Dietary Supplements and the Skin: Focus on Photoprotection and Antioxidant Activity—A Review
Abstract
:1. Introduction
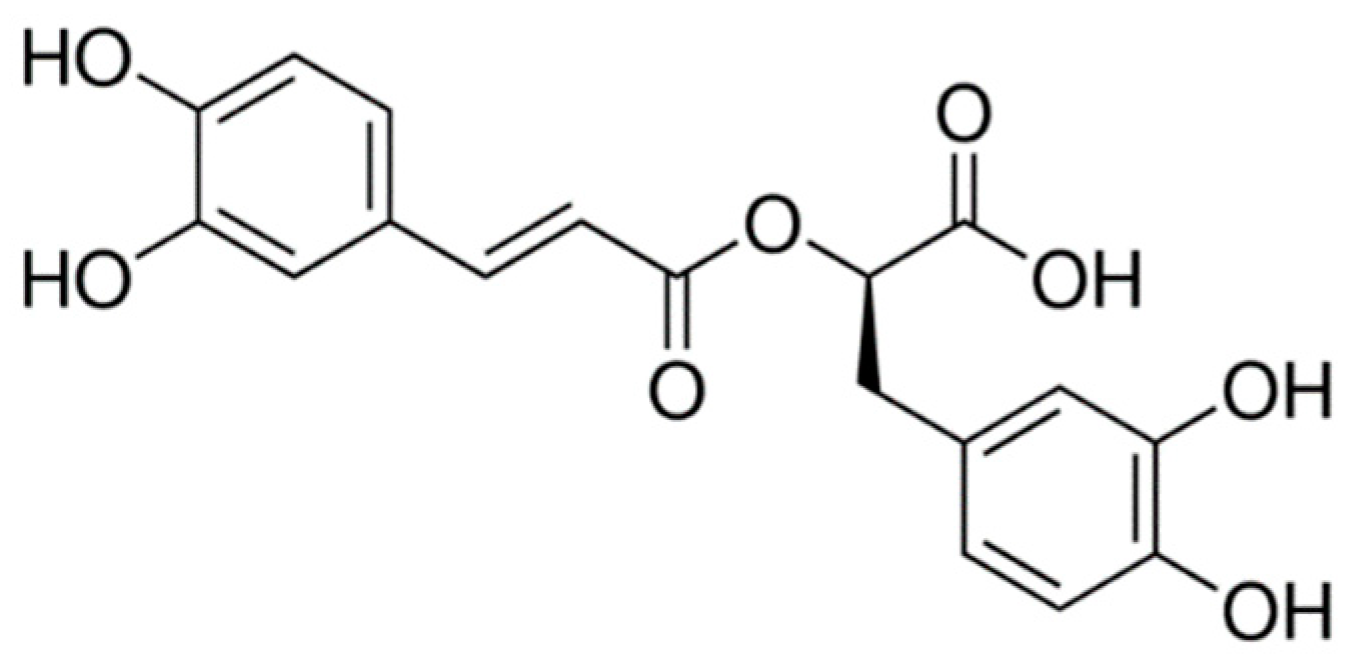
2. Rosmarinic Acid
3. Polypodium leucotomus
4. Pycnogenol®
5. Carotenoids
5.1. Astaxanthin
5.2. Lutein
6. Tranexamic Acid
7. Pomegranate (Punica granatum L.) Extract
8. Orthosilic Acid
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Amoah, S.K.S.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic Acid—Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef] [Green Version]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Palombo, P.; Fabrizi, G.; Ruocco, V.; Ruocco, E.; Fluhr, J.; Roberts, R.; Morganti, P. Beneficial Long-Term Effects of Combined Oral/Topical Antioxidant Treatment with the Carotenoids Lutein and Zeaxanthin on Human Skin: A Double-Blind, Placebo-Controlled Study. Skin Pharmacol. Physiol. 2007, 20, 199–210. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Nobile, V.; Michelotti, A.; Cestone, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Pérez-Sánchez, A.; Micol, V. Skin photoprotective and antiageing effects of a combination of rosemary (Rosmarinus officinalis) and grapefruit (Citrus paradisi) polyphenols. Food Nutr. Res. 2016, 60, 31871. [Google Scholar] [CrossRef] [Green Version]
- Kasai, K.; Yoshimura, M.; Koga, T.; Arii, M.; Kawasaki, S. Effects of Oral Administration of Ellagic Acid-Rich Pomegranate Extract on Ultraviolet-Induced Pigmentation in the Human Skin. J. Nutr. Sci. Vitaminol. 2006, 52, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.O.; Freire, É.S.; Polonini, H.C.; Da Silva, P.J.; Brandão, M.A.; Raposo, N.R. Anti-Aging Effects of Monomethylsilanetriol and Maltodextrin-Stabilized Orthosilicic Acid on Nails, Skin and Hair. Cosmetics 2018, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacký Olomouc Czech Repub. 2006, 150, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Binic, I.; Lazarevic, V.; Ljubenovic, M.; Mojsa, J.; Sokolovic, D. Skin ageing: Natural weapons and strategies. Evid. Based Complement. Altern. Med. 2013, 2013, 827248. [Google Scholar] [CrossRef] [Green Version]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- Szyszkowska, B.; Łepecka-Klusek, C.; Kozłowicz, K.; Jazienicka, I.; Krasowska, D. The influence of selected ingredients of dietary supplements on skin condition. Postep. Derm. Alergol. 2014, 31, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Cândido, T.M.; Ariede, M.B.; Pinto, C.A.; Lourenço, F.R.; Rosado, C.; Velasco, M.V.; Baby, A.R. Prospecting In Vitro Antioxidant and Photoprotective Properties of Rosmarinic Acid in a Sunscreen System Developed by QbD Containing Octyl p-Methoxycinnamate and Bemotrizinol. Cosmetics 2022, 9, 29. [Google Scholar] [CrossRef]
- Fernando, P.M.D.J.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Hewage, S.R.K.M.; Chae, S.W.; Hyun, J.W. Rosmarinic Acid Attenuates Cell Damage against UVB Radiation-Induced Oxidative Stress via Enhancing Antioxidant Effects in Human HaCaT Cells. Biomol. Ther. 2016, 24, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, Y.S.; Park, D. Rosmarinic acid induces melanogenesis through protein kinase A activation signaling. Biochem. Pharmacol. 2007, 74, 960–968. [Google Scholar] [CrossRef]
- Lešnik, S.; Furlan, V.; Bren, U. Rosemary (Rosmarinus officinalis L.): Extraction techniques, analytical methods and health-promoting biological effects. Phytochem. Rev. 2021, 20, 1273–1328. [Google Scholar] [CrossRef]
- Sánchez-Campillo, M.; Gabaldon, J.A.; Castillo, J.; Benavente-García, O.; Del Baño, M.J.; Alcaraz, M.; Vicente, V.; Alvarez, N.; Lozano, J.A. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem. Toxicol. 2009, 47, 386–392. [Google Scholar] [CrossRef]
- Aung, H.T.; Takaya, Y.; Nikai, T.; Niwa, M. cis-trans Interconversion of Rosmarinic Acid. J. Res. Inst. Meijo Univ. 2014, 13, 87–92. [Google Scholar]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Caturla, N.; Castillo, J.; Benavente-García, O.; Alcaraz, M.; Micol, V. Protective effects of citrus and rosemary extracts on UV-induced damage in skin cell model and human volunteers. J. Photochem. Photobiol. B 2014, 136, 12–18. [Google Scholar] [CrossRef]
- Tomazelli, L.C.; de Assis Ramos, M.M.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; de Oliveira Pinto, C.A.S.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef]
- Bors, W.; Christa, M.; Stettmaier, K.; Yinrong, L.; Yeap, F.L. Antioxidant Mechanisms of Polyphenolic Caffeic Acid Oligomers, Constituents of Salvia officinalis. Biol. Res. 2004, 37, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Popov, A.M.; Osipov, A.N.; Korepanova, E.A.; Krivoshapko, O.N.; Artyukov, A.A. Study of antioxidant and membrane activity of rosmarinic acid using different model systems. Biophysics 2013, 58, 607–615. [Google Scholar] [CrossRef]
- Gonzalez, S.; Gilaberte, Y.; Philips, N. Mechanistic insights in the use of a Polypodium leucotomos extract as an oral and topical photoprotective agent. Photochem. Photobiol. Sci. 2010, 9, 559–563. [Google Scholar] [CrossRef]
- Berman, B.; Ellis, C.; Elmets, C. Polypodium Leucotomos—An Overview of Basic Investigative Findings. J. Drugs Dermatol. 2016, 15, 224–228. [Google Scholar]
- Noronha, M.D.M. Tendências Mais Recentes na Fotoproteção. Master’s Thesis, Universidade Lusófona de Humanidades e Tecnologias, Lisboa, Portugal, 2014. [Google Scholar]
- Garcia, F.; Pivel, J.P.; Guerrero, A.; Brieva, A.; Martinez-Alcazar, M.P.; Caamano-Somoza, M.; Gonzalez, S. Phenolic components and antioxidant activity of Fernblock, an aqueous extract of the aerial parts of the fern Polypodium leucotomos. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 157–160. [Google Scholar] [CrossRef]
- Gonzalez, S.; Alonso-Lebrero, J.L.; Rio, R.D.; Jaen, P. Polypodium leucotomos extract: A nutraceutical with photoprotective properties. Drugs Today 2007, 43, 475–485. [Google Scholar] [CrossRef]
- Parrado, C.; Mascaraque, M.; Gilaberte, Y.; Juarranz, A.; Gonzalez, S. Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. Int. J. Mol. Sci. 2016, 17, 1026. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Gilaberte, Y.; Philips, N.; Juarranz, A. Current Trends in Photoprotection—A New Generation of Oral Photoprotectors. Open Dermatol. J. 2011, 5, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Gombau, L.; García, F.; Lahoz, A.; Fabre, M.; Roda-Navarro, P.; Majano, P.; Alonso-Lebrero, J.L.; Pivel, J.P.; Castell, J.V.; Gómez-Lechon, M.J.; et al. Polypodium leucotomos extract: Antioxidant activity and disposition. Toxicol. Vitr. 2006, 20, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp-Hup, M.A.; Bos, J.D.; Rius-Diaz, F.; Gonzalez, S.; Westerhof, W. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: A randomized double-blind placebo-controlled study. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, J.R.; Zapolanski, T.; Magro, C.; Carrington, K.; Prograis, S.; Nussbaum, M.; Gonzalez, S.; Ding, W.; Granstein, R.D. Polypodium leucotomos inhibits ultraviolet B radiation-induced immunosuppression. Photodermatol. Photoimmunol. Photomed. 2008, 24, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bosca, A.; Zapater, P.; Betlloch, I.; Albero, F.; Martínez, A.; Díaz-Alperi, J.; Horga, J.F. Polypodium leucotomos Extract in Atopic Dermatitis: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Actas Dermo-Sifiliogr. 2012, 103, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Tanew, A.; Radakovic, S.; Gonzalez, S.; Venturini, M.; Calzavara-Pinton, P. Oral administration of a hydrophilic extract of Polypodium leucotomos for the prevention of polymorphic light eruption. J. Am. Acad. Dermatol. 2012, 66, 58–62. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Pathak, M.A. Inhibition of ultraviolet-induced formation of reactive oxygen species, lipid peroxidation, erythema and skin photosensitization by Polypodium leucotomos. Photodermatol. Photoimmunol. Photomed. 1996, 12, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Yasuko, K.; Tomohiro, N.; Sei-Itsu, M.; Ai-Na, L.; Yasuo, F.; Takashi, T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim. Biophys. Acta 1984, 792, 92–97. [Google Scholar] [CrossRef]
- Capote, R.; Alonso-Lebrero, J.L.; García, F.; Brieva, A.; Pivel, J.P.; González, S. Polypodium leucotomos extract inhibits trans-urocanic acid photoisomerization and photodecomposition. J. Photochem. Photobiol. B 2006, 82, 173–179. [Google Scholar] [CrossRef]
- Philips, N.; Conte, J.; Chen, Y.-J.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-β by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487. [Google Scholar] [CrossRef]
- Murbach, T.S.; Béres, E.; Vértesi, A.; Glávits, R.; Hirka, G.; Endres, J.R.; Clewell, A.E.; Szakonyiné, I.P. A comprehensive toxicological safety assessment of an aqueous extract of Polypodium leucotomos (Fernblock®). Food Chem. Toxicol. 2015, 86, 328–341. [Google Scholar] [CrossRef] [Green Version]
- Kohli, I.; Shafi, R.; Isedeh, P.; Griffith, J.L.; Al-Jamal, M.S.; Silpa-archa, N.; Jackson, B.; Athar, M.; Kollias, N.; Elmets, C.A.; et al. The impact of oral Polypodium leucotomos extract on ultraviolet B response: A human clinical study. J. Am. Acad. Dermatol. 2017, 77, 33–41.e1. [Google Scholar] [CrossRef]
- Nestor, M.; Bucay, V.; Callender, V.; Cohen, J.L.; Sadick, N.; Waldorf, H. Polypodium leucotomos as an Adjunct Treatment of Pigmentary Disorders. J. Clin. Aesthet. Dermatol. 2014, 7, 13–17. [Google Scholar]
- Ahmed, A.M.; Lopez, I.; Perese, F.; Vasquez, R.; Hynan, L.S.; Chong, B.; Pandya, A.G. A Randomized, Double-Blinded, Placebo-Controlled Trial of Oral Polypodium leucotomos Extract as an Adjunct to Sunscreen in the Treatment of Melasma. JAMA Dermatol. 2013, 149, 981–983. [Google Scholar] [CrossRef] [Green Version]
- Noda, Y.; Anzai, K.; Mori, A.; Kohno, M.; Shinmei, M.; Packer, L. Hydroxyl and superoxide anion radical scavenging activities of natural source antioxidants using the computerized JES-FR30 ESR spectrometer system. Biochem. Mol. Biol. Int. 1997, 42, 35–44. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Wood, J.E.; Senthilmohan, S.T.; Peskin, A.V. Antioxidant activity of procyanidin-containing plant extracts at different pHs. Food Chem. 2002, 77, 155–161. [Google Scholar] [CrossRef]
- D’Andrea, G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef]
- Merah, S.; Dahmane, D.; Krimat, S.; Metidji, H.; Nouasri, A.; Lamari, L.; Dob, T. Chemical analysis of phenolic compounds and determination of antioxidant, antimicrobial and cytotoxic activities of organic extracts of Pinus coulteri. Bangladesh J. Pharm. 2018, 13, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.; Mu, Y.; Gulati, O. Treatment of melasma with Pycnogenol®. Phytother. Res. 2002, 16, 567–571. [Google Scholar] [CrossRef]
- Devaraj, S.; Vega-López, S.; Kaul, N.; Schönlau, F.; Rohdewald, P.; Jialal, I. Supplementation with a pine bark extract rich in polyphenols increases plasma antioxidant capacity and alters the plasma lipoprotein profile. Lipids 2002, 37, 931–934. [Google Scholar] [CrossRef]
- Ryan, J.; Croft, K.; Mori, T.; Wesnes, K.; Spong, J.; Downey, L.; Kure, C.; Lloyd, J.; Stough, C. An examination of the effects of the antioxidant Pycnogenol® on cognitive performance, serum lipid profile, endocrinological and oxidative stress biomarkers in an elderly population. J. Psychopharmacol. 2008, 22, 553–562. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How effective are they to prevent age-related diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef] [Green Version]
- Han, R.M.; Zhang, J.P.; Skibsted, L.H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef] [Green Version]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Dermatol. 2016, 9, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability-The Way of Bioavailability Improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef] [Green Version]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta 2007, 1768, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008, 101, S58–S68. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Pilbrant, Å.; Schannong, M.; Vessman, J. Pharmacokinetics and bioavailability of tranexamic acid. Eur. J. Clin. Pharmacol. 1981, 20, 65. [Google Scholar] [CrossRef]
- Dunn, C.J.; Goa, K.L. Tranexamic Acid. Drugs 1999, 57, 1005–1032. [Google Scholar] [CrossRef]
- Ker, K.; Edwards, P.; Perel, P.; Shakur, H.; Roberts, I. Effect of tranexamic acid on surgical bleeding: Systematic review and cumulative meta-analysis. BMJ Br. Med. J. 2012, 344, e3054. [Google Scholar] [CrossRef] [Green Version]
- Colferai, M.M.T.; Miquelin, G.M.; Steiner, D. Evaluation of oral tranexamic acid in the treatment of melasma. J. Cosmet. Dermatol. 2019, 18, 1495–1501. [Google Scholar] [CrossRef]
- Bagherani, N.; Gianfaldoni, S.; Smoller, B. An Overview on Melasma. J. Pigment. Disord. 2015, 2, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.Y. Mélasma et anomalies pigmentaires chez les asiatiques. Ann. Dermatol. Venereol. 2012, 139, S92–S95. [Google Scholar] [CrossRef]
- Tse, T.W.; Hui, E. Tranexamic acid: An important adjuvant in the treatment of melasma. J. Cosmet. Dermatol. 2013, 12, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Naganuma, M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J. Photochem. Photobiol. B 1998, 47, 136–141. [Google Scholar] [CrossRef]
- Karn, D.; Kc, S.; Amatya, A.; Razouria, E.; Timalsina, M. Oral Tranexamic Acid for the Treatment of Melasma. Kathmandu Univ. Med. J. 2014, 10, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Na, J.I.; Choi, S.Y.; Yang, S.H.; Choi, H.R.; Kang, H.Y.; Park, K.C. Effect of tranexamic acid on melasma: A clinical trial with histological evaluation. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, D.; Bhattacharjee, R.; Vinay, K.; Saikia, U.N.; Parsad, D.; Kumaran, M.S. Efficacy of oral tranexemic acid in refractory melasma: A clinico–immuno-histopathological study. Dermatol. Ther. 2018, 31, e12704. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Yang, J.; Lee, R.-P.; Huang, J.; Hsu, M.; Thames, G.; Gilbuena, I.; Long, J.; Xu, Y.; Park, E.H.; et al. Pomegranate Juice and Extract Consumption Increases the Resistance to UVB-induced Erythema and Changes the Skin Microbiome in Healthy Women: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 14528. [Google Scholar] [CrossRef]
- Kang, S.-J.; Choi, B.-R.; Kim, S.-H.; Yi, H.-Y.; Park, H.-R.; Sung, M.-S.; Song, C.-H.; Cho, I.-J.; Lee, Y.-J.; Ku, S.-K. Evaluation of the skin moisturizing effects and underlying mechanisms of pomegranate concentrate solution and dried pomegranate concentrate powder. J. Korean Med. 2016, 37, 12–22. [Google Scholar] [CrossRef]
- Liu, C.; Guo, H.; DaSilva, N.A.; Li, D.; Zhang, K.; Wan, Y.; Gao, X.-H.; Chen, H.-D.; Seeram, N.P.; Ma, H. Pomegranate (Punica granatum) phenolics ameliorate hydrogen peroxide-induced oxidative stress and cytotoxicity in human keratinocytes. J. Funct. Foods 2019, 54, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Moon, E.; Kim, A.-J.; Kim, M.H.; Lee, S.; Lee, J.B.; Park, Y.K.; Jung, H.-S.; Kim, Y.-B.; Kim, S.Y. Extract of Punica granatum inhibits skin photoaging induced by UVB irradiation. Int. J. Dermatol. 2010, 49, 276–282. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Lee, H.-Y. Composition for Improving Skin, Containing Pomegranate Concentrate as Active Ingredient. WO/2016/013709, 28 January 2016. [Google Scholar]
- Dario, M.F.; Pahl, R.; de Castro, J.R.; de Lima, F.S.; Kaneko, T.M.; Pinto, C.A.S.O.; Baby, A.R.; Velasco, M.V.R. Efficacy of Punica granatum L. hydroalcoholic extract on properties of dyed hair exposed to UVA radiation. J. Photochem. Photobiol. B 2013, 120, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, L.; Bombonatti, B.; Sabo, M.L.; Tadeu, V.R.; Robles, V.M.V.; Bedin, V.; Fortes, B.B.V.A.C. Oral supplementation of orthosilicic acid and its impact on hair quality. Med. Cutan. Ibero Lat. Am. 2017, 45, 29–35. [Google Scholar]
- Kalil, C.L.P.V.; Campos, V.; Cignachi, S.; Izidoro, J.F.; Reinehr, C.P.H.; Chaves, C. Evaluation of cutaneous rejuvenation associated with the use of ortho-silicic acid stabilized by hydrolyzed marine collagen. J. Cosmet. Dermatol. 2018, 17, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Wickett, R.R.; Kossmann, E.; Barel, A.; Demeester, N.; Clarys, P.; Vanden Berghe, D.; Calomme, M. Effect of oral intake of choline-stabilized orthosilicic acid on hair tensile strength and morphology in women with fine hair. Arch. Dermatol. Res. 2007, 299, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Barel, A.; Calomme, M.; Timchenko, A.; Paepe, K.D.; Demeester, N.; Rogiers, V.; Clarys, P.; Vanden Berghe, D. Effect of oral intake of choline-stabilized orthosilicic acid on skin, nails and hair in women with photodamaged skin. Arch. Dermatol. Res. 2005, 297, 147–153. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcílio Cândido, T.; Bueno Ariede, M.; Vieira Lima, F.; de Souza Guedes, L.; Robles Velasco, M.V.; Rolim Baby, A.; Rosado, C. Dietary Supplements and the Skin: Focus on Photoprotection and Antioxidant Activity—A Review. Nutrients 2022, 14, 1248. https://doi.org/10.3390/nu14061248
Marcílio Cândido T, Bueno Ariede M, Vieira Lima F, de Souza Guedes L, Robles Velasco MV, Rolim Baby A, Rosado C. Dietary Supplements and the Skin: Focus on Photoprotection and Antioxidant Activity—A Review. Nutrients. 2022; 14(6):1248. https://doi.org/10.3390/nu14061248
Chicago/Turabian StyleMarcílio Cândido, Thalita, Maíra Bueno Ariede, Fabiana Vieira Lima, Luciana de Souza Guedes, Maria Valéria Robles Velasco, André Rolim Baby, and Catarina Rosado. 2022. "Dietary Supplements and the Skin: Focus on Photoprotection and Antioxidant Activity—A Review" Nutrients 14, no. 6: 1248. https://doi.org/10.3390/nu14061248








