74 | 2.2 | 7 | 0.53 | 2.0 | 8 a | 1.074 | 3.6 | 7 | NA | NA |
| 4–5 years | 0.97 | 3.23 | 23 | 1.20 | 4.0 | 12 | 0.83 | 4.0 | 14 a | 1.390 | 4.6 | 10 | |||
| Nonlactating, nonpregnant women | 0.98 | 3.25 | 35 | 3.30 | 6.8 | 40 | 1.86 | 7 | 40 | 2.9 | 8.9 | 25 | 2.97 | 2.89 | |
| Pregnant women 4 | 1.35 | 4.5 5 | 35 | 3.59 | 9.5 | 40 | 2.56 | 10 | 40 | 3.33 | 10.2 | 25 | 3.36 | 3.59 | |
| Lactating women 4 | 1.88 | 6.23 5 | 35 | 4.55 | 10.4 | 40 | 2.86 | 8 | 40 | 4.03 | 11.3 | 25 | 4.32 | 3.89 | |
| South | North | Yaoundé/Douala | National | |
|---|---|---|---|---|
| Median (P25th, P75th) | Median (P25th, P75th) | Median (P25th, P75th) | Median (P25th, P75th) | |
| Children (N = 860) | ||||
| Total dietary zinc intake (mg/day) | 3.4 (2.4, 4.3) | 4.3 (2.9, 5.8) | 3.4 (2.3, 4.5) | 3.6 (2.5, 4.9) |
| Absorbable zinc intake (mg/day) | 0.8 (0.6, 1.0) | 0.9 (0.7, 1.1) | 0.8 (0.6, 1.0) | 0.9 (0.7, 1.1) |
| Estimated fractional zinc absorption (%) 1 | 28.7 (22.1, 33.2) | 24.2 (19.3, 30.7) | 27.3 (22.2, 31.3) | 27.0 (21.2, 31.8) |
| Phytate intake (mg/day) | 357 (197, 570) | 460 (261, 724) | 331 (160, 545) | 390 (213, 622) |
| Phytate: zinc molar ratio 2 | 13.1 (8.9, 17.6) | 12.8 (8.9, 16.4) | 10.0 (7.7, 13.8) | 11.9 (8.3, 16.0) |
| Women (N = 902) | ||||
| Total dietary zinc intake (mg/day) | 6.3 (5.0, 7.8) | 12.1 (9.8, 14.6) | 6.5 (4.9, 8.1) | 7.6 (5.6, 10.7) |
| Absorbable zinc intake (mg/day) | 2.2 (1.8, 2.5) | 2.9 (2.58, 3.2) | 2.3 (1.9, 2.6) | 2.4 (2.0, 2.8) |
| Estimated fractional zinc absorption (%) 1 | 37.5 (30.0, 49.2) | 25.9 (19.9, 33.7) | 41.1 (32.2, 49.4) | 34.8 (25.2, 45.3) |
| Phytate intake (mg/day) | 772 (567, 1008) | 1353 (1058, 1695) | 661 (466, 878) | 889 (620, 1253) |
| Phytate/zinc molar ratio 2 | 12.8 (8.7, 17.3) | 12.4 (8.4, 15.3) | 9.8 (7.2, 14.5) | 11.6 (8.1, 15.9) |
| Reference Values Applied | Cost Per Effectively Covered Child Per Year | Cost Per Effectively Covered Woman Per Year | |||
|---|---|---|---|---|---|
| Wheat Flour (95 mg/kg) | Bouillon Cube (5 mg/g) | Wheat Flour (95 mg/kg) | Bouillon Cube (5 mg/g) | ||
| WHO | Dietary requirement | 2.48 | 1.84 | 3.03 | 2.26 |
| Physiological requirement | 1.36 | 1.13 | 24.28 | 6.32 | |
| IOM | Dietary requirement | 3.65 | 2.60 | 0.50 | 0.35 |
| Physiological requirement | 1.44 | 1.19 | 0.61 | 0.27 | |
| IZiNCG | Dietary requirement | 4.73 | 3.23 | 0.52 | 0.39 |
| Physiological requirement | 9.45 | 6.65 | 0.89 | 0.54 | |
| EFSA | Dietary requirement | 1.11 | 0.93 | 0.44 | 0.28 |
| Physiological requirement | 1.05 | 0.76 | 0.50 | 0.28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haile, D.; Brown, K.H.; McDonald, C.M.; Luo, H.; Jarvis, M.; Teta, I.; Ndjebayi, A.; Martial, G.A.J.; Vosti, S.A.; Engle-Stone, R. Applying Zinc Nutrient Reference Values as Proposed by Different Authorities Results in Large Differences in the Estimated Prevalence of Inadequate Zinc Intake by Young Children and Women and in Cameroon. Nutrients 2022, 14, 883. https://doi.org/10.3390/nu14040883
Haile D, Brown KH, McDonald CM, Luo H, Jarvis M, Teta I, Ndjebayi A, Martial GAJ, Vosti SA, Engle-Stone R. Applying Zinc Nutrient Reference Values as Proposed by Different Authorities Results in Large Differences in the Estimated Prevalence of Inadequate Zinc Intake by Young Children and Women and in Cameroon. Nutrients. 2022; 14(4):883. https://doi.org/10.3390/nu14040883
Chicago/Turabian StyleHaile, Demewoz, Kenneth H. Brown, Christine M. McDonald, Hanqi Luo, Michael Jarvis, Ismael Teta, Alex Ndjebayi, Guintang Assiene Jules Martial, Stephen A. Vosti, and Reina Engle-Stone. 2022. "Applying Zinc Nutrient Reference Values as Proposed by Different Authorities Results in Large Differences in the Estimated Prevalence of Inadequate Zinc Intake by Young Children and Women and in Cameroon" Nutrients 14, no. 4: 883. https://doi.org/10.3390/nu14040883
APA StyleHaile, D., Brown, K. H., McDonald, C. M., Luo, H., Jarvis, M., Teta, I., Ndjebayi, A., Martial, G. A. J., Vosti, S. A., & Engle-Stone, R. (2022). Applying Zinc Nutrient Reference Values as Proposed by Different Authorities Results in Large Differences in the Estimated Prevalence of Inadequate Zinc Intake by Young Children and Women and in Cameroon. Nutrients, 14(4), 883. https://doi.org/10.3390/nu14040883






