Phenotypes and Endotypes of Peach Allergy: What Is New?
Abstract
:1. Epidemiology
2. Peach Allergens
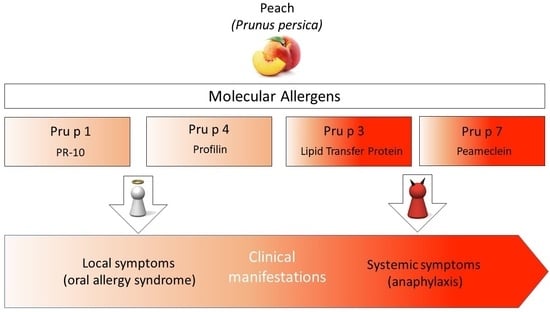
2.1. Pru p 1
2.2. Pru p 3
2.3. Pru p 4
2.4. Pru p 7
2.5. Pru p 9
3. Clinical Manifestations
3.1. Peach Allergy Secondary to Pollen Allergy
3.2. Primary Peach Allergy
3.3. Peamaclein Allergy
4. Diagnosis
4.1. Clinical History
4.2. IgE Sensitization
4.3. Oral Food Challenge
5. Prevention and Management
5.1. Primary and Secondary Prevention
5.2. Management of Peach Allergy
5.3. Allergen Immunotherapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haleema, R.; Shenoy, A.; Shabraya, A.R. A Review on Pharmacological Activities of Prunus persica. Int. J. Pharm. Sci. Rev. Res. 2020, 60, 38–40. [Google Scholar]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef] [Green Version]
- The Plant List: Rosaceae. Available online: http://www.theplantlist.org/1.1/browse/A/Rosaceae (accessed on 3 January 2022).
- The Families of Flowering Plants. Available online: http://www1.biologie.uni-hamburg.de/b-online/delta/angio/index.htm (accessed on 3 January 2022).
- Kant, R.; Shukla, K.R.; Shukla, A. A Review on Peach (Prunus persica): An Asset of Medicinal Phytochemicals. Int. J. Res. Appl. Sci. Eng. Technol. 2018, 6, 2186–2200. [Google Scholar] [CrossRef]
- Cuesta-Herranz, J.; Lázaro, M.; de las Heras, M.; Lluch, M.; Figueredo, E.; Umpierrez, A.; Hernandez, J.; Cuesta, C. Peach allergy pattern: Experience in 70 patients. Allergy 1998, 53, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Burney, P.; Summers, C.; Chinn, S.; Hooper, R.; van Ree, R.; Lidholm, J. Prevalence and distribution of sensitization to foods in the European Community Respiratory Health Survey: A EuroPrevall analysis. Allergy 2010, 65, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Burney, P.G.; Potts, J.; Kummeling, I.; Mills, E.N.C.; Clausen, M.; Dubakiene, R.; Barreales, L.; Fernandez-Perez, C.; Fernandez-Rivas, M.; Le, T.M.; et al. The prevalence and distribution of food sensitization in European adults. Allergy 2014, 69, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyano-Martinez, T.; Pedrosa, M.; Belver, T.; Quirce, S.; Garcia-Ara, C. Peach allergy in Spanish children: Tolerance to the pulp and molecular sensitization profile. Pediatr. Allergy Immunol. 2013, 24, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.A.; Farioli, L.; Stafylaraki, C.; Mascheri, A.; Scibilla, J.; Pravettoni, V.; Primavesi, L.; Piantanida, M.; Nichelatti, M.; Asero, R. Anti-rPru p 3 IgE levels are inversely related to the age at onset of peach-induced severe symptoms reported by peach allergic adults. Int. Arch. Allergy Immunol. 2013, 162, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Le, T.M.; Lindner, T.M.; Pasmans, S.G.; Guikers, C.L.H.; van Hoffen, E.; Bruijnzeel-Koomen, C.A.F.M.; Knulst, A.C. Reported food allergy to peanut, tree nuts and fruit: Comparison of clinical manifestations, prescription of medication and impact on daily life. Allergy 2008, 63, 910–916. [Google Scholar] [CrossRef]
- Allergen Nomenclature. WHO/IUIS Allergne Nomenclature Sub-Committee. Available online: http://www.allergen.org/search.php?allergensource=Prunus+persica (accessed on 3 January 2022).
- Gaier, S.; Marsh, J.; Oberhuber, C.; Rigby, N.M.; Lovegrove, A.; Alessandri, S.; Briza, P.; Radauer, C.; Zuidmeer, L.; van Ree, R.; et al. Purification and structural stability of the peach allergens Pru p 1 and Pru p 3. Mol. Nutr. Food Res. 2008, 52 (Suppl. S2), S220–S229. [Google Scholar] [CrossRef]
- Gaier, S.; Oberhuber, C.; Hemmer, W.; Radauer, C.; Rigby, N.M.; Marsh, J.T.; Mills, C.E.; Shewry, P.R.; Hoffmann-Sommergruber, K. Pru p 3 as a marker for symptom severity for patients with peach allergy in a birch pollen environment. J. Allergy Clin. Immunol. 2009, 124, 166–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastre, J. Molecular diagnosis in allergy. Clin. Exp. Allergy 2010, 40, 1442–1460. [Google Scholar] [CrossRef]
- Carnes, J.; Fernandez-Caldas, E.; Gallego, M.T.; Ferrer, A.; Cuesta-Herranz, J. Pru p 3 (LTP) content in peach extracts. Allergy 2002, 57, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Kleine-Tebbe, J.; Scheurer, S. Stable Plant Food Allergens I: Lipid Transfer Proteins. In Molecular Allergy Diagnostics, 1st ed.; Kleine-Tebbe, J., Jacob, T., Eds.; Springer: Cham, Switzerland, 2017; pp. 57–71. [Google Scholar] [CrossRef]
- Egger, M.; Hauser, M.; Mari, A.; Ferreira, F.; Gadermaier, G. The role of lipid transfer proteins in allergic diseases. Curr. Allergy Asthma Rep. 2010, 10, 326–335. [Google Scholar] [CrossRef]
- Pascal, M.; Munoz-Cano, R.; Reina, Z.; Palacin, A.; Vilella, R.; Picado, C.; Juan, M.; Sánchez-López, J.; Rueda, M.; Salcedo, G.; et al. Lipid transfer protein syndrome: Clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin. Exp. Allergy 2012, 42, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Zamieskova, L.; Žiarovská, J.; Bilčíková, J.; Fialková, V. Natural variability of restriction profiles in non-coding part of Prunus persica (L.) Batsch. Pru p 3 gene. Acta Fytotech. Zootech. 2020, 23, 1–6. [Google Scholar] [CrossRef]
- Salcedo, G.; Sanchez-Monge, R.; Barber, D.; Diaz-Perales, A. Plant non-specific lipid transfer proteins: An interface between plant defence and human allergy. Biochim. Biophys. Acta 2007, 1771, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Herranz, J.; Lazaro, M.; Martinez, A.; Figueredo, E.; Palacios, R.; de-Las-Heras, M.; Martínez, J. Pollen allergy in peach-allergic patients: Sensitization and cross-reactivity to taxonomically unrelated pollens. J. Allergy Clin. Immunol. 1999, 104 Pt 1, 688–694. [Google Scholar] [CrossRef]
- Ando, Y.; Miyamoto, M.; Kato, M.; Nakayama, M.; Fukuda, H.; Yoshihara, S. Pru p 7 Predicts Severe Reactions after Ingestion of Peach in Japanese Children and Adolescents. Int. Arch. Allergy Immunol. 2020, 181, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ma, Y.; Chen, L.; Xie, R.; Zhang, X.; Zhang, B.; Lu, M.; Wu, S.; Gilissen, L.J.W.J.; van Ree, R.; et al. Differential transcript abundance and genotypic variation of four putative allergen-encoding gene families in melting peach. Tree Genet. Genom. 2011, 7, 903–916. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Farioli, L.; Pravettoni, V.; Scibilia, J.; Mascheri, A.; Borgonovo, L.; Piantanida, M.; Primavesi, L.; Stafylaraki, C.; Pasqualetti, S.; et al. Pru p 3-sensitised Italian peach-allergic patients are less likely to develop severe symptoms when also presenting IgE antibodies to Pru p 1 and Pru p 4. Int. Arch. Allergy Immunol. 2011, 156, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Tuppo, L.; Alessandri, C.; Pomponi, D.; Picone, D.; Tamburini, M.; Ferrara, R.; Petriccione, M.; Mangone, I.; Palazzo, P.; Liso, M.; et al. Peamaclein—A new peach allergenic protein: Similarities, differences and misleading features compared to Pru p 3. Clin. Exp. Allergy 2013, 43, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Nahirnak, V.; Rivarola, M.; Almasia, N.I.; Barrios Baron, M.P.; Hopp, H.E.; Vile, D.; Vile, D.; Paniego, N.; Vazquez Rovere, C. Snakin-1 affects reactive oxygen species and ascorbic acid levels and hormone balance in potato. PLoS ONE 2019, 14, e0214165. [Google Scholar] [CrossRef]
- Inomata, N.; Miyakawa, M.; Aihara, M. Gibberellin-regulated protein in Japanese apricot is an allergen cross-reactive to Pru p 7. Immun. Inflamm. Dis. 2017, 5, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Tuppo, L.; Alessandri, C.; Pasquariello, M.S.; Petriccione, M.; Giangrieco, I.; Tamburrini, M.; Mari, A.; Ciardiello, M.A. Pomegranate Cultivars: Identification of the New IgE-Binding Protein Pommaclein and Analysis of Antioxidant Variability. J. Agric. Food Chem. 2017, 65, 2702–2710. [Google Scholar] [CrossRef]
- Inomata, N.; Miyakawa, M.; Ikeda, N.; Oda, K.; Aihara, M. Identification of gibberellin-regulated protein as a new allergen in orange allergy. Clin. Exp. Allergy 2018, 48, 1509–1520. [Google Scholar] [CrossRef]
- Charpin, D.; Pichot, C.; Belmonte, J.; Sutre, J.; Zidkova, J.; Chanez, P.; Shahali, Y.; Sénéchal, H.; Poncet, P. Cypress Pollinosis: From Tree to Clinic. Clin. Rev. Allergy Immunol. 2019, 56, 174–195. [Google Scholar] [CrossRef] [PubMed]
- Klingebiel, C.; Chantran, Y.; Arif-Lusson, R.; Ehrenberg, A.E.; Östling, J.; Poisson, A.; Liabeuf, V.; Agabriel, C.; Birnbaum, J.; Porri, F.; et al. Pru p 7 sensitization is a predominant cause of severe, cypress pollen-associated peach allergy. Clin. Exp. Allergy 2019, 49, 526–536. [Google Scholar] [CrossRef]
- Biagioni, B.; Tomei, L.; Valleriani, C.; Liccioli, G.; Barni, S.; Sarti, L.; Citera, F.; Giovannini, M.; Mori, F. Allergy to Gibberellin-Regulated Proteins (Peamaclein) in Children. Int. Arch. Allergy Immunol. 2021, 182, 1194–1199. [Google Scholar] [CrossRef]
- Allergy & Autoimmune Disease. f95 Peach. Available online: https://www.thermofisher.com/diagnostic-education/hcp/it/it/resource-center/allergen-encyclopedia/whole-allergens.html?key=f95 (accessed on 3 January 2022).
- Palacin, A.; Rivas, L.A.; Gomez-Casado, C.; Aguirre, J.; Tordesillas, L.; Bartra, J.; Blanco, C.; Carrillo, T.; Cuesta-Herranz, J.; Bonny, J.A.; et al. The involvement of thaumatin-like proteins in plant food cross-reactivity: A multicenter study using a specific protein microarray. PLoS ONE 2012, 7, e44088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Mancebo, E.; Gonzalez-de-Olano, D.; Trujillo, M.; Santos, S.; Gandolfo-Cano, M.; Melendez, A.; Juárez, R.; Morales, P.; Calso, A.; Mazuela, O.; et al. Prevalence of Sensitization to Lipid Transfer Proteins and Profilins in a Population of 430 Patients in the South of Madrid. J. Investig. Allergol. Clin. Immunol. 2011, 21, 278–282. [Google Scholar] [PubMed]
- Victorio Puche, L.; Somoza, M.L.; Lopez-Sanchez, J.D.; Garrido-Arandia, M.; Diaz-Peralesm, A.; Blanca, M. Peach Tree Pollen and Prunus persica 9 Sensitisation and Allergy in Children and Adolescents. Int. Arch. Allergy Immunol. 2019, 180, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Somoza, M.L.; Pérez-Sánchez, N.; Victorio-Puche, L.; Martín-Pedraza, L.; Esteban Rodríguez, A.; Blanca-López, N.; Abel Fernández González, E.; Ruano-Zaragoza, M.; Prieto-Moreno Pfeifer, A.; Fernández Caldas, E.; et al. Subjects develop tolerance to Pru p 3 but respiratory allergy to Pru p 9: A large study group from a peach exposed population. PLoS ONE 2021, 16, e0255305. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Asero, R. Peach-induced contact urticaria is associated with lipid transfer protein sensitization. Int. Arch. Allergy Immunol. 2011, 154, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Calderón, R.; Gonzalo-Garijo, M.Á.; Rodríguez-Velasco, F.J.; Sánchez-Vega, S.; Bartolomé-Zavala, B. Occupational respiratory allergy in peach crop workers. Allergy 2017, 72, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rivas, M.; Bolhaar, S.; González-Mancebo, E.; Asero, R.; van Leeuwen, A.; Bohle, B.; Ma, Y.; Ebner, C.; Rigby, N.; Sancho, A.I.; et al. Apple allergy across Europe: How allergen sensitization profiles determine the clinical expression of allergies to plant foods. J. Allergy Clin. Immunol. 2006, 118, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rivas, M.; González-Mancebo, E.; Rodríguez-Pérez, R.; Benito, C.; Sánchez-Monge, R.; Salcedo, G.; Alonso, M.D.; Rosado, A.; Tejedor, M.A.; Vila, C.; et al. Clinically relevant peach allergy is related to peach lipid transfer protein, Pru p 3, in the Spanish population. J. Allergy Clin. Immunol. 2003, 112, 789–795. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, D.H.; Woo, S.H.; Seol, S.H.; Choi, S.P. Targeted temperature management after cardiac arrest with anaphylaxis. Am. J. Emerg. Med. 2017, 35, 807. [Google Scholar] [CrossRef] [PubMed]
- Barradas Lopes, J.; Santa, C.; Valente, C.; Presa, A.R.; Sousa, M.J.; Reis Ferreira, A. Allergy to lipid transfer proteins (LTP) in a pediatric population. Eur. Ann. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Poncet, P.; Sénéchal, H.; Charpin, D. Update on pollen-food allergy syndrome. Expert. Rev. Clin. Immunol. 2020, 16, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Werfel, T.; Asero, R.; Ballmer-Weber, B.K.; Beyer, K.; Enrique, E.; Knulst, A.C.; Mari, A.; Muraro, A.; Ollert, M.; Poulsen, L.K.; et al. Position paper of the EAACI: Food allergy due to immunological cross-reactions with common inhalant allergens. Allergy 2015, 70, 1079–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Sicherer, S.H.; Nowak-Wegrzyn, A. A survey on the management of pollen-food allergy syndrome in allergy practices. J. Allergy Clin. Immunol. 2003, 112, 784–788. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, Y.; Yoo, Y.; Kim, D.K.; Yang, H.J.; Park, H.S.; Lee, H.J.; Kim, M.A.; Jeong, Y.Y.; Kim, B.S.; et al. Clinical Manifestations and Risk Factors of Anaphylaxis in Pollen-Food Allergy Syndrome. Yonsei Med. J. 2019, 60, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S116–S125. [Google Scholar] [CrossRef]
- Sanchez-López, J.; Tordesillas, L.; Pascal, M.; Muñoz-Cano, R.; Garrido, M.; Rueda, M.; Vilella, R.; Valero, A.; Díaz-Perales, A.; Picado, C.; et al. Role of Art v 3 in pollinosis of patients allergic to Pru p 3. J. Allergy Clin. Immunol 2014, 133, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Borghesan, F.; Mistrello, G.; Roncarolo, D.; Amato, S.; Plebani, M.; Asero, R. Respiratory allergy to lipid transfer protein. Int. Arch. Allergy Immunol. 2008, 147, 161–165. [Google Scholar] [CrossRef]
- Lyons, S.A.; Burney, P.G.J.; Ballmer-Weber, B.K.; Fernandez-Rivas, M.; Barreales, L.; Clausen, M.; Dubakiene, R.; Fernandez-Perez, C.; Fritsche, P.; Jedrzejczak-Czechowicz, M.; et al. Food Allergy in Adults: Substantial Variation in Prevalence and Causative Foods Across Europe. J. Allergy Clin. Immunol. Pract. 2019, 7, 1920–1928.e11. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Antonicelli, L.; Arena, A.; Bommarito, L.; Caruso, B.; Colombo, G.; Crivellaro, M.; De Carli, M.; Della Torre, E.; Della Torre, F.; et al. Causes of food-induced anaphylaxis in Italian adults: A multi-centre study. Int. Arch. Allergy Immunol. 2009, 150, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; Bartra, J.; Ebo, D.G.; Antje Faber, M.; Fernández-Rivas, M.; Gomez, F.; Luengo, O.; Till, S.J.; Asero, R.; Barber, D.; et al. The diagnosis and management of allergic reactions in patients sensitized to non-specific lipid transfer proteins. Allergy 2021, 76, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Piantanida, M.; Pinter, E.; Pravettoni, V. The clinical relevance of lipid transfer protein. Clin. Exp. Allergy 2018, 48, 6–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scala, E.; Till, S.J.; Asero, R.; Abeni, D.; Guerra, E.C.; Pirrotta, L.; Paganelli, R.; Pomponi, D.; Giani, M.; De Pità, O.; et al. Lipid transfer protein sensitization: Reactivity profiles and clinical risk assessment in an Italian cohort. Allergy 2015, 70, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Basagaña, M.; Elduque, C.; Teniente-Serra, A.; Casas, I.; Roger, A. Clinical Profile of Lipid Transfer Protein Syndrome in a Mediterranean Area. J. Investig. Allergol. Clin. Immunol. 2018, 28, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Bogas, G.; Muñoz-Cano, R.; Mayorga, C.; Casas, R.; Bartra, J.; Pérez, N.; Pascal, M.; Palomares, F.; Torres, M.J.; Gómez, F. Phenotyping peach-allergic patients sensitized to lipid transfer protein and analysing severity biomarkers. Allergy 2020, 75, 3228–3236. [Google Scholar] [CrossRef]
- NCBI, National Center for Biotechnology Information, Bethesda, USA. Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov (accessed on 10 February 2022).
- Matricardi, P.M.; Salcedo, G.; Sanchez-Monge, R.; Diaz-Perales, A.; Garcia-Casado, G.; Barber, D. Plant non-specific lipid transfer proteins as food and pollen allergens. Clin. Exp. Allergy 2004, 34, 1336–1341. [Google Scholar]
- Ukleja-Sokołowska, N.; Zacniewski, R.; Gawrońska-Ukleja, E.; Żbikowska-Gotz, M.; Lis, K.; Sokołowski, Ł.; Adamczak, R.; Bartuzi, Z. Food-dependent, exercise-induced anaphylaxis in a patient allergic to peach. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418803154. [Google Scholar] [CrossRef] [Green Version]
- Romano, A.; Scala, E.; Rumi, G.; Gaeta, F.; Caruso, C.; Alonzi, C.; Maggioletti, M.; Ferrara, R.; Palazzo, P.; Palmieri, V.; et al. Lipid transfer proteins: The most frequent sensitizer in Italian subjects with food-dependent exercise-induced anaphylaxis. Clin. Exp. Allergy 2012, 42, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, H.; Santrucek, J.; Melcova, M.; Svoboda, P.; Zídková, J.; Charpin, D.; Guilloux, L.; Shahali, Y.; Selva, M.A.; Couderc, R.; et al. A new allergen family involved in pollen food associated syndrome: Snakin/gibberellin regulated proteins. J. Allergy Clin. Immunol. 2018, 141, 411–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inomata, N. Gibberellin-regulated protein allergy: Clinical features and cross reactivity. Allergol. Int. 2020, 69, 11–18. [Google Scholar] [CrossRef]
- Tuppo, L.; Alessandri, C.; Giangrieco, I.; Tamburrini, M.; Arriaza, R.H.; Chruszcz, M.; Mari, A.; Ciardiello, M.A. When the Frequencies of Sensitization and Elicitation of Allergic Reaction Do Not Correlate—The Case of Ap-ple Gibberellin-Regulated Protein Tested in an Italian Population. Front. Allergy 2021, 2, 745825. [Google Scholar] [CrossRef]
- Muraro, A.; Fernandez-Rivas, M.; Beyer, K.; Cardona, V.; Clark, A.; Eller, E.; Hourihane, J.O.B.; Jutel, M.; Sheikh, A.; Agache, I.; et al. The urgent need for a harmonized severity scoring system for acute allergic reactions. Allergy 2018, 73, 1792–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inomata, N.; Miyakawa, M.; Aihara, M. Eyelid edema as a predictive factor for sensitization to Pru p 7 in peach allergy. J. Dermatol. 2016, 43, 900–905. [Google Scholar] [CrossRef]
- Burks, A.W.; Tang, M.; Sicherer, S.; Muraro, A.; Eigenmann, P.A.; Ebisawa, M.; Fiocchi, A.; Chiang, W.; Beyer, K.; Wood, R.; et al. ICON: Food allergy. J. Allergy Clin. Immunol. 2012, 129, 906–920. [Google Scholar] [CrossRef]
- Inomata, N.; Okazaki, F.; Moriyama, T.; Nomura, Y.; Yamaguchi, Y.; Honjoh, T.; Kawamura, Y.; Narita, H.; Aihara, M. Identification of peamaclein as a marker allergen related to systemic reactions in peach allergy. Ann. Allergy Asthma Immunol. 2014, 112, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. S23), 1–250. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Lemanske, R.F.; Castells, M.; Torres, M.J.; Khan, D.; Simon, H.-U.; Bindslev-Jensen, C.; Burks, W.; Poulsen, L.K.; Sampson, H.A.; et al. Precision medicine in allergic disease-food allergy, drug allergy, and anaphylaxis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. Allergy 2017, 72, 1006–1021. [Google Scholar] [CrossRef] [Green Version]
- Asero, R.; Aruanno, A.; Bresciani, M.; Brusca, I.; Carollo, M.; Cecchi, L.; Cortellini, G.; Deleonardi, G.; Farsi, A.; Ferrarini, E.; et al. Evaluation of two commercial peach extracts for skin prick testing in the diagnosis of hypersensitivity to lipid transfer protein. A multicenter study. Eur. Ann. Allergy Clin. Immunol. 2021, 53, 168–170. [Google Scholar] [CrossRef]
- Somoza, M.L.; Prieto-Moreno Pfeifer, A.; Martín-Pedraza, L.; Victorio Puche, L.; Esteban Rodríguez, A.; Blanca-López, N.; Eva Fernández González, A.; Fernández-Caldas, E.; Morán Morales, M.; Fernández-Sánchez-, F.J.; et al. Skin Testing With Peach Peel Extract Versus Serum IgE to Pru p 3 as a Stronger Predictor of Peach-Induced Anaphylaxis. Allergy Asthma Immunol. Res. 2021, 13, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Kennard, L.; Thomas, I.; Rutkowski, K.; Azzu, V.; Yong, P.F.K.; Kasternow, B.; Hunter, H.; Cabdi, N.M.O.; Nakonechna, A.; Wagner, A. A Multicenter Evaluation of Diagnosis and Management of Omega-5 Gliadin Allergy (Also Known as Wheat-Dependent Exercise-Induced Anaphylaxis) in 132 Adults. J. Allergy Clin. Immunol. Pract. 2018, 6, 1892–1897. [Google Scholar] [CrossRef]
- O’Keefe, A.W.; De Schryver, S.; Mill, J.; Mill, C.; Dery, A.; Ben-Shosha, M. Diagnosis and management of food allergies: New and emerging options: A systematic review. J. Asthma Allergy 2014, 7, 141–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caimmi, D.; Caffarelli, C.; Licari, A.; Miraglia del Giudice, M.; Calvani, M.; Marseglia, G.L.; Marseglia, A.; Ricci, G.; Martelli, A.; Cravidi, C.; et al. Food allergy in primary care. Acta Biomed. 2021, 92 (Suppl. S7), e2021521. [Google Scholar] [CrossRef]
- Fleischer, D.M.; Chan, E.S.; Venter, C.; Spergel, J.M.; Abrams, E.M.; Stukus, D.; Groetch, M.; Shaker, M.; Greenhawt, M. A consensus approach to the primary prevention of food allergy through nutrition: Guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J. Allergy Clin. Immunol. Pract. 2021, 9, 22–43. [Google Scholar] [CrossRef]
- Fiocchi, A.; Vickery, B.P.; Wood, R.A. The use of biologics in food allergy. Clin. Exp. Allergy 2021, 51, 1006–1018. [Google Scholar] [CrossRef]
- Food Safety. EU Law on Food Information to Consumers. Available online: https://ec.europa.eu/food/safety/labelling-and-nutrition/food-information-consumers-legislation_it (accessed on 5 January 2022).
- Patriarca, G.; Nucera, E.; Roncallo, C.; Pollastrini, E.; Bartolozzi, F.; De Pasquale, T.; Buonomo, A.; Gasbarrini, G.; Di Campli, C.; Schiavino, D. Oral desensitizing treatment in food allergy: Clinical and immunological results. Aliment. Pharmacol. Ther. 2003, 17, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Navarro, B.; Alarcon, E.; Claver, A.; Pascal, M.; Diaz-Perales, A.; Cistero-Bahima, A. Oral immunotherapy with peach juice in patients allergic to LTPs. Allergy Asthma Clin. Immunol. 2019, 15, 60. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Rivas, M.; Garrido Fernández, S.; Nadal, J.A.; Díaz de Durana, M.D.; García, B.E.; González-Mancebo, E.; Martín, S.; Barber, D.; Rico, P.; Tabar, A.I. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy 2009, 64, 876–883. [Google Scholar] [CrossRef]
- Moura, A.L.; Pereira, C.; Regateiro, F.S.; Azevedo, J.; Todo Bom, A.; Carrapatoso, I. Pru p 3 sublingual immunotherapy ultra-rush protocol is safe and clinically effective. Eur. Ann. Allergy Clin. Immunol. 2019, 51, 206–212. [Google Scholar] [CrossRef]
- Beitia, J.M.; Castro, A.V.; Cardenas, R.; Pena-Arellano, M.I. Pru p 3 sublingual immunotherapy in patients with Lipid Tranfer Protein Syndrome: Is it worth? Int. Arch. Allergy Immunol. 2021, 182, 447–454. [Google Scholar] [CrossRef] [PubMed]
- González Pérez, A.; Carbonell Martínez, A.; Escudero Pastor, A.I.; Navarro Garrido, C.; Miralles López, J.C. Pru p 3 oral immunotherapy efficacy, induced immunological changes and quality of life improvement in patients with LTP syndrome. Clin. Transl. Allergy 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, I.; Medellín, D.R.; Noguerado-Mellado, B.; Ordoñez, C.L.; Abreu, M.G.; Jimeno Nogales, L.; Rojas-Pérez-Ezquerra, P. Treatment with lipid transfer protein sublingual immunotherapy: Slowing down new sensitizations. Asia Pac. Allergy 2021, 11, e6. [Google Scholar] [CrossRef] [PubMed]
- Caimmi, D.; Demoly, P. Guidelines for the prescription of allergen immunotherapy and patient’s follow-up—Clinical questions and revision of the literature. Rev. Fr. Allergol. 2021, 61, 35–56. [Google Scholar] [CrossRef]
- Hamada, M.; Kagawa, M.; Tanaka, I. Evaluation of subcutaneous immunotherapy with birch pollen extract for pollen-food allergy syndrome. Asia Pac. Allergy 2021, 11, e39. [Google Scholar] [CrossRef]
- Van der Valk, J.P.M.; Nagl, B.; van Wljk, R.G.; Bohle, B.; de Jong, N.W. The Effect of Birch Pollen Immunotherapy on Apple and rMal d 1 Challenges in Adults with Apple Allergy. Nutrients 2020, 12, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy 2018, 73, 799–815. [Google Scholar] [CrossRef] [Green Version]

| Allergen | Biochemical Name | Molecular Weight (kDa) | Main Characteristic |
|---|---|---|---|
| Pru p 1 | Pathogenesis-related protein group 10, (PR-10), Bet v 1 family member | 18 | Mainly found in areas with high birch pollen exposure [10]. |
| Pru p 2 | Thaumatin-like protein (TLP) | 25–28 | Pru p 2 from peach was one of the probable allergens causing fruit allergies [36]. |
| Pru p 3 | Non-specific lipid-transfer protein 1 (nsLTP1) | 10 | Major allergen [10]. Present in the outer surface of peach [27]. In total, 54 (96%) out of 57 children showed positive Pru p 3-sIgE in a Spanish study [10]. |
| Pru p 4 | Profilin | 14 | Minor allergen [10]. In total, 52 (12.1%) out of 430 patients were sensitized to profilins in an adult study [37]. |
| Pru p 7 | Gibberellin-regulated protein (GRP) | 6910.84 Da (Mass spectrometry) | Major allergen [33]. Identified in 2012 [13]. Present both in the pulp and in the peel [27]. Sensitization to Pru p7 was present in 171 (54%) out of 316 subjects with suspected peach allergy [33]. Pru p 7 sensitization was more frequent in peach-allergic (123/198, 62%) than in peach-tolerant (48/118, 41%) patients, p-value = 0.0002 [33]. |
| Pru p 9 | Pathogenesis-related protein PR-1 | 18 | Identified in 2018 [13]. Sensitization to peach-tree pollen was rated third, after olive tree and grass [38], in areas with peach-tree cultivars. In total, 205 (30%) out of 685 children were sensitized to Pru p 9 on skin prick test [38]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barni, S.; Caimmi, D.; Chiera, F.; Comberiati, P.; Mastrorilli, C.; Pelosi, U.; Paravati, F.; Marseglia, G.L.; Arasi, S. Phenotypes and Endotypes of Peach Allergy: What Is New? Nutrients 2022, 14, 998. https://doi.org/10.3390/nu14050998
Barni S, Caimmi D, Chiera F, Comberiati P, Mastrorilli C, Pelosi U, Paravati F, Marseglia GL, Arasi S. Phenotypes and Endotypes of Peach Allergy: What Is New? Nutrients. 2022; 14(5):998. https://doi.org/10.3390/nu14050998
Chicago/Turabian StyleBarni, Simona, Davide Caimmi, Fernanda Chiera, Pasquale Comberiati, Carla Mastrorilli, Umberto Pelosi, Francesco Paravati, Gian Luigi Marseglia, and Stefania Arasi. 2022. "Phenotypes and Endotypes of Peach Allergy: What Is New?" Nutrients 14, no. 5: 998. https://doi.org/10.3390/nu14050998
APA StyleBarni, S., Caimmi, D., Chiera, F., Comberiati, P., Mastrorilli, C., Pelosi, U., Paravati, F., Marseglia, G. L., & Arasi, S. (2022). Phenotypes and Endotypes of Peach Allergy: What Is New? Nutrients, 14(5), 998. https://doi.org/10.3390/nu14050998