The Role of Nutrients in Prevention, Treatment and Post-Coronavirus Disease-2019 (COVID-19)
Abstract
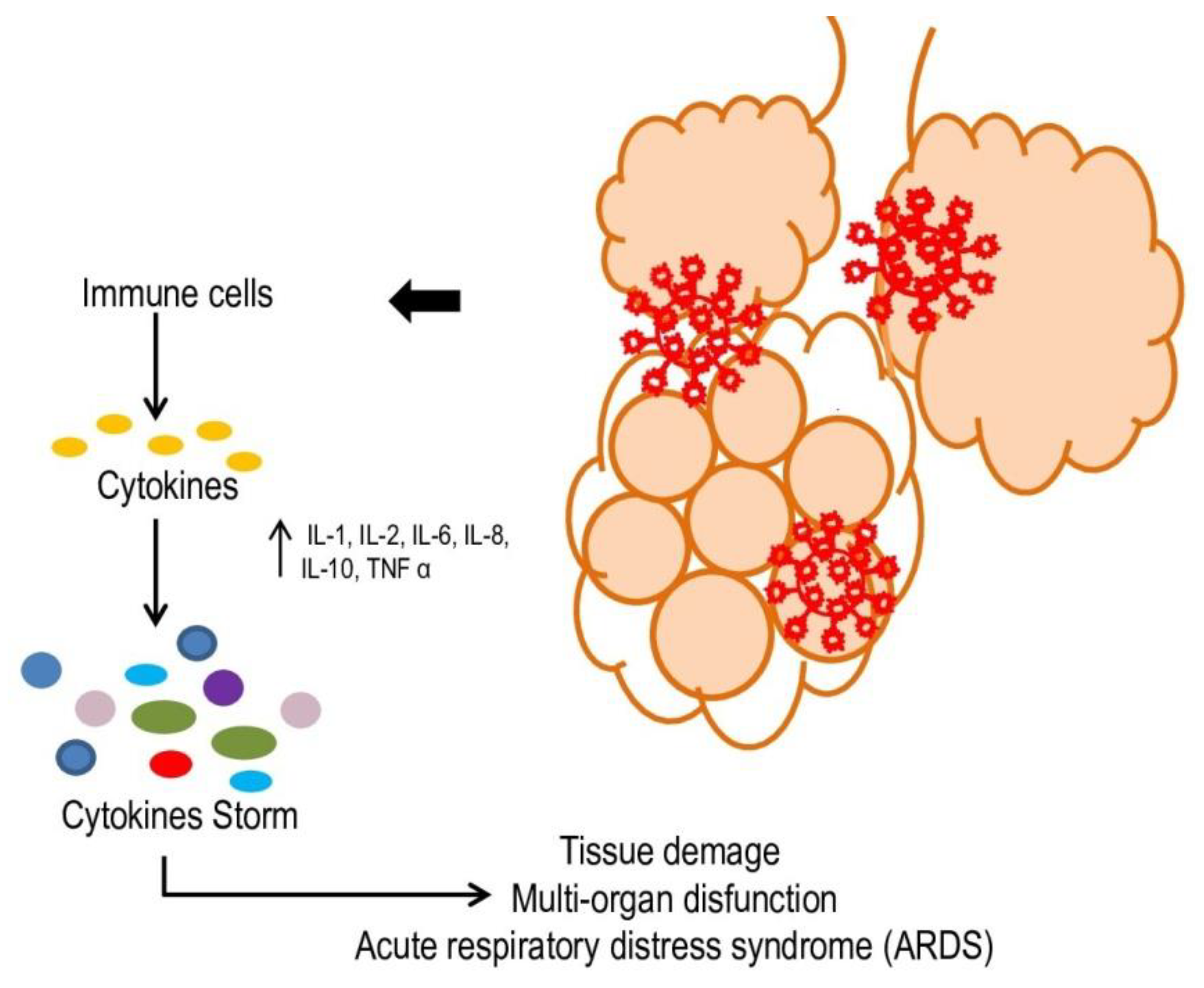
:1. Introduction
2. Materials and Methods
3. Results
3.1. Nutrients in COVID-19 Prevention
3.2. Nutrients in COVID-19 Treatment
3.2.1. Vitamin C
3.2.2. Vitamin D
3.2.3. Zinc
3.2.4. N-3 PUFAs
3.2.5. Lactoferrin
3.2.6. Hesperidin
3.3. Nutrients Post COVID-19
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus Disease-2019 |
| SARS-CoV-2 | severe acute respiratory syndrome Coronavirus 2 |
| WHO | World Health Organization |
| ACE2 | angiotensin converting enzyme 2 receptor |
| S protein | Spike protein |
| TMPRSS2 | transmembrane protease serine 2 |
| IFN-β | interferon beta |
| CXCL-1O | CXC motif chemokine ligand 10 |
| ARDS | acute respiratory distress syndrome |
| Se | selenium |
| Fe | iron |
| Zn | zinc |
| N-3 PUFAs | omega-3 fatty acids |
| EPA | enzymatic conversion of omega-3 eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| SPM | pro-resolution mediators |
| Lf | Lactoferrin |
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Wu, J.T.; Leung, K.; Leung, G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet 2020, 395, 689–697. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization Coronavirus 19. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. (accessed on 25 October 2020).
- Yoshimoto, F.K. The proteins of severe acute respiratory syndrome coronavirus-2 (SARS CoV-2 or n-COV19), the cause of COVID-19. Protein. J. 2020, 39, 198–216. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nagata, N.; Shirato, K.; Kawase, M.; Takeda, M.; Taguchi, F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010, 84, 12658–12664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belouzard, S.; Chu, V.C.; Whittaker, G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA 2009, 106, 5871–5876. [Google Scholar] [CrossRef] [Green Version]
- Parasher, A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2020, 97, 312–320. [Google Scholar] [CrossRef]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. HLH Across Speciality Collaboration, U.K. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.; Perlman, S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welt, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.F.; Brindisi, G.; Indolfi, C.; Diaferio, L.; Marchese, G.; Ghiglioni, D.G.; Zicari, A.M.; Miraglia Del Giudice, M. Upper airway involvement in pediatric COVID-19. Pediatric Allergy Immunol. 2020, 31 (Suppl. S26), 85–88. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, H.J.; Tsang, R.S.; Byford, R.; Andrews, N.J.; Sherlock, J.; Pillai, P.S.; Williams, J.; Button, E.; Campbell, H.; Sinnathamby, M.; et al. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response among individuals in clinical risk groups. J. Infect. 2022, 3. [Google Scholar] [CrossRef] [PubMed]
- Stratton, C.W.; Tang, Y.W.; Lu, H. Pathogenesis-directed therapy of 2019 novel coronavirus disease. J. Med. Virol. 2021, 93, 1320–1342. [Google Scholar] [CrossRef]
- Li, J.Z.; Gandhi, R.T. Realizing the Potential of Anti-SARS-CoV-2 Monoclonal Antibodies for COVID-19 Management. JAMA 2022, 327, 427–429. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kannan, S.; Shaik Syed Ali, P.; Sheeza, A. Omicron (B.1.1.529)—Variant of concern—Molecular profile and epidemiology: A mini review. Eur. Rev. Med. Pharmacol. Sci. 2021, 24, 8019–8022. [Google Scholar]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gois, P.; Ferreira, D.; Olenski, S.; Seguro, A. Vitamin D and infectious diseases: Simple bystander or contributing factor? Nutrients 2017, 9, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber-Bzura, B.M. Vitamin D and influenza—Prevention or therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef] [Green Version]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olliver, M.; Spelmink, L.; Hiew, J.; Meyer-Hoffert, U.; Henriques-Normark, B.; Bergman, P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J. Infect. Dis. 2013, 208, 1474–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiller, C.L.; Martineau, A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [Green Version]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Griffiths, C.J.; Martineau, A.R. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Antibacterial effects of vitamin D. Nat. Rev. Endocrinol. 2011, 7, 337–345. [Google Scholar] [CrossRef]
- Zemb, P.; Bergman, P.; Camargo, C.A.; Cavalier, E.; Cormier, C.; Courbebaisse, M.; Hollis, B.; Joulia, F.; Minisola, S.; Pilz, S.; et al. Vitamin D Deficiency and the COVID-19 Pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Ali, N. Role of Vitamin D in Preventing of COVID-19 Infection, Progression and Severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef]
- Ueland, P.M.; McCann, A.; Midttun, O.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Aspects Med. 2017, 53, 10–27. [Google Scholar] [CrossRef]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447S–463S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimzadeh-Attari, V.; Panahi, G.; Hebert, J.R.; Ostadrahimi, A.; Saghafi-Asl, M.; Lotfi-Yaghin, N.; Baradaran, B. Nutritional approach for increasing public health during pandemic of COVID-19: A comprehensive review of antiviral nutrients and nutraceuticals. Health Promot. Perspect. 2021, 11, 119–136. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Zhong, N.; Carlson, B.A.; Perella, C.M.; Hatfield, D.L.; Beck, M.A. Decreased selenoprotein expression alters the immune response during influenza virus infection in mice. J. Nutr. 2007, 137, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Omega-3 fatty acids and inflammation. Br. J. Clin. Pharmacol. 2012, 75, 645–662. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=search (accessed on 5 March 2020).
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef]
- Lai, Y.J.; Chang, H.S.; Yang, Y.P.; Lin, T.W.; Lai, W.Y.; Lin, Y.Y.; Chang, C.C. The role of micronutrient and immunomodulation effect in the vaccine era of COVID-19. J. Chin. Med. Assoc. 2021, 84, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Cherian, J.J.; Sharma, A. Exploring links between vitamin D deficiency and COVID-19. PLoS Pathog. 2020, 16, e1008874. [Google Scholar] [CrossRef] [PubMed]
- WHO EMRO. Nutrition Advice for Adults during the COVID-19 Outbreak. Available online: http://www.emro.who.int/nutrition/nutrition-infocus/nutrition-advice-for-adults-during-the-COVID-19-outbreak.html. (accessed on 30 September 2020).
- Dissanayake, H.A.; de Silva, N.L.; Sumanatilleke, M.; de Silva, S.D.N.; Gamage, K.K.K.; Dematapitiya, C.; Kuruppu, D.C.; Ranasinghe, P.; Pathmanathan, S.; Katulanda, P. Prognostic and therapeutic role of vitamin D in COVID-19: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Clavé, P.; Collins, P.F.; Holdoway, A.; Wischmeyer, P.E. Recovery Focused Nutritional Therapy across the Continuum of Care: Learning from COVID-19. Nutrients 2021, 13, 3293. [Google Scholar] [CrossRef] [PubMed]
- Hawryłkowicz, V.; Lietz-Kijak, D.; Kaźmierczak-Siedlecka, K.; Sołek-Pastuszka, J.; Stachowska, L.; Folwarski, M.; Parczewski, M.; Stachowska, E. Patient Nutrition and Probiotic Therapy in COVID-19: What Do We Know in 2021? Nutrients 2021, 13, 3385. [Google Scholar] [CrossRef]
- Khatiwada, S.; Subedi, A. A Mechanistic Link Between Selenium and Coronavirus Disease 2019 (COVID-19) Curr. Nutr. Rep. 2021, 10, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.O.; Jamshaid, H.; Saif, A.; Hussain, T. Immunomodulatory role and potential utility of various nutrients and dietary components in SARS-CoV-2 infection. Int. J. Vitam. Nutr. Res. 2021, 2, 45–50. [Google Scholar] [CrossRef]
- Daei Sorkhabi, A.; Sarkesh, A.; Daei Sorkhabi, A.; Entezari-Maleki, T.; Rashedi, J.; Bannazadeh Baghi, H. Vitamin supplementation as a potential adjunctive therapeutic approach for COVID-19: Biological and clinical plausibility. J. Basic Clin. Physiol. Pharmacol. 2021, 33, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Luo, G.; Yuan, J.; Wang, Y.; Yang, X.; Wang, X.; Li, G.; Liu, Z.; Zhong, N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat. Inflamm. 2014, 2014, 426740. [Google Scholar] [CrossRef] [Green Version]
- Hagel, A.F.; Layritz, C.M.; Hagel, W.H.; Hagel, H.J.; Hagel, E.; Dauth, W.; Kressel, J.; Regnet, T.; Rosenberg, A.; Neurath, M.F.; et al. Intravenous infusion of ascorbic acid decreases serum histamine concentrations in patients with allergic and non-allergic diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Roy, A.; Maitra, S.; Gulati, A.; Khanna, P.; Baidya, D.K. Vitamin C and COVID-19 treat-ment: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102324. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Chaurasia, R.; Sengar, N.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- D’Avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.L.; Nan, D.; Fernandez-Ayala, M.; García-Unzueta, M.; Hernández- Hernández, M.A.; López-Hoyos, M.; Muñoz-Cacho, P.; Olmos, J.M.; Gutiérrez- Cuadra, M.; Ruiz-Cubillán, J.; et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J. Clin. Endocrinol. Metab. 2020, 106, e1343–e1353. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Jahaj, E.; Pratikaki, M.; Orfanos, S.E.; Dimopoulou, I.; Kotanidou, A. Low 25-hydroxyvitamin D levels on admission to the intensive care unit may predispose COVID-19 pneumonia patients to a higher 28- day mortality risk: A pilot study on a Greek ICU cohort. Nutrients 2020, 12, 3773. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled study (SHADE study). Postgrad. Med. J. 2020, 98, 87–90. [Google Scholar] [CrossRef]
- Loucera, C.; Peña-Chilet, M.; Esteban-Medina, M.; Muñoyerro-Muñiz, D.; Villegas, R.; Lopez-Miranda, J.; Rodriguez-Baño, J.; Túnez, I.; Bouillon, R.; Dopazo, J.; et al. Real world evidence of calcifediol or vitamin D prescription and mortality rate of COVID-19 in a retrospective cohort of hospitalized Andalusian patients. Sci. Rep. 2021, 11, 23380. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Quesada-Gomez, J.M. Vitamin D Endocrine System and COVID-19. JBMR Plus 2021, 5, e10576. [Google Scholar] [CrossRef]
- Nogues, X.; Ovejero, D.; Pineda-Moncusí, M.; Bouillon, R.; Arenas, D.; Pascual, J.; Ribes, A.; Guerri-Fernandez, R.; Villar-Garcia, J.; Rial, A.; et al. Calcifediol Treatment and COVID-19-Related Outcomes. J. Clin. Endocrinol. Metab. 2021, 106, e4017–e4027. [Google Scholar] [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F.; Jude, E.B. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: A cross-sectional multi-centre observational study. Nutrients 2020, 12, 3799. [Google Scholar] [CrossRef]
- Alcala-Diaz, J.F.; Limia-Perez, L.; Gomez-Huelgas, R.; Martin-Escalante, M.D.; Cortes-Rodriguez, B.; Zambrana-Garcia, J.L.; Entrenas-Castillo, M.; Perez-Caballero, A.I.; López-Carmona, M.D.; Garcia-Alegria, J.; et al. Calcifediol Treatment and Hospital Mortality Due to COVID-19: A Cohort Study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef] [PubMed]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Barrios, J.M.V.; Alcalá Díaz, J.F.; Miranda, J.L.; Bouillon, R.; Gomez, J.M.Q. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Treatment Guidelines. Vitamin D. NIH, Bethesda, MD. NIH. Available online: https://www.COVID19treatmentguidelines.nih.gov/adjunctive-therapy/vitamin-d/ (accessed on 17 July 2020).
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, M.A.; McKean, M.; Rutman, A.; Myint, B.S.; Silverman, M.; O’Callaghan, C. The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur. Respir. J. 2001, 18, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Woodworth, B.A.; Zhang, S.; Tamashiro, E.; Bhargave, G.; Palmer, J.N.; Cohen, N.A. Zinc increases ciliary beat frequency in a calcium-dependent manner. Am. J. Rhinol. Allergy 2010, 24, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Darma, A.; Athiyyah, A.F.; Ranuh, R.G.; Merbawani, W.; Setyoningrum, R.A.; Hidajat, B.; Siti Nurul Hidayati, S.N.; Endaryanto, A.; Sudarmo, S.M. Zinc supplementation effect on the bronchial cilia length, the number of cilia, and the number of intact bronchial cell in zinc deficiency rats. Indones. Biomed. J. 2020, 12, 78–84. [Google Scholar] [CrossRef]
- Ishida, T. Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ Ions for host cell-virus growth inhibition. AJBSR 2019, 2, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Wen, W.; Su, W.; Tang, H.; Le, W.; Zhang, X.; Zheng, Y.; Liu, X.; Xie, L.; Li, J.; Ye, J.; et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020, 6, 31. [Google Scholar] [CrossRef]
- Military Hospital of Tunis. A Study of Hydroxychloroquine and Zinc in the Prevention of COVID-19 Infection in Military Healthcare Workers (COVID-Milit). ClinicalTrials.gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04377646 (accessed on 17 July 2020).
- Zhang, L.; Liu, Y. Potential interventions for novel coronavirus in China: A systematic review. J. Med. Virol. 2020, 92, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Basil, M.C.; Levy, B.D. Specialized pro-resolution mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Moss, J.W.E.; Ramji, D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Office of the Commissioner FDA. Food Safety Modernization Act (FSMA). Available online: https://www.fda.gov/food/guidance-regulation-food-and-dietary-supplements/food-safety-modernization-act-fsma (accessed on 2 December 2021).
- Panigrahy, D.; Gilligan, M.M.; Huang, S.; Gartung, A.; Cortés-Puch, I.; Sime, P.J.; Phipps, R.P.; Serhan, C.N.; Hammock, B.D. Inflammation resolution: A dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev. 2020, 39, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Regidor, P.A.; Santos, F.G.; Rizo, J.M.; Egea, F.M. Pro resolving inflammatory effects of the lipid mediators of omega 3 fatty acids and its implication in SARS COVID-19. Med. Hypotheses 2020, 145, 110340. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhao, Y.; Huang, Q.; Li, J. n-3 Polyunsaturated fatty acids improve inflammation via inhibiting sphingosine kinase 1 in a rat model of parenteral nutrition and CLP-induced sepsis. Lipids 2016, 51, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Polyunsaturated fatty acids and sepsis. Nutrition 2019, 65, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, X.; Qiu, Y.; Song, Y.; Feng, F.; Feng, J.; Song, Q.; Jia, Q.; Wang, J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE 2020, 15, e0235458. [Google Scholar] [CrossRef] [PubMed]
- Coperchinia, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, J.; Gowdy, K.M.; Shaikh, S.R. N-3 polyunsaturated fatty acids modulate B cell activity in pre-clinical models: Implications for the immune response to infections. Eur. J. Pharmacol. 2016, 785, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, A.; Tintle, N.L.; Myers, M.; Lockshon, L.; Bacareza, H.; Harris, W.S. Omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot. Essent. Fat. Acids 2021, 166, 102250. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and cardiovascular disease: Are marine phospholipids the answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef] [PubMed]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2021, 45, 100694. [Google Scholar] [CrossRef] [PubMed]
- William, J.; Gorog, D.A. Incidence of thrombotic complications in COVID-19. J. Thromb. Thrombolysis 2021, 28, 1–8. [Google Scholar]
- Montreuil, J.; Tonnelat, J.; Mullet, S. Preparation and properties of lactotransferrin of human milk. Biochim. Biophys. Acta 1960, 45, 413–421. [Google Scholar] [CrossRef]
- Groves, M.L. The isolation of a red protein from milk. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Telang, S. Lactoferrin: A critical player in neonatal host defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, P.P.; Uribe-Luna, S.; Conneely, O.M. Lactoferrin and host defense. Biochem. Cell Biol. 2002, 80, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in aseptic and septic inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutone, A.; Lepanto, M.S.; Rosa, L.; Scotti, M.G.; Rossi, A.; Ranucci, S.; De Fino, I.; Bragonzi, A.; Valenti, P.; Musci, G.; et al. Aerosolized bovine lactoferrin counteracts infection, inflammation and iron dysbalance in a cystic fibrosis mouse model of pseudomonas aeruginosa chronic lung infection. Int. J. Mol. Sci. 2019, 20, 2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin Efficiently Counteracts the Inflammation-Induced Changes of the Iron Homeostasis System in Macrophages. Front. Immunol. 2017, 8, 705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Miesbach, W.; Makris, M. COVID-19: Coagulopathy, risk of thrombosis, and the rationale for anticoagulation. Clin. Appl. Thromb. 2020, 26, 1076029620938149. [Google Scholar] [CrossRef]
- Zuo, Y.; Warnock, M.; Harbaugh, A.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Knight, J.S.; Kanthi, Y.; Lawrence, D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as protective natural barrier of respiratory and intestinal mucosa against coronavirus infection and inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin against SARS-CoV-2: In vitro and in silico evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Tripepi, G.; Naldi, E.; Aimati, M.; Santangeli, S.; Venditto, F.; Caldarelli, M.; Valenti, P. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med. 2021, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Health 2021, 18, 10985. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Saha, R.K.; Takahashi, T.; Suzuki, T. Glucosyl hesperidin prevents influenza a virus replication in vitro by inhibition of viral sialidase. Biol. Pharm. Bull. 2009, 32, 1188–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Sun, G.; Zhu, Z. Hesperidin attenuates influenza A virus (H1N1) induced lung injury in rats through its anti-inflammatory effect. Antivir. Ther. 2018, 23, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.A.; El-Ashmawy, N.E.; Okasha, K.M. Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection? Med. Hypotheses 2020, 144, 109957. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef]
- Balmeh, N.; Mahmoudi, S.; Mohammadi, N.; Karabedianhajiabadi, A. Predicted therapeutic targets for COVID-19 disease by inhibiting SARS-CoV-2 and its related receptors. Inform. Med. Unlocked 2020, 20, 100407. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Bellavite, P.; Zanolin, E.; McCullough, P.A.; Pandolfi, S.; Affuso, F. Retrospective Study of Outcomes and Hospitalization Rates of Patients in Italy with a Confirmed Diagnosis of Early COVID-19 and Treated at Home Within 3 Days or After 3 Days of Symptom Onset with Prescribed and Non-Prescribed Treatments Between November 2020 and August 2021. Med. Sci. Monit. 2021, 27, e935379. [Google Scholar]
- Cawood, A.L.; Walters, E.R.; Smith, T.R.; Sipaul, R.H.; Stratton, R.J. A Review of Nutrition Support Guidelines for Individuals with or Recovering from COVID-19 in the Community. Nutrients 2020, 12, 3230. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.; Hatch, R.A.; Bishop, J.; Morgan, K.; Jenkinson, C.; Cuthbertson, B.H.; Brett, S.J. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: A 12-month follow-up study. Crit. Care 2013, 17, R100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol. Neurobiol. 2022, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Sigfrid, L.; Jesudason, E.; Lim, W.S.; Rello, J.; Amuasi, H.; Drake, T.M.; Bozza, F.; Palmieri, C.; Munblit, D.; Holter, J.C.; et al. What Is the Recovery Rate and Risk of Long-Term Consequences from COVID-19? A Harmonised, Global Longitudinal Observational Study Protocol. BMJ Open 2021, 11, e043887. [Google Scholar] [CrossRef] [PubMed]
- Javid Mishamandani, Z.; Norouzy, A.; Hashemian, S.M.; Khoundabi, B.; Rezaeisadrabadi, M.; Safarian, M.; Nematy, M.; Pournik, O.; Jamialahmadi, T.; Shadnoush, M.; et al. Nutritional status of patients hospitalized in the intensive care unit: A comprehensive report from Iranian hospitals, 2018. J. Crit. Care 2019, 54, 151–158. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. Endorsed by the EC: ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Englund, K. Clinical presentation and course of COVID-19. Clevel. Clin. J. Med. 2020, 87, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E. Nutrition therapy in sepsis. Crit. Care Clin. 2018, 34, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Martinez Garcia, R.M.; Jimenez Ortega, A.I.; Lopez Sobaler, A.M.; Ortega, R.M. Nutrition strategies that improve cognitive function. Nutr. Hosp. 2018, 35, 16–19. [Google Scholar] [PubMed]
- Beilharz, J.E.; Morris, M.G. Role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients 2015, 7, 6719–6738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective actions of dietary choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, L.; Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakke, M.; Banati, D.; Calabrese, V.; Cederholm, T.; Cryan, J.; et al. Poor cognitive ageing vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warthon-Medina, M.; Moran, V.H.; Stammers, A.L.; Dillon, S.; Qualter, P.; Nissensohn, M.; Serra Majem, L.; Lowe, N.M. Zinc intake, status and indices of cognitive function in adults and children: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 649–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlogl, M.; Holick, M.F. Vitamin D and neurocognitive function. Clin. Interv. Aging 2014, 9, 559–568. [Google Scholar] [PubMed] [Green Version]
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Aparicio Vizuete, A.; Robles, F.; Rodriguez-Rodriguez, E.; Lopez-Sobaler, A.M.; Ortega, R.M. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur. J. Nutr. 2010, 49, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M. Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 2: Macronutrients. J. Nutr. Health Aging 2006, 10, 386–399. [Google Scholar]
- Kim, S.H.; Park, Y.M.; Choi, B.Y.; Kim, M.K.; Roh, S.; Kim, K.; Yang, Y.J. Associations of serum levels of vitamins A, C, and E with the risk of cognitive impairment among elderly Koreans. Nutr. Res. Pract. 2018, 12, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Markiewicz-Zukowska, R.; Gutowska, A.; Borawska, M.H. Serum zinc concentrations correlate with mental and physical status of nursing home residents. PLoS ONE 2015, 10, e0117257. [Google Scholar] [CrossRef]
- Andruchow, N.D.; Konishi, K.; Shatenstein, B.; Bohbot, V.D. A lower ratio of omega-6 to omega-3 fatty acids predicts better hippocampus-dependent spatial memory and cognitive status in older adults. Neuropsychology 2017, 31, 724–734. [Google Scholar] [CrossRef]
- Guo, X.M.; Liu, H.; Qian, J. Daily iron supplementation on cognitive performance in primary-school-aged children with and without anemia: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 16107–16111. [Google Scholar]
- Bourre, J.M. Effects of nutrients (in food) on the structure and function of the nervous system: Update on dietary requirements for brain. Part 1: Micronutrients. J. Nutr. Health Aging 2006, 10, 377–385. [Google Scholar]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Seetharaman, S.; Andel, R.; McEvoy, C.; Dahl Aslan, A.K.; Finkel, D.; Pedersen, N.L. Blood glucose, diet-based glycemic load and cognitive aging among dementia-free older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2015, 70, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.S.; Portales-Cervantes, L.; Leong, E. Mast cell responses to viruses and pathogen products. Int. J. Mol. Sci. 2019, 20, 4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theoharides, T.C.; Tsilioni, I.; Ren, H. Recent advances in our understanding of mast cell activation—Or should it be mast cell mediator disorders? Expert Rev. Clin. Immunol. 2019, 15, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef]
- Theoharides, T.C. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors 2020, 46, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. LICORN and the Lille COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Louca, P.; Murra, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health 2021, 4, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, F.; Santamaria, S.; Montesano, P. Gender difference, nutritional supplements and drug use in sport to enhancing performance: An italian revision over the last decade. Sport Mont. 2019, 17, 69–73. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motti, M.L.; Tafuri, D.; Donini, L.; Masucci, M.T.; De Falco, V.; Mazzeo, F. The Role of Nutrients in Prevention, Treatment and Post-Coronavirus Disease-2019 (COVID-19). Nutrients 2022, 14, 1000. https://doi.org/10.3390/nu14051000
Motti ML, Tafuri D, Donini L, Masucci MT, De Falco V, Mazzeo F. The Role of Nutrients in Prevention, Treatment and Post-Coronavirus Disease-2019 (COVID-19). Nutrients. 2022; 14(5):1000. https://doi.org/10.3390/nu14051000
Chicago/Turabian StyleMotti, Maria Letizia, Domenico Tafuri, Lorenzo Donini, Maria Teresa Masucci, Valentina De Falco, and Filomena Mazzeo. 2022. "The Role of Nutrients in Prevention, Treatment and Post-Coronavirus Disease-2019 (COVID-19)" Nutrients 14, no. 5: 1000. https://doi.org/10.3390/nu14051000
APA StyleMotti, M. L., Tafuri, D., Donini, L., Masucci, M. T., De Falco, V., & Mazzeo, F. (2022). The Role of Nutrients in Prevention, Treatment and Post-Coronavirus Disease-2019 (COVID-19). Nutrients, 14(5), 1000. https://doi.org/10.3390/nu14051000









