A Double-Blind, Randomized Intervention Study on the Effect of a Whey Protein Concentrate on E. coli-Induced Diarrhea in a Human Infection Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants, Ethics and Trial Registration
2.3. Randomization and Stratification
2.4. Medical Guidelines
2.5. Dietary Guidelines and Calcium Restriction
2.6. Probiotics and Prebiotics/Fibers
2.7. Dietary Intervention Products
Nutritional Values
2.8. Compliance with Dietary Guidelines and Supplement Intake
2.9. Diarrheagenic E. coli Challenge
2.10. Reported Stool Consistency, Stool Frequency and Gastrointestinal Symptoms
2.11. Fecal Sample Collection
2.12. Microbiota Composition Profiling by 16S rRNA Gene Sequencing
2.13. Fecal SCFA Analysis
2.14. Data and Statistical Analysis
Sample Size Calculation
2.15. Statistical Analysis of Primary Outcomes
- -
- Subjects who did not fulfill the in- and exclusion criteria;
- -
- Subjects who reported protocol violations or major protocol deviations;
- -
- Subjects for whom IP compliance was <80%;
- -
- Subjects for whom >50% of the data points were missing.
2.16. Outcome Analysis
2.17. Microbiota Analysis
3. Results
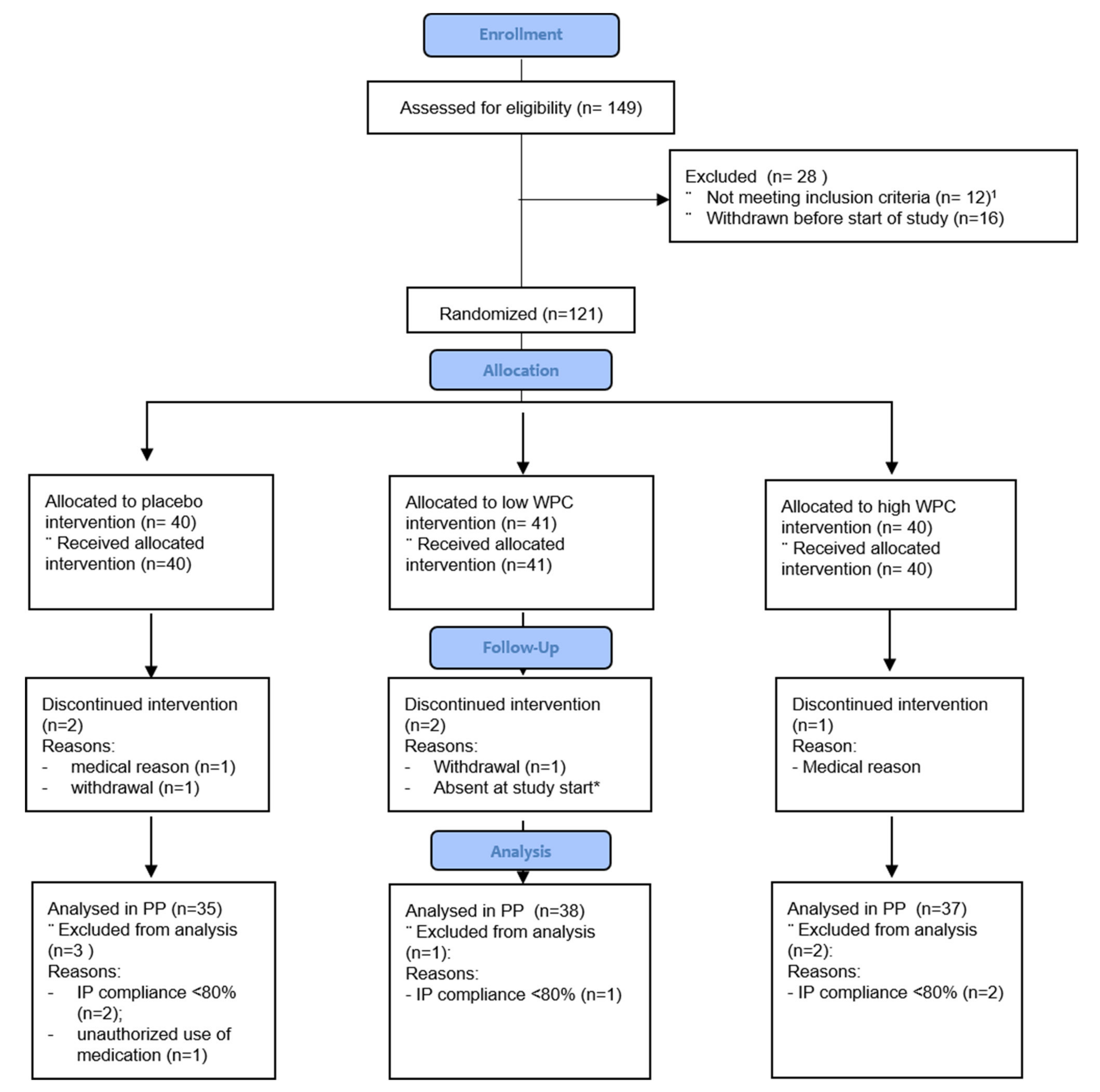
3.1. Baseline Characteristics and Compliance with Diet and Investigational Products
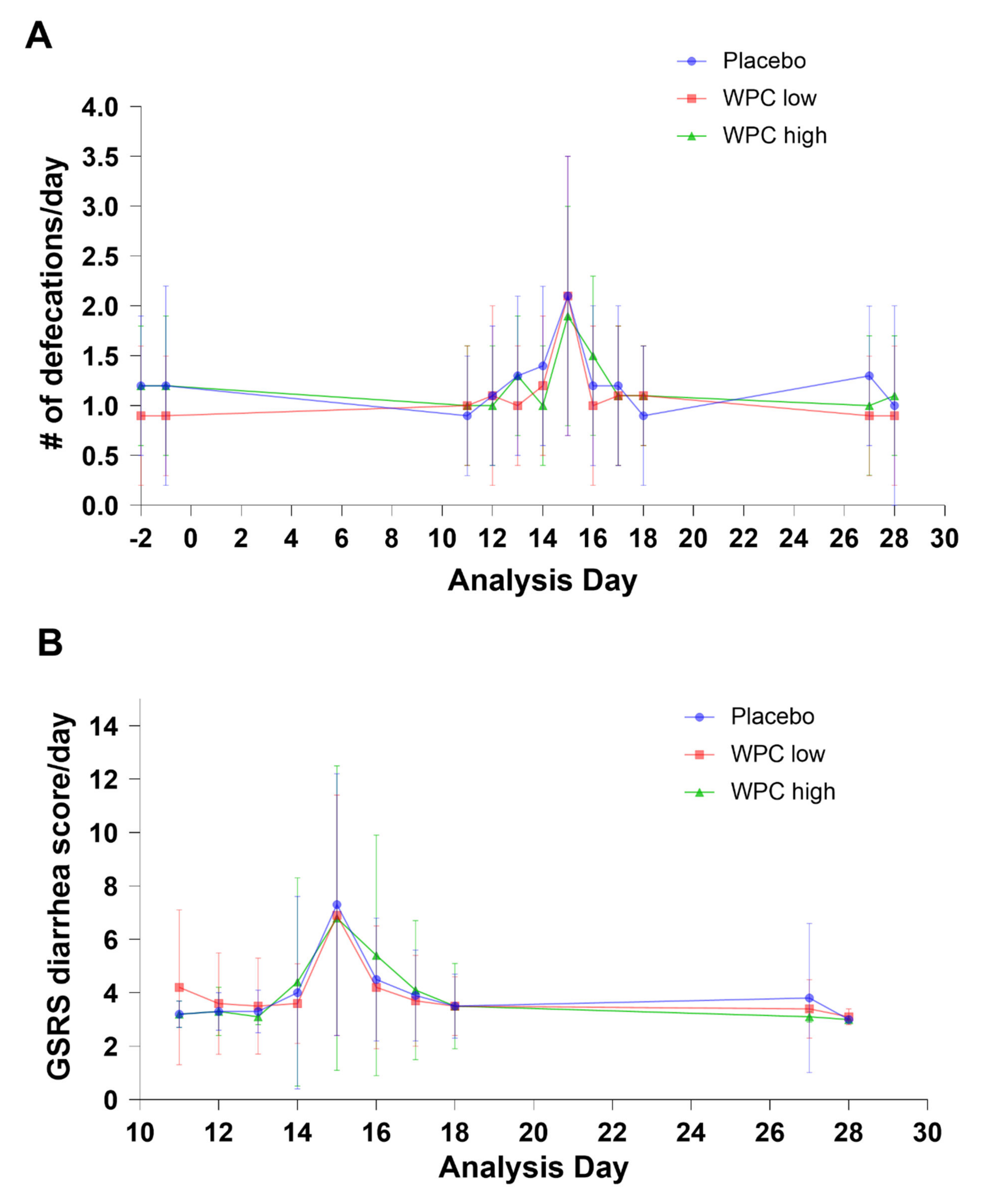
3.2. Primary Outcomes
3.3. Secondary Outcome
3.4. Post Hoc Analysis
3.5. Microbiota Results
3.6. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse events |
| AUC | Area under the curve |
| BSS | Bristol Stool Scale |
| CFA | Colonization factor antigen |
| CFU | Colony-forming unit |
| ETEC | Enterotoxigenic Escherichia coli |
| GI | Gastrointestional |
| GIGA | Gastrointestinal benefit of dairy ingredients to prevent symptoms of gut infection in healthy adult subjects |
| GSRS | Gastrointestinal Symptom Rating Score |
| IP | Investigational product |
| ITT | Intention to treat |
| LT | Heat labile |
| METC | Medisch Etisch Toetsings Commissie |
| MFGM | Milk fat globular membrane |
| MMRM | Mixed model repeated measures |
| NTR | Netherlands Trial Register |
| OTU | Operational taxonomic unit |
| PCR | Polymeric chain reaction |
| PP | Per protocol |
| RDA | Redundancy analysis |
| (S)AE | Severe adverse events |
| SCFA | Short-chain fatty acids |
| ST | Heat stable |
| WPC | Whey protein concentrate |
References
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner, R.C., Jr.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M.; et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef] [Green Version]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Brennhofer, S.; Reifsnider, E.; Bruening, M. Malnutrition coupled with diarrheal and respiratory infections among children in Asia: A systematic review. Public Health Nurs. 2017, 34, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Mokomane, M.; Kasvosve, I.; De Melo, E.; Pernica, J.M.; Goldfarb, D. The global problem of childhood diarrhoeal diseases: Emerging strategies in prevention and management. Ther. Adv. Infect. Dis. 2018, 5, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Ulfman, L.H.; Leusen, J.; Savelkoul, H.F.J.; Warner, J.O.; Van Neerven, R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front Nutr. 2018, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Hernell, O.; Timby, N.; Domellöf, M.; Lönnerdal, B. Clinical Benefits of Milk Fat Globule Membranes for Infants and Children. J. Pediatr. 2016, 173, S60–S65. [Google Scholar] [CrossRef] [Green Version]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; De La Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef] [Green Version]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef]
- Timby, N.; Hernell, O.; Vaarala, O.; Melin, M.; Lönnerdal, B.; Domellöf, M. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 384–389. [Google Scholar] [CrossRef]
- Zavaleta, N.; Kvistgaard, A.S.; Graverholt, G.; Respicio, G.; Guija, H.; Valencia, N.; Lönnerdal, B. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia and micronutrient status in infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 561–568. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; Li, Z.; Christensen, B.; Heckmann, A.B.; Stenlund, H.; Lönnerdal, B.; Hernell, O. Feeding Infants Formula With Probiotics or Milk Fat Globule Membrane: A Double-Blind, Randomized Controlled Trial. Front Pediatr. 2019, 7, 347. [Google Scholar] [CrossRef]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved Neurodevelopmental Outcomes Associated with Bovine Milk Fat Globule Membrane and Lactoferrin in Infant Formula: A Randomized, Controlled Trial. J. Pediatr. 2019, 215, 24–31. [Google Scholar] [CrossRef]
- Veereman-Wauters, G.; Staelens, S.; Rombaut, R.; Dewettinck, K.; Deboutte, D.; Brummer, R.-J.; Boone, M.; Le Ruyet, P. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012, 28, 749–752. [Google Scholar] [CrossRef]
- Poppitt, S.D.; McGregor, R.A.; Wiessing, K.R.; Goyal, V.K.; Chitkara, A.J.; Gupta, S.; Palmano, K.; Kuhn-Sherlock, B.; McConnell, M.A. Bovine complex milk lipid containing gangliosides for prevention of rotavirus infection and diarrhoea in northern Indian infants. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Van Hoffen, E.; Mercenier, A.; Vidal, K.; Benyacoub, J.; Schloesser, J.; Kardinaal, A.; Lucas-van de Bos, E.; van Alen, I.; Roggero, I.; Duintjer, K.; et al. Characterization of the pathophysiological determinants of diarrheagenic Escherichia coli infection using a challenge model in healthy adults. Sci. Rep. 2021, 11, 6060. [Google Scholar] [CrossRef]
- Ten Bruggencate, S.J.M.; Girard, S.A.; Floris-Vollenbroek, E.G.M.; Bhardwaj, R.; Tompkins, T.A. The effect of a multi-strain probiotic on the resistance toward Escherichia coli challenge in a randomized, placebo-controlled, double-blind intervention study. Eur. J. Clin. Nutr. 2015, 69, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Bovee-Oudenhoven, I.M.; Lettink-Wissink, M.L.; Van Doesburg, W.; Witteman, B.J.; Van Der Meer, R. Diarrhea caused by enterotoxigenic Escherichia coli infection of humans is inhibited by dietary calcium. Gastroenterology 2003, 125, 469–476. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; ten Bruggencate, S.J.M.; Schonewille, A.J.; Alhoniemi, E.; Forssten, S.D.; Bovee-Oudenhoven, I.M.J. Lactobacillus acidophilus supplementation in human subjects and their resistance to enterotoxigenic Escherichia coli infection. Br. J. Nutr. 2014, 111, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Levine, M.M.; Tacket, C.O. Vaccines against Enterotoxigenic Escherichia Coli Infections. Part II: Live Oral Vaccines and Subunit (Purified Fimbriae and Toxin Subunit) Vaccines. In New Generation Vaccins; Marcel Dekker, Inc.: New York, NY, USA, 1997; pp. 875–883. [Google Scholar]
- Levine, M.M.; Barry, E.M.; Chen, W.H. A roadmap for enterotoxigenic Escherichia coli vaccine development based on volunteer challenge studies. Hum. Vaccines Immunother. 2019, 15, 1357–1378. [Google Scholar] [CrossRef] [Green Version]
- Ten Bruggencate, S.J.; Frederiksen, P.D.; Pedersen, S.M.; Floris-Vollenbroek, E.G.; Van De Bos, E.L.; Van Hoffen, E.; Wejse, P.L. Dietary milk-fat-globule membrane affects resistance to diarrheagenic escherichia coli in healthy adults in a randomized, placebo-controlled, double-blind study. J. Nutr. 2016, 146, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Van Rossum, C.T.M.; Buurma-Rethans, E.J.M.; Dinnissen, C.S.; Beukers, M.H.; Brants, H.A.M.; Ocké, M.C. The Diet of the Dutch. Results of the Dutch National Food Consumption Survey 2012–2016; National Institute for Public Health and the Environment: Utrecht, The Netherlands, 2016. [Google Scholar]
- Heaton, K.W.; Ghosh, S.; Braddon, E.F. How bad are the symptoms and bowel dysfunction of patients with the irritable bowel syndrome? A prospective, controlled study with emphasis on stool form. Gut 1991, 32, 73–79. [Google Scholar] [CrossRef]
- Svedlund, J.; Sjödin, I.; Dotevall, G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis. Sci. 1988, 33, 129–134. [Google Scholar] [CrossRef]
- Rahman, S.; Kortman, G.A.M.; Boekhorst, J.; Lee, P.; Khan, M.R.; Ahmed, F. Effect of low-iron micronutrient powder (MNP) on the composition of gut microbiota of Bangladeshi children in a high-iron groundwater setting: A randomized controlled trial. Eur. J. Nutr. 2021, 60, 3423–3436. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic. Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef] [Green Version]
- Gommers, L.M.M.; Ederveen, T.H.A.; Wijst, J.; Overmars-Bos, C.; Kortman, G.A.M.; Boekhorst, J.; Bindels, R.J.M.; Baaij, J.H.F.; Hoenderop, J.G.J. Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI-induced hypomagnesemia. FASEB J. 2019, 33, 11235–11246. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988, 75, 800–802. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Van Neerven, J. The effects of milk and colostrum on allergy and infection: Mechanisms and implications. Anim. Front. 2014, 4, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Hammarström, L.; Weiner, C.K. Targeted antibodies in dairy-based products. Adv. Exp. Med. Biol. 2008, 606, 321–343. [Google Scholar]
- Hageman, J.H.; Danielsen, M.; Nieuwenhuizen, A.; Feitsma, A.L.; Dalsgaard, T.K. Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. Int. Dairy J. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Pierce, A.; Colavizza, D.; Benaissa, M.; Maes, P.; Tartar, A.; Montreuil, J.; Spik, G. Molecular cloning and sequence analysis of bovine lactotransferrin. Eur. J. Biochem. 1991, 196, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Den Hartog, G.; Jacobino, S.; Bont, L.; Cox, L.; Ulfman, L.H.; Leusen, J.H.W.; van Neerven, R.J.J. Specificity and Effector Functions of Human RSV-Specific IgG from Bovine Milk. PLoS ONE 2014, 9, e112047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuller, K.L.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Jiménez-Flores, R.; Donovan, S.M. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J. Dairy Sci. 2013, 96, 3488–3497. [Google Scholar] [CrossRef] [Green Version]
- Tellez, A.; Corredig, M.; Guri, A.; Zanabria, R.; Griffiths, M.W.; Delcenserie, V. Bovine milk fat globule membrane affects virulence expression in Escherichia coli O157:H7. J. Dairy Sci. 2012, 95, 6313–6319. [Google Scholar] [CrossRef] [Green Version]
- Guri, A.; Griffiths, M.; Khursigara, C.; Corredig, M. The effect of milk fat globules on adherence and internalization of Salmonella Enteritidis to HT-29 cells. J. Dairy Sci. 2012, 95, 6937–6945. [Google Scholar] [CrossRef]
- Berding, K.; Wang, M.; Monaco, M.H.; Alexander, L.S.; Mudd, A.T.; Chichlowski, M.; Waworuntu, R.V.; Berg, B.M.; Miller, M.; Dilger, R.; et al. Prebiotics and bioactive milk fractions affect gut development, microbiota, and neurotransmitter expression in piglets. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 688–697. [Google Scholar] [CrossRef]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Zavaleta, N.; Chen, S.-Y.; Lönnerdal, B.; Slupsky, C. Effect of bovine milk fat globule membranes as a complementary food on the serum metabolome and immune markers of 6-11-month-old Peruvian infants. NPJ Sci. Food 2018, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Brink, L.R.; Herren, A.W.; McMillen, S.; Fraser, K.; Agnew, M.; Roy, N.; Lönnerdal, B. Omics analysis reveals variations among commercial sources of bovine milk fat globule membrane. J. Dairy Sci. 2020, 103, 3002–3016. [Google Scholar] [CrossRef] [Green Version]
- Kiewiet, M.B.G.; Faas, M.M.; de Vos, P. Immunomodulatory protein hydrolysates and their application. Nutrients 2018, 10, 904. [Google Scholar] [CrossRef] [Green Version]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, Production and Clinical Treatments of Milk. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef]
- Kurpad, A.V. The requirements of protein & amino acid during acute & chronic infections. Indian J. Med. Res. 2006, 124, 129–148. [Google Scholar]
- Blackburn, G.L. Nutritional assessment and support during infection. Am. J. Clin. Nutr. 1977, 30, 1493–1497. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef]
- Swarte, J.C.; Eelderink, C.; Douwes, R.M.; Said, M.Y.; Hu, S.; Post, A.; Westerhuis, R.; Bakker, S.J.; Harmsen, H.J. Effect of high versus low dairy consumption on the gut microbiome: Results of a randomized, cross-over study. Nutrients 2020, 12, 2129. [Google Scholar] [CrossRef]
- Porter, C.K.; Talaat, K.R.; Isidean, S.D.; Kardinaal, A.; Chakraborty, S.; Gutiérrez, R.L.; Sack, D.A.; Bourgeois, A.L. The Controlled Human Infection Model for Enterotoxigenic Escherichia coli. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Sprong, R.C.; Hulstein, M.F.E.; Lambers, T.T.; van der Meer, R. Sweet buttermilk intake reduces colonisation and translocation of Listeria monocytogenes in rats by inhibiting mucosal pathogen adherence. Br. J. Nutr. 2012, 108, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.-J.; Martín-Sosa, S.; Alonso, J.M.; Hueso, P. Enterotoxigenic Escherichia coli strains bind bovine milk gangliosides in a ceramide-dependent process. Lipids 2003, 38, 761–768. [Google Scholar] [CrossRef]
- Idota, T.; Kawakami, H. Inhibitory effects of milk gangliosides on the adhesion of Escherichia coli to human intestinal carcinoma cells. Biosci. Biotechnol. Biochem. 1995, 59, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Otnaess, A.B.; Laegreid, A.; Ertresvåg, K. Inhibition of enterotoxin from Escherichia coli and Vibrio cholerae by gangliosides from human milk. Infect. Immun. 1983, 40, 563–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprong, R.C.; Hulstein, M.F.; Van der Meer, R. Bactericidal activities of milk lipids. Antimicrob. Agents Chemother. 2001, 45, 1298–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Description | Unit | Placebo | WPC Low | WPC High |
|---|---|---|---|---|
| Fat | w/w% | 5 | 10 | 16 |
| - of which Phospholipids | w/w% | 0 | 4.4 | 7.0 |
| Protein | w/w% | 60 | 58 | 57 |
| - of which HA300 | w/w% | 60 | 31 | 0 |
| Lactose | w/w% | 1.7 | 1.0 | 1.0 |
| Maltodextrin | w/w% | 18 | 18 | 18 |
| Moisture | w/w% | 4.4 | 4.7 | 4.0 |
| Ash 525 °C | w/w% | 7.7 | 5.3 | 4.0 |
| Calcium content | mg/kg | 7700 | 7967 | 8200 |
| Variable | Placebo | Ingredient Low Dose | Ingredient High Dose | |
|---|---|---|---|---|
| Gender | n | 35 | 38 | 37 |
| Male | 100% | 100% | 100% | |
| Age | Mean (SD) | 36.29 (11.6) | 34.16 (11.93) | 33.7 (9.98) |
| BMI (kg/m2) | Mean (SD) | 24.43 (2.24) | 23.85 (2.93) | 24.02 (2.81) |
| Parameter | Variable | Placebo | WPC Low | WPC High | p-Value Trend |
|---|---|---|---|---|---|
| Change in stool frequency (# defecations/day) d16 vs. d15 P | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.01 |
| Mean (SD) | −0.9 (1.5) | −1.2 (1.6) | −0.4 (1.4) | ||
| Percentage change in stool frequency d16 vs. d15 S | n (Nmiss) | 33 (2) | 37 (1) | 35 (2) | p 0.053 |
| Mean (SD) | −36.2 (45.2) | −38.8 (67.3) | −3.2 (80.8) | ||
| Stool frequency d15 PH | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.931 |
| Mean (SD) | 2.1 (1.4) | 2.1 (1.4) | 1.9 (1.1) | ||
| Stool frequency d16 S | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.034 |
| Mean (SD) | 1.2 (0.8) | 1.0 (0.8) | 1.5 (0.8) | ||
| AUC for stool frequency d11-d18 PH | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.707 |
| Mean (SD) | 9.2 (3.9) | 8.5 (2.8) | 8.9 (2.8) |
| Parameter | Variable | Placebo | WPC Low | WPC High | Statistics |
|---|---|---|---|---|---|
| Change in GSRS diarrhea d16 vs. d15 P | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.13 |
| Mean (SD) | −2.8 (4.7) | −2.7 (4.6) | −1.4 (4.7) | ||
| Percentage change in GSRS diarrhea d16 vs. d15 S | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.09 |
| Mean (SD) | −20.1 (37.9) | −21.3 (46.6) | −0.7 (61.0) | ||
| GSRS max diarrhea score day 14–day 18 S | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.84 |
| Mean (SD) | 8.3 (4.9) | 7.4 (4.4) | 7.9 (5.9) | ||
| GSRS diarrhea d15 PH | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.48 |
| Mean (SD) | 7.3 (4.9) | 6.9 (4.5) | 6.8 (5.7) | ||
| AUC GSRS diarrhea d11-d18 PH | n (Nmiss) | 35 (0) | 38 (0) | 37 (0) | p 0.54 |
| Mean (SD) | 29.7 (8.8) | 29.2 (8.1) | 30.4 (14.7) |
| Parameter | Variable | Placebo | WPC Low | WPC High | Statistics |
|---|---|---|---|---|---|
| Change in BSS max d16 vs. d15 | n (Nmiss) | 28 (7) | 26 (12) | 34 (3) | p 0.79 |
| Mean (SD) | −0.82 (2.06) | −1.00 (1.7) | −0.38 (1.54) | ||
| Percentage change in BSS max d16 vs. d15 | n (Nmiss) | 28 (7) | 26 (12) | 34 (3) | p 0.58 |
| Mean (SD) | −7.53 (43.01) | −14.88 (27.51) | −1.51 (36.11) | ||
| BSS max d15 | n (Nmiss) | 33 (2) | 37 (1) | 35 (2) | p 0.07 |
| Mean (SD) | 5.61 (1.60) | 5.57 (1.48) | 4.83 (1.64) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulfman, L.H.; Schloesser, J.E.L.; Kortman, G.A.M.; van den Belt, M.; Lucas-van de Bos, E.; Roggekamp, J.; van Neerven, R.J.J.; Porbahaie, M.; van Hoffen, E.; Kardinaal, A.F.M. A Double-Blind, Randomized Intervention Study on the Effect of a Whey Protein Concentrate on E. coli-Induced Diarrhea in a Human Infection Model. Nutrients 2022, 14, 1204. https://doi.org/10.3390/nu14061204
Ulfman LH, Schloesser JEL, Kortman GAM, van den Belt M, Lucas-van de Bos E, Roggekamp J, van Neerven RJJ, Porbahaie M, van Hoffen E, Kardinaal AFM. A Double-Blind, Randomized Intervention Study on the Effect of a Whey Protein Concentrate on E. coli-Induced Diarrhea in a Human Infection Model. Nutrients. 2022; 14(6):1204. https://doi.org/10.3390/nu14061204
Chicago/Turabian StyleUlfman, Laurien H., Joyce E. L. Schloesser, Guus A. M. Kortman, Maartje van den Belt, Elly Lucas-van de Bos, Joris Roggekamp, R. J. Joost van Neerven, Mojtaba Porbahaie, Els van Hoffen, and Alwine F. M. Kardinaal. 2022. "A Double-Blind, Randomized Intervention Study on the Effect of a Whey Protein Concentrate on E. coli-Induced Diarrhea in a Human Infection Model" Nutrients 14, no. 6: 1204. https://doi.org/10.3390/nu14061204
APA StyleUlfman, L. H., Schloesser, J. E. L., Kortman, G. A. M., van den Belt, M., Lucas-van de Bos, E., Roggekamp, J., van Neerven, R. J. J., Porbahaie, M., van Hoffen, E., & Kardinaal, A. F. M. (2022). A Double-Blind, Randomized Intervention Study on the Effect of a Whey Protein Concentrate on E. coli-Induced Diarrhea in a Human Infection Model. Nutrients, 14(6), 1204. https://doi.org/10.3390/nu14061204






