Association between Dietary Inflammatory Index and Sarcopenia in Crohn’s Disease Patients
Abstract
:1. Introduction
2. Materials and Methods
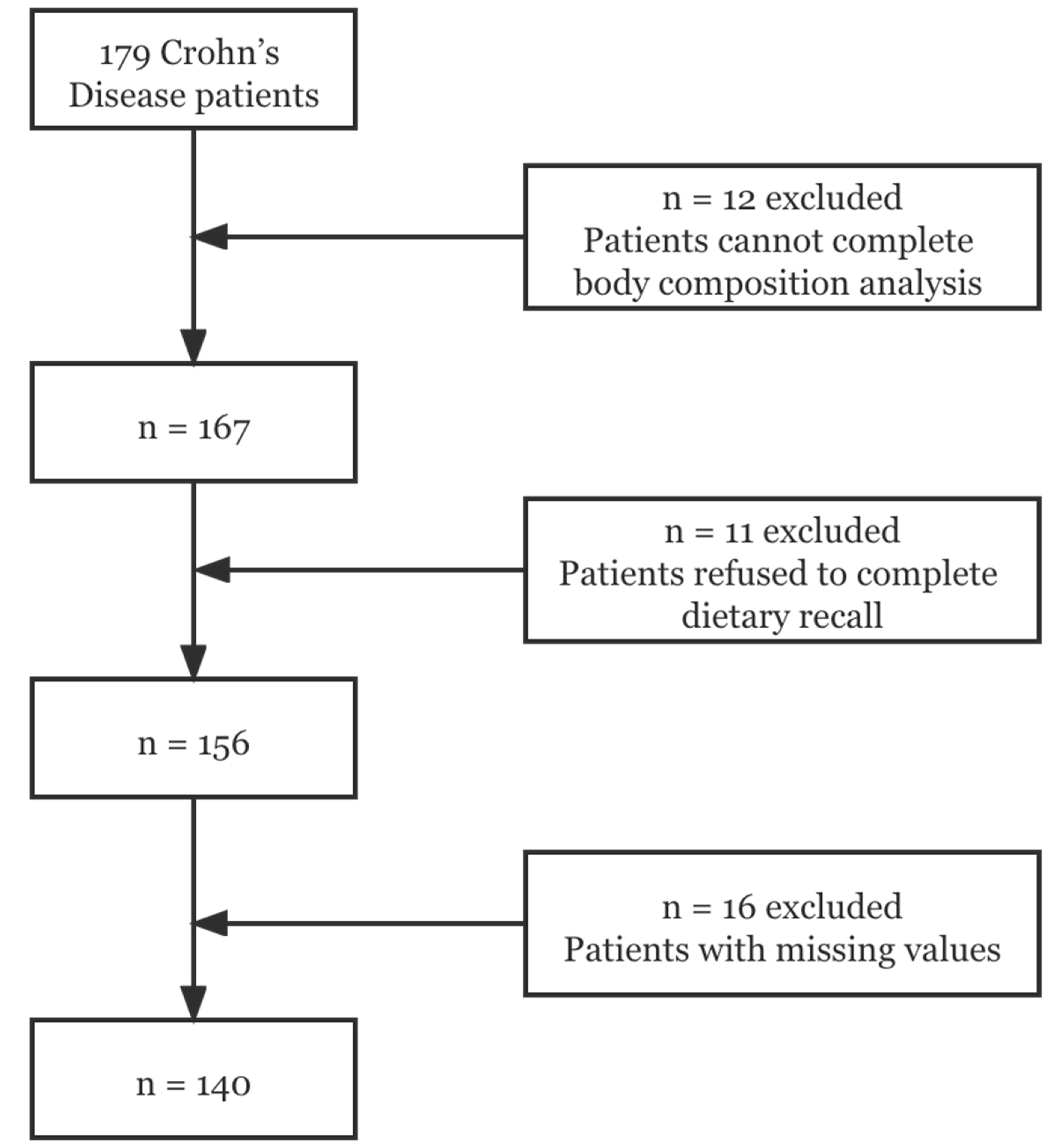
2.1. Participants
2.2. Data Collection
2.3. Dietary Intake Measurement
2.4. Dietary Inflammatory Index
2.5. Sarcopenia
2.6. Nutritional Assessment
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Dietary Intakes of CD Patients across Quartiles of the DII
3.3. DII was Associated with Sarcopenia in CD Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Witkowski, M.; Witkowski, M.; Gagliani, N.; Huber, S. Recipe for IBD: Can we use food to control inflammatory bowel disease? Semin. Immunopathol. 2018, 40, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triggs, C.M.; Munday, K.; Hu, R.; Fraser, A.G.; Gearry, R.B.; Barclay, M.L.; Ferguson, L.R. Dietary factors in chronic inflammation: Food tolerances and intolerances of a New Zealand Caucasian Crohn’s disease population. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2010, 690, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; James, D. Dietary Patterns and Self-Reported Associations of Diet with Symptoms of Inflammatory Bowel Disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Lochhead, P.; Khalili, H.; Song, M.; Tabung, F.K.; Burke, K.E.; Richter, J.M.; Giovannucci, E.L.; Chan, A.T.; Ananthakrishnan, A.N. Dietary Inflammatory Potential and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology 2020, 159, 873–883. [Google Scholar] [CrossRef]
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015, 148, 1087–1106. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Zhuang, X.; Zhao, M.; Zhuo, S.; Li, X.; Ma, R.; Li, N.; Liu, C.; Zhu, Y.; Tang, C.; et al. Index-Based Dietary Patterns and Inflammatory Bowel Disease: A Systematic Review of Observational Studies. Adv. Nutr. 2021, 12, 2288–2300. [Google Scholar] [CrossRef]
- De Vries, J.H.M.; Dijkhuizen, M.; Tap, P.; Witteman, B.J.M. Patient’s Dietary Beliefs and Behaviours in Inflammatory Bowel Disease. Dig. Dis. 2019, 37, 131–139. [Google Scholar] [CrossRef]
- Bian, D.; Shi, Y.; Jiang, Y.; Zhong, J.; Sun, J.; Gu, Y. Combined Patient-Generated Subjective Global Assessment and body composition facilitates nutritional support in inflammatory bowel disease: An ambulatory study in Shanghai. Asia Pac. J. Clin. Nutr. 2018, 27, 1230–1238. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Quinlan, J.I.; Overthrow, K.; Greig, C.; Lord, J.M.; Armstrong, M.J.; Cooper, S.C. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients 2021, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landi, F.; Liperoti, R.; Russo, A.; Giovannini, S.; Tosato, M.; Capoluongo, E.; Bernabei, R.; Onder, G. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin. Nutr. 2012, 31, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, L.; Ferrucci, L.; Cherubini, A.; Maggio, M.; Bandinelli, S.; Savino, E.; Brombo, G.; Zuliani, G.; Guralnik, J.M.; Landi, F.; et al. The Predictive Value of the EWGSOP Definition of Sarcopenia: Results from the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 259–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, E.; Lee, S.R.; Park, B.S.; Kim, J.S. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin. Exp. Res. 2012, 24, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Budui, S.L.; Rossi, A.P.; Zamboni, M. The pathogenetic bases of sarcopenia. Clin. Cases Miner. Bone Metab. 2015, 12, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabung, F.K.; Liu, L.; Wang, W.; Fung, T.T.; Wu, K.; Smith-Warner, S.A.; Cao, Y.; Hu, F.B.; Ogino, S.; Fuchs, C.S.; et al. Association of Dietary Inflammatory Potential With Colorectal Cancer Risk in Men and Women. JAMA Oncol. 2018, 4, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Kang, M.; Wilkens, L.R.; Shvetsov, Y.B.; Harmon, B.E.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Haiman, C.A.; Le Marchand, L.; et al. The Dietary Inflammatory Index and All-Cause, Cardiovascular Disease, and Cancer Mortality in the Multiethnic Cohort Study. Nutrients 2018, 10, 1844. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Canela, M.; Bes-Rastrollo, M.; Martínez-González, M.A. The Role of Dietary Inflammatory Index in Cardiovascular Disease, Metabolic Syndrome and Mortality. Int. J. Mol. Sci. 2016, 17, 1265. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Stubbs, B.; Hébert, J.R.; Cesari, M.; Schofield, P.; Soysal, P.; Maggi, S.; Veronese, N. The Relationship Between the Dietary Inflammatory Index and Incident Frailty: A Longitudinal Cohort Study. J. Am. Med. Dir. Assoc. 2018, 19, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Canto-Osorio, F.; Denova-Gutierrez, E.; Sánchez-Romero, L.M.; Salmerón, J.; Barrientos-Gutierrez, T. Dietary Inflammatory Index and metabolic syndrome in Mexican adult population. Am. J. Clin. Nutr. 2020, 112, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhu, J.; Fan, J.; Sun, L.; Cai, S.; Fan, C.; Zhong, Y.; Li, Y. Dietary Inflammatory Index in relation to bone mineral density, osteoporosis risk and fracture risk: A systematic review and meta-analysis. Osteoporos. Int. 2021, 32, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Shivappa, N.; de Souza Genaro, P.; Martini, L.A.; Schuch, N.J.; Hebert, J.R.; Pinheiro, M.M. Lack of association between dietary inflammatory index and low impact fractures in the Brazilian population: The Brazilian Osteoporosis Study (BRAZOS). Adv. Rheumatol. 2019, 59, 16. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef]
- IBD expert group of the Chinese Society of Gastroenterology. Expert Consensus of clinical nutrition therapy in inflammatory bowel disease. Chin. J. Intern. Med. 2013, 52, 1082–1087. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nakamura, S.; Miyazaki, T.; Kakimoto, K.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Inflammatory Bowel Disease and Sarcopenia: Its Mechanism and Clinical Importance. J. Clin. Med. 2021, 10, 4214. [Google Scholar] [CrossRef] [PubMed]
- Cravo, M.L.; Velho, S.; Torres, J.; Costa Santos, M.P.; Palmela, C.; Cruz, R.; Strecht, J.; Maio, R.; Baracos, V. Lower skeletal muscle attenuation and high visceral fat index are associated with complicated disease in patients with Crohn’s disease: An exploratory study. Clin. Nutr. ESPEN 2017, 21, 79–85. [Google Scholar] [CrossRef]
- Atlan, L.; Cohen, S.; Shiran, S.; Sira, L.B.; Pratt, L.T.; Yerushalmy-Feler, A. Sarcopenia is a Predictor for Adverse Clinical Outcome in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 883–888. [Google Scholar] [CrossRef]
- Vagianos, K.; Shafer, L.A.; Witges, K.; Targownik, L.E.; Haviva, C.; Graff, L.A.; Sexton, K.A.; Lix, L.M.; Sargent, M.; Bernstein, C.N. Association Between Change in Inflammatory Aspects of Diet and Change in IBD-related Inflammation and Symptoms Over 1 Year: The Manitoba Living With IBD Study. Inflamm. Bowel Dis. 2021, 27, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sazuka, S.; Katsuno, T.; Nakagawa, T.; Saito, M.; Saito, K.; Matsumura, T.; Arai, M.; Sato, T.; Yokosuka, O. Concomitant use of enteral nutrition therapy is associated with sustained response to infliximab in patients with Crohn’s disease. Eur. J. Clin. Nutr. 2012, 66, 1219–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamers, C.R.; de Roos, N.M.; Witteman, B.J.M. The association between inflammatory potential of diet and disease activity: Results from a cross-sectional study in patients with inflammatory bowel disease. BMC Gastroenterol. 2020, 20, 316. [Google Scholar] [CrossRef]
- Mirmiran, P.; Moslehi, N.; Morshedzadeh, N.; Shivappa, N.; Hébert, J.R.; Farsi, F.; Daryani, N.E. Does the inflammatory potential of diet affect disease activity in patients with inflammatory bowel disease? Nutr. J. 2019, 18, 65. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.; McNicholas, D.; Creavin, B.; Kelly, M.E.; Walsh, T.; Beddy, D. Sarcopenia and Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2019, 25, 67–73. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Nardone, O.M.; de Sire, R.; Petito, V.; Testa, A.; Villani, G.; Scaldaferri, F.; Castiglione, F. Inflammatory Bowel Diseases and Sarcopenia: The Role of Inflammation and Gut Microbiota in the Development of Muscle Failure. Front. Immunol. 2021, 12, 694217. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ma, X.; Chen, Y. Dietary practices of Chinese patients with inflammatory bowel disease: A naturalistic inquiry. Gastroenterol. Nurs. 2014, 37, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Morton, H.; Pedley, K.C.; Stewart, R.J.C.; Coad, J. Inflammatory Bowel Disease: Are Symptoms and Diet Linked? Nutrients 2020, 12, 2975. [Google Scholar] [CrossRef] [PubMed]
| Variable | DII | p-Value | |||
|---|---|---|---|---|---|
| Quartile 1 (n = 35) | Quartile 2 (n = 35) | Quartile 3 (n = 35) | Quartile 4 (n = 35) | ||
| (−3.24, −1.05) | (−1.05, 1.13) | (1.13, 2.67) | (2.67, 4.89) | ||
| Age (years) | 32.37 ± 2.75 | 32.63 ± 4.05 | 31.43 ± 3.28 | 33.91 ± 3.93 | 0.792 |
| BMI (kg/m2) | 20.29 ± 1.10 | 21.13 ± 1.04 | 21.61 ± 1.11 | 19.76 ± 1.12 | 0.071 |
| Handgrip strength (kg) | 31.43 ± 3.13 | 30.78 ± 2.94 | 37.44 ± 3.20 | 30.91 ± 2.96 | 0.005 * |
| Disease duration (years) | 4.80 ± 15.4 | 5.17 ± 1.39 | 4.49 ± 1.56 | 7.83 ± 2.43 | 0.030 * |
| C-Reactive Protein(mg/L) | 3.43 ± 7.68 | 8.23 ± 15.25 | 9.53 ± 18.79 | 15.55 ± 25.36 | 0.048 * |
| Hemoglobin(mg/L) | 133.83 ± 6.64 | 130.26 ± 9.18 | 133.46 ± 7.95 | 121.91 ± 6.58 | 0.096 |
| Albumin (mg/L) | 42.92 ± 1.54 | 41.80 ± 2.25 | 41.15 ± 1.83 | 41.36 ± 1.95 | 0.530 |
| ASMI (kg/m2) | 7.52 ± 0.39 | 7.33 ± 0.30 | 7.50 ± 0.41 | 6.84 ± 0.38 | 0.034 * |
| PG-SGA | 2.66 ± 0.84 | 4.32 ± 1.04 | 3.46 ± 0.90 | 5.86 ± 1.80 | 0.002 * |
| DII | −2.09 ± 0.23 | 0.06 ± 0.21 | 1.88 ± 0.16 | 3.39 ± 0.18 | <0.001 * |
| Male (%) | 82.86% | 68.57% | 71.43% | 65.71% | 0.406 |
| Smoking status | |||||
| Current smoker (%) | 2.86% | 2.86% | 2.86% | 8.57% | 0.710 |
| Former smoker (%) | 8.57% | 8.57% | 8.57% | 8.57% | |
| Nonsmoker (%) | 85.71% | 88.57% | 85.71% | 82.86% | |
| Drinker (%) | 2.86% | 0 | 2.86% | 2.86% | 0.801 |
| Montreal Classification-Location (%) | |||||
| L1 | 31.14% | 51.43% | 51.43% | 31.43% | 0.294 |
| L2 | 17.14% | 22.86% | 14.29% | 34.28% | |
| L3 | 42.86% | 25.71% | 34.29% | 34.28% | |
| L4 | 2.86% | 0 | 0 | 0 | |
| Montreal Classification-Behavior (%) | |||||
| B1 | 60.00% | 74.29% | 62.86% | 68.57% | 0.905 |
| B2 | 34.29% | 22.86% | 31.43% | 28.57% | |
| B3 | 5.71% | 2.86% | 5.71% | 2.86% | |
| Sarcopenia % | 20.00% | 20.00% | 8.57% | 57.14% | <0.001 * |
| Malnutrition % | 22.86% | 51.43% | 42.86% | 62.86% | 0.006 * |
| CDAI score | 82.30 ± 62.26 | 57.66 ± 73.60 | 67.54 ± 76.99 | 95.82 ± 67.49 | 0.394 |
| Active phase (%) | 17.14% | 17.14% | 11.42% | 14.28% | 0.893 |
| Food Components (Mean ± SD) | Quartile 1 (n = 35) (−3.24, −1.05) | Quartile 2 (n = 35) (−1.05, 1.13) | Quartile 3 (n = 35) (1.13, 2.67) | Quartile 4 (n = 35) (2.67, 4.89) | p-Value |
|---|---|---|---|---|---|
| PEN (kcal) | 555.42 ± 397.02 | 234.77 ± 191.24 | 43.31 ± 84.27 | 18.15 ± 55.25 | <0.001 * |
| Alcohol (g) | 0.31 ± 0.64 | 0 ± 0 | 0.12 ± 0.25 | 0.06 ± 0.12 | 0.591 |
| Vitamin B12 (μg) | 8.39 ± 2.19 | 5.59 ± 1.45 | 4.19 ± 1.36 | 6.32 ± 2.42 | 0.018 * |
| Vitamin B6 (mg) | 2.86 ± 0.24 | 2.14 ± 0.30 | 1.52 ± 0.14 | 1.15 ± 0.15 | <0.001 * |
| β-Carotene (μg) | 5156.52 ± 1962.81 | 3375.32 ± 1459.11 | 3233.56 ± 1062.02 | 1516.66 ± 429.70 | 0.002 * |
| Carbohydrate (g) | 278.88 ± 28.01 | 244.26 ± 27.44 | 203.47 ± 19.27 | 200.43 ± 27.08 | <0.001 * |
| Cholesterol (mg) | 552.57 ± 111.37 | 547.23 ± 86.69 | 451.38 ± 72.76 | 369.83 ± 58.37 | 0.006 * |
| Energy (kcal) | 1953.05 ± 142.23 | 1775.61 ± 184.59 | 1421.34 ± 113.24 | 1192.53 ± 327.51 | <0.001 * |
| Total fat (g) | 60.51 ± 6.38 | 60.60 ± 10.48 | 44.77 ± 6.97 | 29.95 ± 5.02 | <0.001 * |
| Saturated fat (g) | 16.45 ± 6.03 | 20.02 ± 17.41 | 13.90 ± 7.37 | 9.32 ± 4.93 | <0.001 * |
| Fiber (g) | 6.53 ± 1.45 | 5.20 ± 0.76 | 5.70 ± 0.79 | 4.88 ± 0.69 | 0.085 |
| Folic acid (μg) | 541.02 ± 45.25 | 341.12 ± 22.55 | 235.04 ± 17.89 | 184.74 ± 17.41 | <0.001 * |
| Fe (mg) | 23.82 ± 3.61 | 18.53 ± 3.61 | 14.11 ± 2.73 | 11.80 ± 1.90 | <0.001 * |
| Mg (mg) | 346.57 ± 22.26 | 260.50 ± 24.63 | 207.68 ± 13.55 | 164.59 ± 16.37 | <0.001 * |
| MUFA (g) | 18.21 ± 2.32 | 20.41 ± 4.07 | 15.97 ± 2.89 | 9.95 ± 2.01 | <0.001 * |
| Niacin (mg) | 26.56 ± 2.14 | 19.87 ± 1.94 | 14.74 ± 1.47 | 10.77 ± 1.26 | <0.001 * |
| n-3 Fatty acids (g) | 0.99 ± 0.18 | 1.13 ± 0.44 | 0.67 ± 0.23 | 0.49 ± 0.16 | 0.004 * |
| n-6 Fatty acids (g) | 16.36 ± 2.30 | 12.60 ± 4.01 | 6.27 ± 0.74 | 5.33 ± 2.78 | <0.001 * |
| Protein (g) | 79.26 ± 5.06 | 68.79 ± 6.86 | 55.84 ± 4.32 | 43.83 ± 4.74 | <0.001 * |
| PUFA (g) | 10.19 ± 1.09 | 9.14 ± 1.14 | 6.60 ± 0.84 | 4.56 ± 0.93 | <0.001 * |
| Riboflavin (mg) | 1.82 ± 0.21 | 1.11 ± 0.09 | 0.71 ± 0.08 | 0.56 ± 0.06 | <0.001 * |
| Saturated fat (g) | 16.45 ± 2.07 | 20.02 ± 5.98 | 13.90 ± 2.53 | 9.32 ± 1.70 | <0.001 * |
| Se (μg) | 65.59 ± 5.59 | 52.35 ± 4.78 | 37.54 ± 3.34 | 30.42 ± 3.09 | <0.001 * |
| Thiamin (mg) | 1.75 ± 0.17 | 1.23 ± 0.12 | 0.85 ± 0.10 | 0.69 ± 0.08 | <0.001 * |
| Vitamin A (RE) | 2149.20 ± 338.99 | 1082.67 ± 125.33 | 558.26 ± 111.47 | 381.02 ± 81.47 | <0.001 * |
| Vitamin C (mg) | 164.95 ± 16.42 | 86.94 ± 7.25 | 63.23 ± 11.99 | 44.96 ± 8.37 | <0.001 * |
| Vitamin D (μg) | 9.11 ± 1.45 | 5.12 ± 0.73 | 2.39 ± 0.49 | 2.21 ± 0.47 | <0.001 * |
| Vitamin E (mg) | 22.24 ± 2.61 | 13.49 ± 1.09 | 8.74 ± 1.10 | 6.50 ± 1.08 | <0.001 * |
| Zn (mg) | 16.64 ± 1.09 | 13.11 ± 1.06 | 9.78 ± 0.69 | 7.27 ± 0.75 | <0.001 * |
| Isoflavones (mg) | 6.36 ± 1.82 | 5.31 ± 1.66 | 4.17 ± 1.95 | 3.11 ± 2.00 | 0.074 |
| Model | DII, OR (95% Cl) | p-Trend | |||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| Sarcopenia | |||||
| Model 1 | 1 | 1.00 (0.31, 3.23) | 0.38 (0.09, 1.59) | 5.33 (1.84, 15.47) | 0.012 |
| Model 2 | 1 | 1.24 (0.30, 5.06) | 0.45 (0.08, 2.45) | 7.22 (1.95, 26.80) | 0.009 |
| Model 3 | 1 | 1.09 (0.23, 5.04) | 0.52 (0.08, 3.41) | 9.59 (1.69, 54.42) | 0.031 |
| Muscle Mass (ASMI a) | |||||
| Model 1 | 1 | 0.63 (0.21, 1.89) | 0.42 (0.13, 1.38) | 4.23 (1.55, 11.55) | 0.022 |
| Model 2 | 1 | 0.69 (0.18, 2.73) | 0.48 (0.11, 2.23) | 5.48 (1.51, 19.87) | 0.018 |
| Model 3 | 1 | 0.63 (0.15, 2.72) | 0.51 (0.09, 2.79) | 5.77 (1.06, 31.35) | 0.106 |
| Muscle Function (Handgrip strength b) | |||||
| Model 1 | 1 | 0.53 (0.20, 1.44) | 0.33 (0.12, 0.97) | 2.00 (0.77, 5.18) | 0.432 |
| Model 2 | 1 | 0.53 (0.18, 1.55) | 0.33 (0.10, 1.06) | 1.87 (0.67, 5.21) | 0.481 |
| Model 3 | 1 | 0.48 (0.15, 1.58) | 0.23 (0.06, 0.89) | 1.26 (0.33, 4.83) | 0.804 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, D.; Liu, X.; Wang, C.; Jiang, Y.; Gu, Y.; Zhong, J.; Shi, Y. Association between Dietary Inflammatory Index and Sarcopenia in Crohn’s Disease Patients. Nutrients 2022, 14, 901. https://doi.org/10.3390/nu14040901
Bian D, Liu X, Wang C, Jiang Y, Gu Y, Zhong J, Shi Y. Association between Dietary Inflammatory Index and Sarcopenia in Crohn’s Disease Patients. Nutrients. 2022; 14(4):901. https://doi.org/10.3390/nu14040901
Chicago/Turabian StyleBian, Dongsheng, Xutong Liu, Cenyu Wang, Yongmei Jiang, Yubei Gu, Jie Zhong, and Yongmei Shi. 2022. "Association between Dietary Inflammatory Index and Sarcopenia in Crohn’s Disease Patients" Nutrients 14, no. 4: 901. https://doi.org/10.3390/nu14040901
APA StyleBian, D., Liu, X., Wang, C., Jiang, Y., Gu, Y., Zhong, J., & Shi, Y. (2022). Association between Dietary Inflammatory Index and Sarcopenia in Crohn’s Disease Patients. Nutrients, 14(4), 901. https://doi.org/10.3390/nu14040901






