Clinical Potential of Fruit in Bladder Cancer Prevention and Treatment
Abstract
:1. Introduction
2. Pomegranate
2.1. Pomegranate—Basic Information
2.2. Pomegranate in Bladder Cancer Prevention
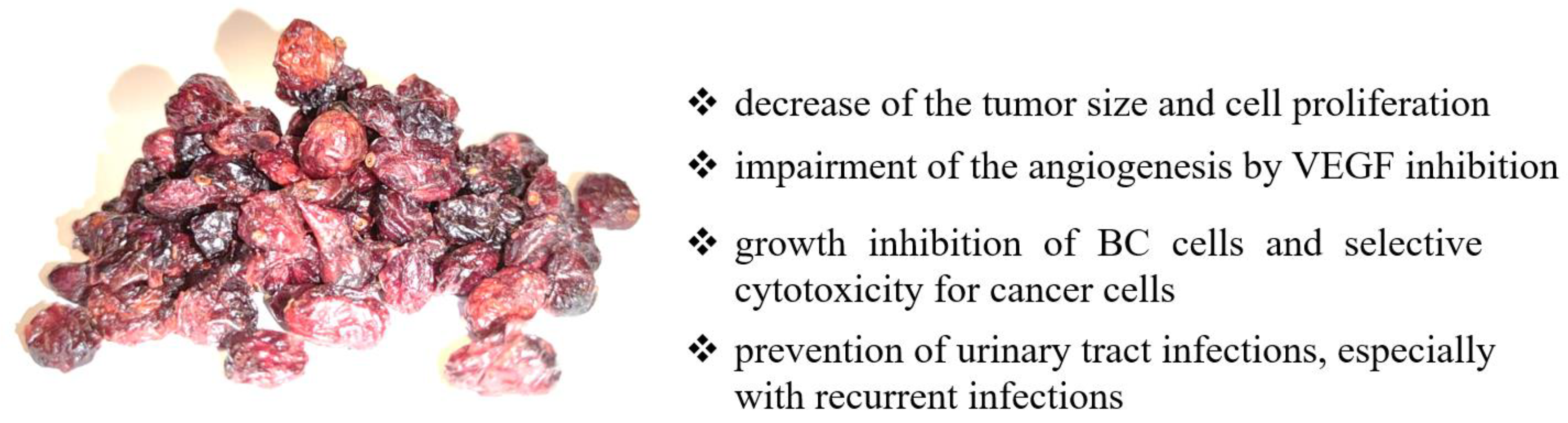
3. Cranberry
3.1. Cranberry—Basic Information
3.2. Cranberry in Bladder Cancer Prevention
4. Citrus
4.1. Citrus—Basic Information
4.2. Citrus in Bladder Cancer Prevention
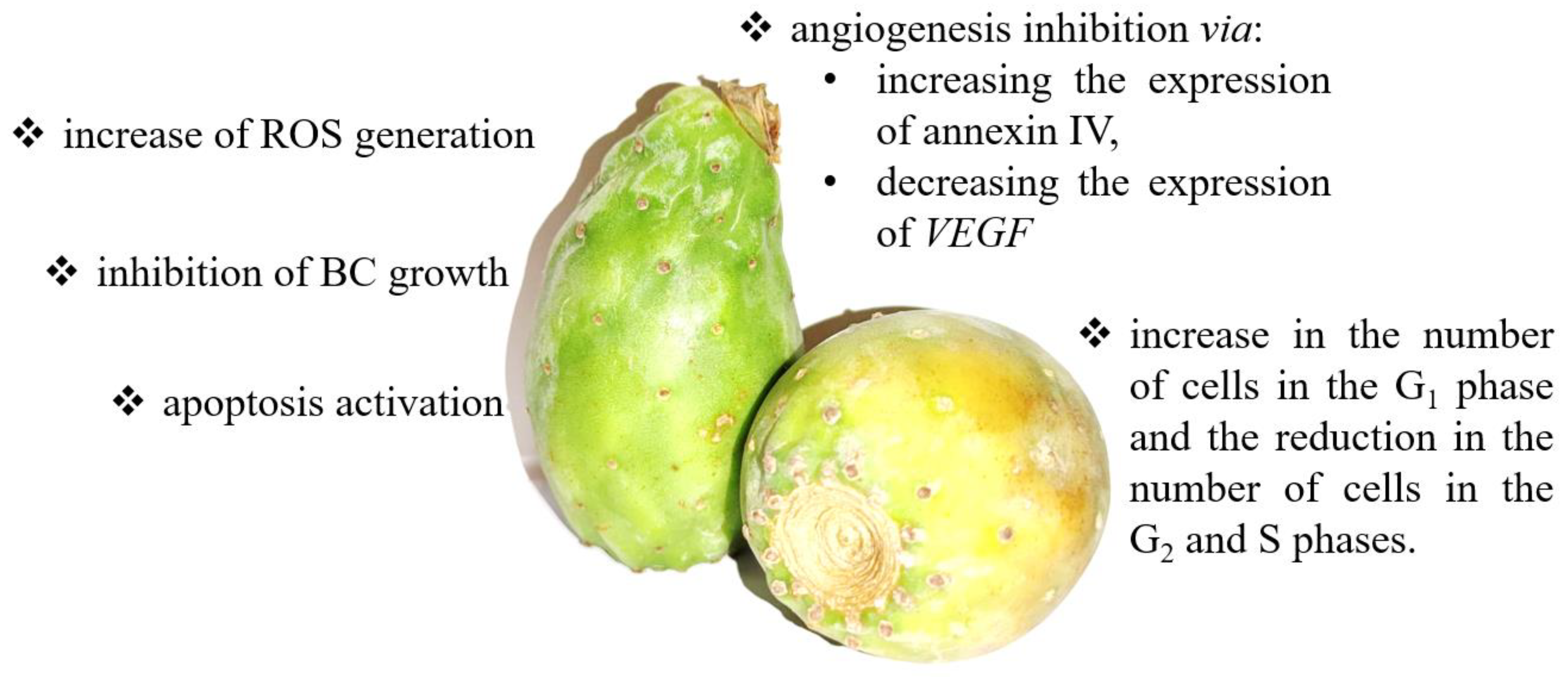
5. Cactus Pear
5.1. Cactus Pear—Basic Information
5.2. Cactus Pear in Bladder Cancer Prevention
6. Apple
6.1. Apple—Basic Information
6.2. Apple in Bladder Cancer Prevention
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grasso, M. Bladder cancer: A major public health issue. Eur. Urol. Suppl. 2008, 7, 510–515. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeegers, M.P.; Tan, F.E.; Dorant, E.; van den Brandt, P.A. The impact of characteristics of cigarette smoking on urinary tract cancer risk: A meta-analysis of epidemiologic studies. Cancer 2000, 89, 630–639. [Google Scholar] [CrossRef]
- Kogevinas, M.; Mannetje, A.; Cordier, S.; Ranft, U.; González, C.A.; Vineis, P. Occupation and bladder cancer among men in Western Europe. Canc. Causes Cont. 2003, 14, 907–914. [Google Scholar] [CrossRef]
- Wolff, D. The genetics of bladder cancer: A cytogeneticist’s perspective. Cytogenet. Genome Res. 2007, 118, 177–181. [Google Scholar] [CrossRef]
- Kwan, M.L.; Garren, B.; Nielsen, M.E.; Tang, L. Lifestyle and nutritional modifiable factors in the prevention and treatment of bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 37, 380–386. [Google Scholar] [CrossRef]
- Dianatinasab, M.; Wesselius, A.; Salehi-Abargouei, A.; Yu, E.Y.W.; Brinkman, M.; Fararouei, M.; van den Brandt, P.; White, E.; Weiderpass, E.; Le Calvez-Kelm, F.; et al. Adherence to a Western dietary pattern and risk of bladder cancer: A pooled analysis of 13 cohort studies of the Bladder Cancer Epidemiology and Nutritional Determinants international study. Int. J. Cancer 2020, 147, 3394–3403. [Google Scholar] [CrossRef]
- Olsen, A.; Egeberg, R.; Halkjær, J.; Christensen, J.; Overvad, K.; Tjønneland, A. Healthy aspects of the Nordic diet are related to lower total mortality. J. Nutr. 2011, 141, 639–644. [Google Scholar] [CrossRef]
- Gunge, V.B.; Andersen, I.; Kyrø, C.; Hansen, C.P.; Dahm, C.C.; Christensen, J.; Tjønneland, A.; Olsen, A. Adherence to a healthy Nordic Food Index and risk of myocardial infarction in middle-aged Danes: The diet, cancer and health cohort study. Eur. J. Clin. Nutr. 2017, 71, 652–658. [Google Scholar] [CrossRef]
- Luo, J.; Xu, X. Dietary fiber intake and the risk of bladder cancer in the Prostate, Lung, Colorectal and Ovarian (PLCO) cohort. Carcinogenesis 2020, 41, 478–482. [Google Scholar] [CrossRef]
- Park, S.Y.; Ollberding, N.J.; Woolcott, C.G.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Fruit and vegetable intakes are associated with lower risk of bladder cancer among women in the multiethnic cohort study. J. Nutr. 2013, 143, 1283–1292. [Google Scholar] [CrossRef] [Green Version]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Dinh, T.; Lee, J. The health economics of bladder cancer: An updated review of the published literature. Pharmacoeconomics 2014, 32, 1093–1104. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Dalbagni, G. Bladder cancer: Restaging TUR reduces recurrence and progression risk. Nat. Rev. Urol. 2010, 7, 649. [Google Scholar] [CrossRef] [PubMed]
- Zuiverloon, T.C.; Theodorescu, D. Pharmacogenomic considerations in the treatment of muscle-invasive bladder cancer. Pharmacogenomics 2017, 18, 1167–1178. [Google Scholar] [CrossRef]
- Nagano, J.; Kono, S.; Preston, D.L.; Moriwaki, H.; Sharp, G.B.; Koyama, K.; Mabuchi, K. Bladder-cancer incidence in relation to vegetable and fruit consumption: A prospective study of atomic-bomb survivors. Int. J. Cancer 2000, 86, 132–138. [Google Scholar] [CrossRef]
- Wakai, K.; Hirose, K.; Takezaki, T.; Hamajima, N.; Ogura, Y.; Nakamura, S.; Hayashi, N.; Tajima, K. Foods and beverages in relation to urothelial cancer: Case-control study in Japan. Int. J. Urol. 2004, 11, 11–19. [Google Scholar] [CrossRef]
- McGuire, S.U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv. Nutr. 2011, 2, 293–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, K.; Boeing, H.; Volatier, J.L.; Becker, W. Evaluating the potential health gain of the World Healt’ Organization’s recommendation concerning vegetable and fruit consumption. Public Health Nutr. 2003, 6, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.P.; Chan, Y.Y.; Li, C.F.; Chien, L.H.; Lee, S.T.; Wu, T.F. Deciphering the Molecular Mechanism Underlying the Inhibitory Efficacy of Taiwanese Local Pomegranate Peels against Urinary Bladder Urothelial Carcinoma. Nutrients. 2018, 10, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hort. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Elfalleh, W.; Hannachi, H.; Tlili, N.; Yahia, Y.; Nasri, N.; Ferchichi, A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Zand, R.S.; Jenkins, D.J.; Diamandis, E.P. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res. Treat. 2000, 62, 35–49. [Google Scholar] [CrossRef]
- Paladini, A.C.; Marder, M.; Viola, H.; Wolfman, C.; Wasowski, C.; Medina, J.H. Flavonoids and the central nervous system: From forgotten factors to potent anxiolytic compounds. J. Pharm. Pharmacol. 1999, 51, 519–526. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. Cancer 2019, 72, 386–397. [Google Scholar] [CrossRef]
- Cáceres, A.; Girón, L.M.; Alvarado, S.R.; Torres, M.F. Screening of antimicrobial activity of plants popularly used in Guatemala for the treatment of dermatomucosal diseases. J. Ethnopharmacol. 1987, 20, 223–237. [Google Scholar] [CrossRef]
- Naqvi, S.; Khan, M.; Vohora, S. Anti-bacterial, anti-fungal and anthelmintic investigations on Indian medicinal plants. Fitoterapia 1991, 62, 221–228. [Google Scholar]
- Saxena, A.; Vikram, N.K. Role of selected Indian plants in management of type 2 diabetes: A review. J. Altern. Complement. Med. 2004, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Zaid, H.; Silbermann, M.; Ben-Arye, E.; Saad, B. Greco-Arab and Islamic herbal-derived anticancer modalities: From tradition to molecular mechanisms. Evid. Based Complement. Altern. Med. 2012, 2012, 349040. [Google Scholar] [CrossRef]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Vidal, A.; Fallarero, A.; Peña, B.R.; Medina, M.E.; Gra, B.; Rivera, F.; Gutierrez, Y.; Vuorela, P.M. Studies on the toxicity of Punica granatum L. (Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2003, 89, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Shravan Kumar, Y.; Adukondalu, D.; Bhargavi Latha, A.; Vamshi Vishnu, Y.; Ramesh, G.; Shiva Kumar, R.; Madhusudan Rao, Y.; Sarangapani, M. Effect of pomegranate pretreatment on the oral bioavailability of buspirone in male albino rabbits. Daru 2011, 19, 266–269. [Google Scholar]
- Nagata, M.; Hidaka, M.; Sekiya, H.; Kawano, Y.; Yamasaki, K.; Okumura, M.; Arimori, K. Effects of pomegranate juice on human cytochrome P450 2C9 and tolbutamide pharmacokinetics in rats. Drug Metab. Dispos. 2007, 35, 302–305. [Google Scholar] [CrossRef]
- Misaka, S.; Nakamura, R.; Uchida, S.; Takeuchi, K.; Takahashi, N.; Inui, N.; Kosuge, K.; Yamada, S.; Watanabe, H. Effect of 2 weeks’ consumption of pomegranate juice on the pharmacokinetics of a single dose of midazolam: An open-label, randomized, single-center, 2-period crossover study in healthy Japanese volunteers. Clin. Ther. 2011, 33, 246–252. [Google Scholar] [CrossRef]
- Lee, S.T.; Lu, M.H.; Chien, L.H.; Wu, T.F.; Huang, L.C.; Liao, G.I. Suppression of urinary bladder urothelial carcinoma cell by the ethanol extract of pomegranate fruit through cell cycle arrest and apoptosis. BMC Complement. Altern. Med. 2013, 13, 364. [Google Scholar] [CrossRef] [Green Version]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef]
- Hay, N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell 2005, 8, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Srinivasula, S.M.; Ahmad, M.; Lin, J.H.; Poyet, J.L.; Fernandes-Alnemri, T.; Tsichlis, P.N.; Alnemri, E.S. CLAP, a novel caspase recruitment domain-containing protein in the tumor necrosis factor receptor pathway, regulates NF-kappaB activation and apoptosis. J. Biol Chem. 1999, 274, 17946–17954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.; Cheng, Z.; Taylor, C.A.; McConkey, B.J.; Thompson, J.E. Thompson, Apoptosis induction by eIF5A1 involves activation of the intrinsic mitochondrial pathway. J. Cell Physiol. 2010, 223, 798–809. [Google Scholar]
- Cho, S.; Ko, H.M.J.; Lee, J.A.; Park, J.E.; Jang, M.S.; Park, S.G.; Lee, D.H.; Ryu, S.E.; Park, B.C. Positive regulation of apoptosis signal-regulating kinase 1 by hD53L1. J. Biol Chem. 2004, 279, 16050–16056. [Google Scholar] [CrossRef] [Green Version]
- Kitaura, Y.; Matsumoto, S.; Satoh, H.; Hitomi, K.; Maki, M. Peflin and ALG-2, members of the penta-EF-hand protein family, form a heterodimer that dissociates in a Ca2+-dependent manner. J. Biol Chem. 2001, 276, 14053–14058. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Q.; Zhang, H.Y.; Hao, F.J.; Meng, X.; Guan, Y.; Du, Z.X. Induction of BAG2 protein during proteasome inhibitor-induced apoptosis in thyroid carcinoma cells. Br. J. Pharmacol. 2008, 155, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Mani, A.; Gelmann, E.P. The ubiquitin-proteasome pathway and its role in cancer. J. Clin. Oncol. 2005, 23, 4776–4789. [Google Scholar] [CrossRef]
- McCutchen-Maloney, S.L.; Matsuda, K.; Shimbara, N.; Binns, D.D.; Tanaka, K.; Slaughter, C.A.; DeMartino, G.N. cDNA cloning, expression, and functional characterization of PI31, a proline-rich inhibitor of the proteasome. J. Biol Chem. 2000, 275, 18557–18565. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Wang, C.; Spencer, E.; Yang, L.; Braun, A.; You, J.; Slaughter, C.; Pickart, C.; Chen, Z.J. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 2000, 103, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Bu, R.; Hussain, A.R.; Al-Obaisi, K.A.; Ahmed, M.; Uddin, S.; Al-Kuraya, K.S. Bortezomib inhibits proteasomal degradation of IκB α and induces mitochondrial dependent apoptosis in activated B-cell diffuse large B-cell lymphoma. Leuk. Lymphoma. 2014, 55, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.F.; Hsu, L.T.; Tsang, B.X.; Huang, L.C.; Shih, W.Y.; Chen, L.Y. Clarification of the molecular pathway of Taiwan local pomegranate fruit juice underlying the inhibition of urinary bladder urothelial carcinoma cell by proteomics strategy. BMC Complement. Altern Med. 2016, 16, 96. [Google Scholar] [CrossRef] [Green Version]
- Küffner, R.; Rohr, A.; Schmiede, A.; Krüll, C.; Schulte, U. Involvement of two novel chaperones in the assembly of mitochondrial NADH:Ubiquinone oxidoreductase (complex I). J. Mol. Biol. 1998, 283, 409–417. [Google Scholar] [CrossRef]
- Marsh, L.; Shah, K. Nowatorski inhibitor izomerazy triosefosforanowej u ssaków znaleziony przez podejście in silico. Int. J. Med. Chem. 2014, 2014, 469125. [Google Scholar] [PubMed]
- Sun, R.; Zhang, J.; Chen, L.; Zhang, N.; Wang, X.; Chen, W. Punica granatum Extract Inhibits Bladder Cancer Cell Viability, Invasion and Migration through Down-Regulation of HOXD10 Signalling Pathway. Dokl. Biochem. Biophys. 2021, 497, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bahena-Ocampo, I.; Espinosa, M.; Ceballos-Cancino, G.; Lizarraga, F.; Campos-Arroyo, D.; Schwarz, A.; Maldonado, V.; Melendez-Zajgla, J.; Garcia-Lopez, P. miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation. EMBO Rep. 2016, 17, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Li, H.; Yu, G.; Xiao, W.; Hu, J.; Tang, K.; Zeng, J.H.W.; Zeng, G. MicroRNA-10b promotes migration and invasion through KLF4 and HOXD10 in human bladder cancer. Oncol. Rep. 2014, 31, 1832–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, M.; Su, Y.; Li, K.; Jin, D.; Li, Q.; Li, Y.; Zhou, B. Gallic Acid Inhibits Bladder Cancer T24 Cell Progression Through Mitochondrial Dysfunction and PI3K/Akt/NF-κB Signaling Suppression. Front. Pharmacol. 2020, 11, 1222. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, H.; Chen, Q. Oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX) as secondline therapy for patients with advanced urothelial cancer. Oncotarget 2016, 7, 58579–58585. [Google Scholar] [CrossRef] [Green Version]
- Bi, L.; Yan, X.; Chen, W.; Gao, J.; Qian, L.; Qiu, S. Antihepatocellular carcinoma potential of tetramethylpyrazine induces cell cycle modulation and mitochondrial-dependent apoptosis: Regulation of P53 signaling pathway in HepG2 cells in vitro. Interact. Cancer Ther. 2016, 15, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, X.; Heng, H.; Yu, H.L.; Dan, M.; Jie, C.; Zeng, Y. Anticancer effects of 10-hydroxycamptothecin induce apoptosis of human osteosarcoma through activating caspase-3, p53 and cytochrome c pathways. Oncol. Lett. 2018, 15, 2459–2464. [Google Scholar] [PubMed] [Green Version]
- Siveen, K.S.; Prabhu, K.; Krishnankutty, R.; Kuttikrishnan, S.; Tsakou, M.; Alali, F. Vascular endothelial growth factor (VEGF) signaling in tumour vascularization: Potential and challenges. Curr. Vasc. Pharmacol. 2017, 15, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Melincovici, C.S.; Bosca, A.B.; Susman, S.; Marginean, M.; Mihu, C.; Istrate, M. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Zhou, B.; Yi, H.; Tan, J.; Wu, Y.; Liu, G.; Qiu, Z. Anti-proliferative effects of polyphenols from pomegranate rind (Punica granatum L.) on EJ bladder cancer cells via regulation of p53/miR-34a axis. Phytother. Res. 2015, 29, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell. 2007, 26, 731–743. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Han, G.; Li, Y.; Shi, L.H.; Li, H. Expression and role of miR-34a in bladder cancer. Indian J. Biochem. Biophys. 2013, 50, 87–92. [Google Scholar]
- Li, H.; Yu, G.; Shi, R.; Lang, B.; Chen, X.; Xia, D.; Xiao, H.; Guo, X.; Guan, W.; Ye, Z.; et al. Cisplatin-induced epigenetic activation of miR-34a sensitizes bladder cancer cells to chemotherapy. Mol. Cancer 2014, 13, 8. [Google Scholar] [CrossRef] [Green Version]
- Mortada, W.I.; Awadalla, A.; Khater, S.M.; Barakat, N.M.; Husseiny, S.M.; Shokeir, A.A. Preventive effect of pomegranate juice against chemically induced bladder cancer: An experimental study. Heliyon 2020, 6, e05192. [Google Scholar] [CrossRef]
- Basiri, S. Evaluation of antioxidant and antiradical properties of Pomegranate (Punica granatum L.) seed and defatted seed extracts. J. Food Sci. Technol. 2015, 52, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Lansky, E.P.; Jiang, W.; Mo, H.; Bravo, L.; Froom, P.; Yu, W. Possible synergistic prostate cancer suppression by anatomically discrete pomegranate fractions. Investig. New Drugs 2005, 23, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xi, Q.; Meng, Q.; Liu, J.; Zhang, Y.; Han, Y. Interleukin-6 promotes tumor progression in colitis-associated colorectal cancer through HIF-1α regulation. Oncol. Lett. 2016, 12, 4665–4670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef]
- Guay, D.R. Cranberry and urinary tract infections. Drugs 2009, 69, 775–807. [Google Scholar] [CrossRef] [PubMed]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Neto, C.C.; Amoroso, J.W.; Liberty, A.M. Anticancer activities of cranberry phytochemicals: An update. Mol. Nutr. Food Res. 2008, 52, 18–27. [Google Scholar] [CrossRef]
- Ruel, G.; Couillard, C. Evidences of the cardioprotective potential of fruits: The case of cranberries. Mol. Nutr. Food Res. 2007, 51, 692–701. [Google Scholar] [CrossRef]
- Cimolai, N.; Cimolai, T. The cranberry and the urinary tract. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 767–776. [Google Scholar] [CrossRef]
- Saito, M.; Hirata-Koizumi, M.; Matsumoto, M.; Urano, T.; Hasegawa, R. Undesirable effects of citrus juice on the pharmacokinetics of drugs: Focus on recent studies. Drug Saf. 2005, 28, 677–694. [Google Scholar] [CrossRef]
- Srinivas, N.R. Cranberry juice ingestion and clinical drug-drug interaction potentials; review of case studies and perspectives. J. Pharm. Pharm. Sci. 2013, 16, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Gettman, M.T.; Ogan, K.; Brinkley, L.J.; Adams-Huet, B.; Pak, C.Y.; Pearle, M.S. Effect of cranberry juice consumption on urinary stone risk factors. J. Urol. 2005, 174, 590–594. [Google Scholar] [CrossRef]
- Howell, A.B.; Foxman, B. Cranberry juice and adhesion of antibiotic-resistant uropathogens. JAMA 2002, 287, 3082–3083. [Google Scholar] [CrossRef] [PubMed]
- Hisano, M.; Bruschini, H.; Nicodemo, A.C.; Srougi, M. Cranberries and lower urinary tract infection prevention. Clinics 2012, 67, 661–668. [Google Scholar] [CrossRef]
- Lavigne, J.P.; Bourg, G.; Combescure, C.; Botto, H.; Sotto, A. In-vitro and in-vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin. Microbiol. Infect. 2008, 14, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Romaniello, L.; Vitelli, O.; Gatto, A.; Serva, M.; Cataldi, L. Cranberry juice for the prevention of recurrent urinary tract infections: A randomized controlled trial in children. Scand. J. Urol. Nephrol. 2009, 43, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.C.; Hutchison, C.; Cole, T.; Barry, S.J.; Paul, J.; Reed, N.S.; Russell, J.M. A randomised double-blind placebo-controlled trial to determine the effect of cranberry juice on decreasing the incidence of urinary symptoms and urinary tract infections in patients undergoing radiotherapy for cancer of the bladder or cervix. Clin. Oncol. R Coll Radiol. 2012, 24, e31–e38. [Google Scholar] [CrossRef]
- Prasain, J.K.; Rajbhandari, R.; Keeton, A.B.; Piazza, G.A.; Barnes, S. Metabolism and growth inhibitory activity of cranberry derived flavonoids in bladder cancer cells. Food Funct. 2016, 7, 4012–4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Khanna, S.; Alessio, H.M.; Vider, J.; Bagchi, D.; Bagchi, M.; Sen, C.K. Anti-angiogenic property of edible berries. Free Radic Res. 2002, 36, 1023–1031. [Google Scholar] [CrossRef]
- Prasain, J.K.; Jones, K.; Moore, R.; Barnes, S.; Leahy, M.; Roderick, R.; Juliana, M.M.; Grubbs, C.J. Effect of cranberry juice concentrate on chemically-induced urinary bladder cancers. Oncology Rep. 2008, 19, 1565–1570. [Google Scholar]
- Ho, S.-C.; Lin, C.-C. Investigation of heat treating conditions for enhancing the anti-inflammatory activity of Citrus fruit (Citrus reticulata) peels. J. Agric. Food Chem. 2008, 56, 7976–7982. [Google Scholar] [CrossRef]
- Economos, C.; Clay, W.D. Nutritional and health benefits of citrus fruits. Food Nutr. Agric. 1999, 24, 11–18. [Google Scholar]
- Committee, N.P. Pharmacopoeia of People’s Republic of China. Beijing China Med. Sci. Technol. Press 2010, 2, 157–159. [Google Scholar]
- Ahn, K.I.; Choi, E.O.; Kwon, D.H.; Hwang Bo, H.; Kim, M.Y.; Kim, H.J.; Ji, S.Y.; Hong, S.H.; Jeong, J.W.; Park, C.; et al. Induction of apoptosis by ethanol extract of Citrus unshiu Markovich peel in human bladder cancer T24 cells through ROS-mediated inactivation of the PI3K/Akt pathway. Biosci. Trends 2017, 11, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Nachmias, B.; Ashhab, Y.; Ben-Yehuda, D. The inhibitor of apoptosis protein family (IAPs): An emerging therapeutic target in cancer. Semin. Cancer Biol. 2004, 14, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Vucic, D. Targeting IAP proteins for therapeutic intervention in cancer. Nat. Rev. Drug Discov. 2012, 11, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Billen, L.P.; Shamas-Din, A.; Andrews, D.W. Bid: A Bax-like BH3 protein. Oncogene 2008, 27, 93–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantari, C.; Walczak, H. Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta. 2011, 1813, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Glinsky, V.V.; Raz, A. Modified citrus pectin anti-metastatic properties: One bullet, multiple targets. Carbohydr. Res. 2009, 344, 1788–1791. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; AlSadek, D.M. Galectin-3 as a potential target to prevent cancer metastasis. Clin. Med. Insights Oncol. 2015, 9, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Gunning, A.P.; Bongaerts, R.J.; Morris, V.J. Recognition ofgalactan components of pectin by galectin-3. FASEB J. 2009, 23, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, M.; Adelinik, A.; Papian, S.; Khorramdelazad, H.; Abedinzadeh, M. Role of galectin-3 in the pathogenesis of bladder transitional cell carcinoma. Hum. Immunol. 2015, 76, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Canesin, G.; Gonzalez-Peramato, P.; Palou, J.; Urrutia, M.; Cordón-Cardo, C.; Sánchez-Carbayo, M. Galectin-3 expression is associated with bladder cancer progression and clinical outcome. Tumour. Biol. 2010, 31, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, J.; Liu, X.; Li, L.; Zheng, J. Cleavage and phosphorylation: Important post-translational modifications of galectin-3. Cancer Metastas. Rev. 2017, 36, 367–374. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta. 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P. Galectin 3 as a guardian of the tumor microenvironment. Biochim. Biophys. Acta. 2016, 1863, 427–437. [Google Scholar] [CrossRef]
- Fang, T.; Liu, D.D.; Ning, H.M.; Dan, L.; Sun, J.Y.; Huang, X.J.; Dong, Y.; Geng, M.Y.; Yun, S.F.; Yan, J.; et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol. Sin. 2018, 39, 1885–1893. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef]
- Lee, C.H.; Jeong, T.S.; Choi, Y.K.; Hyun, B.H.; Oh, G.T.; Kim, E.H.; Kim, J.R.; Han, J.I.; Bok, S.H. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem. Biophys. Res. Commun. 2001, 284, 681–688. [Google Scholar] [CrossRef]
- Kaul, T.N.; Middleton, E., Jr.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985, 15, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ueng, Y.F.; Chang, Y.L.; Oda, Y.; Park, S.S.; Liao, J.F.; Lin, M.F.; Chen, C.F. In vitro and in vivo effects of naringin on cytochrome P450-dependent monooxygenase in mouse liver. Life Sci. 1999, 65, 2591–2602. [Google Scholar] [CrossRef]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Moussa, M.; Carroll, K.K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr. Cancer 1996, 26, 167–181. [Google Scholar] [CrossRef]
- Le Marchand, L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of flavonoids and lung cancer. J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.I.; Lee, S.J.; Lee, S.B.; Park, K.; Kim, W.J.; Moon, S.K. Requirement for Ras/Raf/ERK pathway in naringin-induced G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis 2008, 29, 1701–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.I.; Strock, C.J.; Ball, D.W.; Nelkin, B.D. The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine-paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway. Mol. Cell. Biol. 2003, 23, 543–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.K.; Jung, S.Y.; Choi, Y.H.; Lee, Y.C.; Patterson, C.; Kim, C.H. PDTC, metal chelating compound, induces G1 phase cell cycle arrest in vascular smooth muscle cells through inducing p21Cip1 expression: Involvement of p38 mitogen activated protein kinase. J. Cell. Physiol. 2004, 198, 310–323. [Google Scholar] [CrossRef]
- Lee, B.; Kim, C.H.; Moon, S.K. Honokiol causes the p21WAF1-mediated G(1)-phase arrest of the cell cycle through inducing p38 mitogen activated protein kinase in vascular smooth muscle cells. FEBS Lett. 2006, 580, 5177–5184. [Google Scholar] [CrossRef] [Green Version]
- Li, W.Q.; Kuriyama, S.; Li, Q.; Nagai, M.; Hozawa, A.; Nishino, Y.; Tsuji, I. Citrus consumption and cancer incidence: The Ohsaki cohort study. Int. J. Cancer 2010, 127, 1913–1922. [Google Scholar] [CrossRef]
- Liang, S.; Lv, G.; Chen, W.; Jiang, J.; Wang, J. Citrus fruit intake and bladder cancer risk: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2014, 65, 893–898. [Google Scholar] [CrossRef]
- Vieira, A.R.; Vingeliene, S.; Chan, D.S.; Aune, D.; Abar, L.; Navarro Rosenblatt, D.; Greenwood, D.C.; Norat, T. Fruits, vegetables, and bladder cancer risk: A systematic review and meta-analysis. Cancer Med. 2015, 4, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Zalabani, A.H.; Stewart, K.F.; Wesselius, A.; Schols, A.M.; Zeegers, M.P. Modifiable risk factors for the prevention of bladder cancer: A systematic review of meta-analyses. Eur. J. Epidemiol. 2016, 31, 811–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xenou, D.; Tzelves, L.; Terpos, E.; Stamatelopoulos, K.; Sergentanis, T.N.; Psaltopoulou, T. Consumption of Fruits, Vegetables and Bladder Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutr. Cancer 2021, 2, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.A.; Almela, L.; Obón, J.M.; Castellar, R. Determination of antioxidant constituents in cactus pear fruits. Plant. Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, M.S.; Badr, S.E.A.; Khalil, E.M.; Elsaid, A.S. Feed efficiency, some blood parameters and In- vitro chemoprevention of prickly pear (Opuntia ficus indica L.) seeds oil growing in Egypt. Issues Biol. Sci. Pharma. Res. 2020, 8, 20–27. [Google Scholar]
- Sobieraj, D.M.; Freyer, C.W. Probable hypoglycemic adverse drug reaction associated with prickly pear cactus, glipizide, and metformin in a patient with type 2 diabetes mellitus. Ann. Pharmacother. 2010, 44, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Brewer, M.; Garcia, F. Cactus pear: A natural product in cancer chemoprevention. Nutr. J. 2005, 4, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magloire, F.J.; Zou, D.J. Comparison of Chinese and American cactus pear (Opuntia cacti) in induction of ROS and growth inhibition in bladder cancer cells. In Proceedings of the VI International Congress on Cactus Pear and Cochineal, Joao Pessoa, Brazil, 22–26 October 2007; Volume 811, pp. 187–196. [Google Scholar] [CrossRef]
- Kao, Y.-L.; Kuo, Y.-M.; Lee, Y.-R.; Chen, W.-R.; Lee, H.-J.; Lee, H.-J. Apple polyphenol decelerates bladder cancer growth involving apoptosis and cell cycle arrest in N-butyl-N-(4-hydroxybutyl) nitrosamine-induced experimental animal model. J. Funct. Foods 2017, 36, 1–8. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C. Fruit juice, organic anion transporting polypeptides, and drug interactions in psychiatry. J. Clin. Psychiatry 2014, 75, e1323–e1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, Y.-L.; Kuo, Y.-M.; Lee, Y.-R.; Yang, S.-F.; Chen, W.-R.; Lee, H.-J. Apple polyphenol induces cell apoptosis, cell cycle arrest at G2/M phase, and mitotic catastrophe in human bladder transitional carcinoma cells. J. Funct. Foods 2015, 14, 384–394. [Google Scholar] [CrossRef]
- Shoji, T.; Akazome, Y.; Kanda, T.; Ikeda, M. The toxicology and safety of apple polyphenol extract. Food Chem Toxicol. 2004, 42, 959–967. [Google Scholar] [CrossRef]
- Candé, C.; Cohen, I.; Daugas, E.; Ravagnan, L.; Larochette, N.; Zamzami, N.; Kroemer, G. Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie 2002, 84, 215–222. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Mailand, N.; Gibbs-Seymour, I.; Bekker-Jensen, S. Regulation of PCNA-protein interactions for genome stability. Nat. Rev. Mol. Cell Biol. 2013, 14, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Chau, L.Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015, 22, 22. [Google Scholar] [CrossRef] [Green Version]
- Büchner, F.L.; Bueno-de-Mesquita, H.B.; Ros, M.M.; Kampman, E.; Egevad, L.; Overvad, K.; Raaschou-Nielsen, O.; Tjønneland, A.; Roswall, N.; Clavel-Chapelon, F.; et al. Consumption of vegetables and fruit and the risk of bladder cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2009, 125, 2643–2651. [Google Scholar] [CrossRef]
- Sacerdote, C.; Matullo, G.; Polidoro, S.; Gamberini, S.; Piazza, A.; Karagas, M.R.; Rolle, L.; De Stefanis, P.; Casetta, G.; Morabito, F.; et al. Intake of fruits and vegetables and polymorphisms in DNA repair genes in bladder cancer. Mutagenesis 2007, 22, 281–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wigner, P.; Bijak, M.; Saluk-Bijak, J. Clinical Potential of Fruit in Bladder Cancer Prevention and Treatment. Nutrients 2022, 14, 1132. https://doi.org/10.3390/nu14061132
Wigner P, Bijak M, Saluk-Bijak J. Clinical Potential of Fruit in Bladder Cancer Prevention and Treatment. Nutrients. 2022; 14(6):1132. https://doi.org/10.3390/nu14061132
Chicago/Turabian StyleWigner, Paulina, Michał Bijak, and Joanna Saluk-Bijak. 2022. "Clinical Potential of Fruit in Bladder Cancer Prevention and Treatment" Nutrients 14, no. 6: 1132. https://doi.org/10.3390/nu14061132
APA StyleWigner, P., Bijak, M., & Saluk-Bijak, J. (2022). Clinical Potential of Fruit in Bladder Cancer Prevention and Treatment. Nutrients, 14(6), 1132. https://doi.org/10.3390/nu14061132







