Combination of Texture-Induced Oral Processing and Vegetable Preload Strategy Reduced Glycemic Excursion but Decreased Insulin Sensitivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
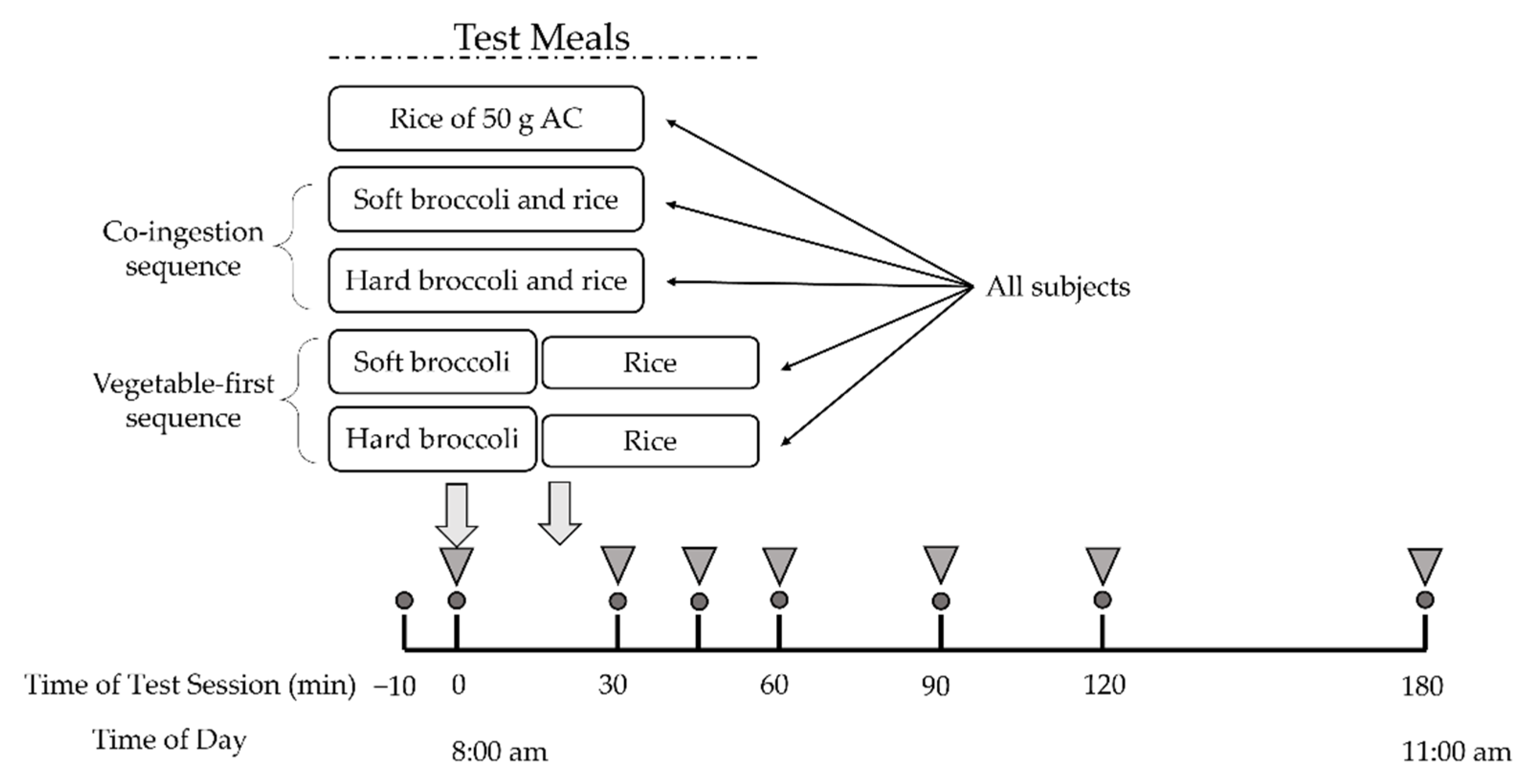
2.2. Study Design
2.3. Meals
2.4. Texture Analysis
2.5. Analysis of Oral Processing Behaviors
2.6. Blood Collection and Metabolite Measurements
2.7. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Instrumental Texture Characteristics of Broccoli
3.3. Oral Processing Behaviours
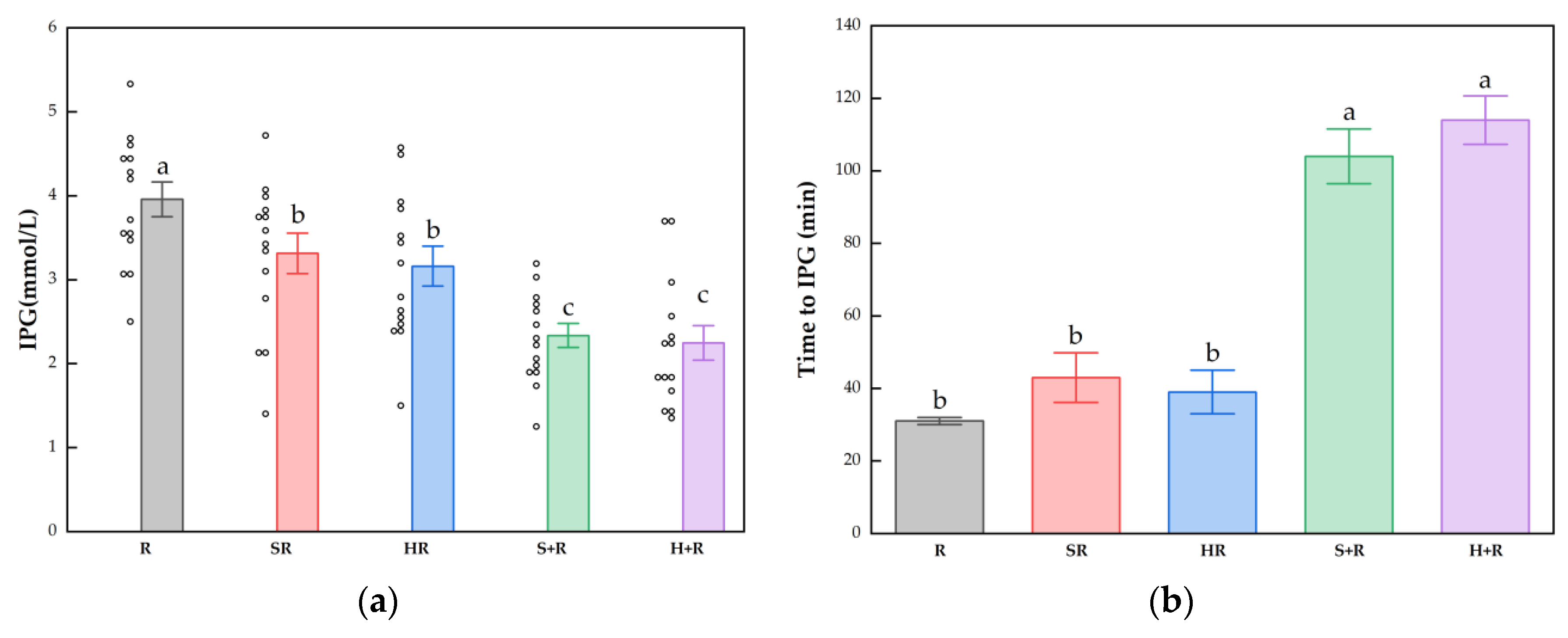
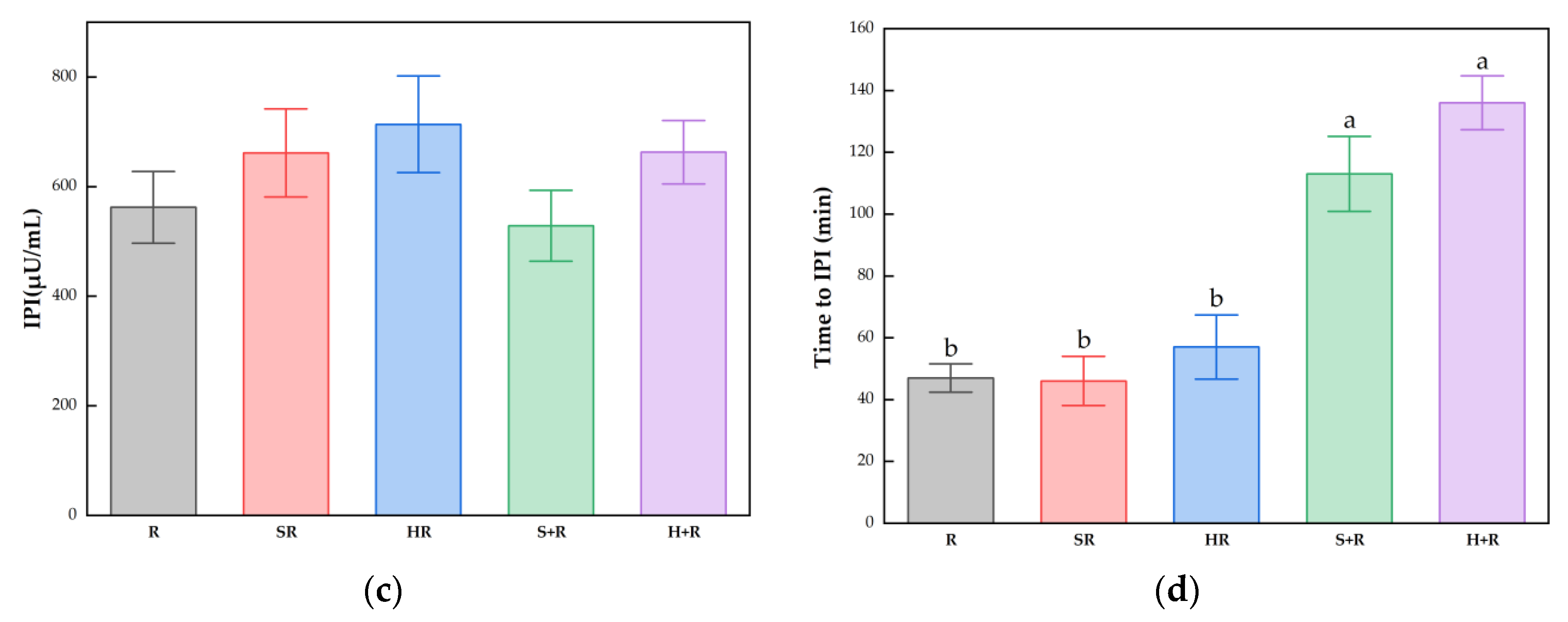
3.4. Postprandial Glucose and Insulin Response
3.5. Postprandial Glucose and Insulin Response Characteristics
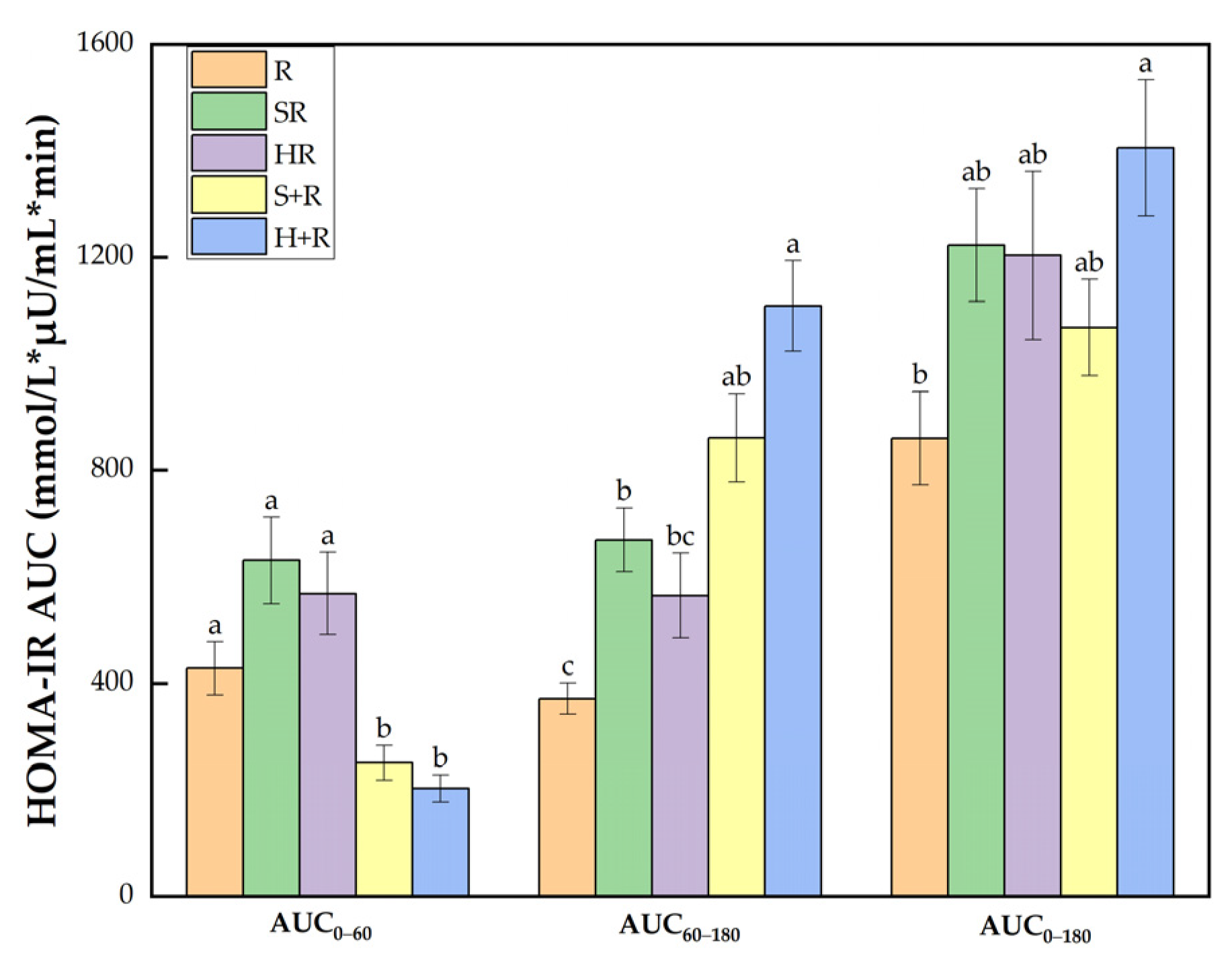
3.6. Insulin Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolb, H.; Eizirik, D.L. Resistance to type 2 diabetes mellitus: A matter of hormesis? Nat. Rev. Endocrinol. 2011, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.S.; Sharp, S.J.; Imamura, F.; Chowdhury, R.; Gundersen, T.E.; Steur, M.; Sluijs, I.; van der Schouw, Y.T.; Agudo, A.; Aune, D.; et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ 2020, 370, m2194. [Google Scholar] [CrossRef] [PubMed]
- Mamluk, L.; O’Doherty, M.G.; Orfanos, P.; Saitakis, G.; Woodside, J.V.; Liao, L.M.; Sinha, R.; Boffetta, P.; Trichopoulou, A.; Kee, F. Fruit and vegetable intake and risk of incident of type 2 diabetes: Results from the consortium on health and ageing network of cohorts in Europe and the United States (CHANCES). Eur. J. Clin. Nutr. 2017, 71, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.Y.; Fang, J.C.; Gao, Z.H.; Zhang, C.; Xie, S.Y. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhong, L.; Song, Y.; Hu, Y.; Wang, G.; Sun, S. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim. Care Diabetes 2016, 10, 272–280. [Google Scholar] [CrossRef]
- Chiao-Hsin, Y.; Chia-Wei, C.; Jenshinn, L. White Rice Glycemic Index Measured in Venous and Capillary Blood Samples. Food Sci. Technol. Res. 2017, 23, 297–304. [Google Scholar]
- Gustafsson, K.; Asp, N.G.; Hagander, B.; Nyman, M. Effects of different vegetables in mixed meals on glucose homeostasis and satiety. Eur. J. Clin. Nutr. 1993, 47, 192–200. [Google Scholar]
- Imai, S.; Matsuda, M.; Hasegawa, G.; Fukui, M.; Obayashi, H.; Ozasa, N.; Kajiyama, S. A simple meal plan of ‘eating vegetables before carbohydrate’ was more effective for achieving glycemic control than an exchange-based meal plan in Japanese patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2011, 20, 161–168. [Google Scholar]
- Gopirajah, R.; Raichurkar, K.P.; Wadhwa, R.; Anandharamakrishnan, C. The glycemic response to fibre rich foods and their relationship with gastric emptying and motor functions: An MRI study. Food Funct. 2016, 7, 3964–3972. [Google Scholar] [CrossRef]
- Argyri, K.; Sotiropoulos, A.; Psarou, E.; Papazafiropoulou, A.; Zampelas, A.; Kapsokefalou, M. Dessert formulation using sucralose and dextrin affects favorably postprandial response to glucose, insulin, and C-peptide in type 2 diabetic patients. Rev. Diabet. Stud. 2013, 10, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Coe, S.; Ryan, L. Impact of polyphenol-rich sources on acute postprandial glycaemia: A systematic review. J. Nutr. Sci. 2016, 5, e24. [Google Scholar] [CrossRef] [PubMed]
- Della, G.L.; Thomas, M.A.; Cena, H. Insulin Sensitivity and Glucose Homeostasis Can Be Influenced by Metabolic Acid Load. Nutrients 2018, 10, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della, G.L.; Roggi, C.; Cena, H. Diet-induced acidosis and alkali supplementation. Int. J. Food Sci. Nutr. 2016, 67, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, M.; Han, Y.; Wang, L.; Ye, T.; Lu, J.; Fan, Z. Acute effects of non-homogenised and homogenised vegetables added to rice-based meals on postprandial glycaemic responses and in vitro carbohydrate digestion. Br. J. Nutr. 2018, 120, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Engelen, L.; Fontijn-Tekamp, A.; van der Bilt, A. The influence of product and oral characteristics on swallowing. Arch. Oral Biol. 2005, 50, 739–746. [Google Scholar] [CrossRef]
- Ranawana, V.; Monro, J.A.; Mishra, S.; Henry, C.J. Degree of particle size breakdown during mastication may be a possible cause of interindividual glycemic variability. Nutr. Res. 2010, 30, 246–254. [Google Scholar] [CrossRef]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. Increased number of chews during a fixed-amount meal suppresses postprandial appetite and modulates glycemic response in older males. Physiol. Behav. 2014, 133, 136–140. [Google Scholar] [CrossRef]
- Madhu, V.; Shirali, A.; Pawaskar, P.N.; Madi, D.; Chowta, N.; Ramapuram, J.T. Mastication Frequency and Postprandial Blood Sugar Levels in Normoglycaemic and Dysglycaemic Individuals: A Cross-Sectional Comparative Study. J. Clin. Diagn. Res. 2016, 10, C6–C8. [Google Scholar] [CrossRef]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. Increasing the number of masticatory cycles is associated with reduced appetite and altered postprandial plasma concentrations of gut hormones, insulin and glucose. Br. J. Nutr. 2013, 110, 384–390. [Google Scholar] [CrossRef]
- Slyper, A. Oral Processing, Satiation and Obesity: Overview and Hypotheses. Diabetes Metab. Syndr. Obes. 2021, 14, 3399–3415. [Google Scholar] [CrossRef]
- Read, N.W.; Welch, I.M.; Austen, C.J.; Barnish, C.; Bartlett, C.E.; Baxter, A.J.; Brown, G.; Compton, M.E.; Hume, K.E.; Storie, I.; et al. Swallowing food without chewing; a simple way to reduce postprandial glycaemia. Br. J. Nutr. 1986, 55, 43–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranawana, V.; Leow, M.K.; Henry, C.J. Mastication effects on the glycaemic index: Impact on variability and practical implications. Eur. J. Clin. Nutr. 2014, 68, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, A.; Ohtsuka, Y.; Yamanaka, Y. Morning Mastication Enhances Postprandial Glucose Metabolism in Healthy Young Subjects. Tohoku J. Exp. Med. 2019, 249, 193–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, V.M.H.; Ooi, D.S.Q.; Kapur, J.; Wu, T.; Chan, Y.H.; Henry, C.J.; Lee, Y.S. The role of digestive factors in determining glycemic response in a multiethnic Asian population. Eur. J. Nutr. 2016, 55, 1573–1581. [Google Scholar] [CrossRef]
- Aguayo-Mendoza, M.G.; Ketel, E.C.; van der Linden, E.; Forde, C.G.; Piqueras-Fiszman, B.; Stieger, M. Oral processing behavior of drinkable, spoonable and chewable foods is primarily determined by rheological and mechanical food properties. Food Qual. Prefer. 2019, 71, 87–95. [Google Scholar] [CrossRef]
- Forde, C.G.; Leong, C.; Chia-Ming, E.; McCrickerd, K. Fast or slow-foods? Describing natural variations in oral processing characteristics across a wide range of Asian foods. Food Funct. 2017, 8, 595–606. [Google Scholar] [CrossRef]
- Mosca, A.C.; Torres, A.P.; Slob, E.; de Graaf, K.; McEwan, J.A.; Stieger, M. Small food texture modifications can be used to change oral processing behaviour and to control ad libitum food intake. Appetite 2019, 142, 104375. [Google Scholar] [CrossRef]
- McCrickerd, K.; Lim, C.M.H.; Leong, C.; Chia, E.M.; Forde, C.G. Texture-Based Differences in Eating Rate Reduce the Impact of Increased Energy Density and Large Portions on Meal Size in Adults. J. Nutr. 2017, 147, 1208–1217. [Google Scholar] [CrossRef]
- De Wijk, R.A.; Zijlstra, N.; Mars, M.; de Graaf, C.; Prinz, J.F. The effects of food viscosity on bite size, bite effort and food intake. Physiol. Behav. 2008, 95, 527–532. [Google Scholar] [CrossRef]
- Goh, A.T.; Choy, J.; Chua, X.H.; Ponnalagu, S.; Khoo, C.M.; Whitton, C.; van Dam, R.M.; Forde, C.G. Increased oral processing and a slower eating rate increase glycaemic, insulin and satiety responses to a mixed meal tolerance test. Eur. J. Nutr. 2021, 60, 2719–2733. [Google Scholar] [CrossRef]
- Laboure, H.; Van Wymelbeke, V.; Fantino, M.; Nicolaidis, S. Behavioral, plasma, and calorimetric changes related to food texture modification in men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1501–R1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Goh, H.J.; Govindharajulu, P.; Leow, M.K.; Henry, C.J. Postprandial glucose, insulin and incretin responses differ by test meal macronutrient ingestion sequence (PATTERN study). Clin. Nutr. 2020, 39, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.P.; Dickison, M.; Coughlin, N.; Karan, A.; Mauer, E.; Truong, W.; Casper, A.; Emiliano, A.B.; Kumar, R.B.; Saunders, K.H.; et al. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes Obes. Metab. 2019, 21, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.P.; Andono, J.; Touhamy, S.H.; Casper, A.; Iliescu, R.G.; Mauer, E.; Shan, Z.Y.; Ludwig, D.S.; Aronne, L.J. Carbohydrate-last meal pattern lowers postprandial glucose and insulin excursions in type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e440. [Google Scholar] [CrossRef] [Green Version]
- Bhavadharini, B.; Mohan, V.; Dehghan, M.; Rangarajan, S.; Swaminathan, S.; Rosengren, A.; Wielgosz, A.; Avezum, A.; Lopez-Jaramillo, P.; Lanas, F.; et al. White Rice Intake and Incident Diabetes: A Study of 132,373 Participants in 21 Countries. Diabetes Care 2020, 43, 2643–2650. [Google Scholar] [CrossRef]
- Kameyama, N.; Maruyama, C.; Matsui, S.; Araki, R.; Yamada, Y.; Maruyama, T. Effects of consumption of main and side dishes with white rice on postprandial glucose, insulin, glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 responses in healthy Japanese men. Br. J. Nutr. 2014, 111, 1632–1640. [Google Scholar] [CrossRef]
- Ranawana, V.; Clegg, M.E.; Shafat, A.; Henry, C.J. Postmastication digestion factors influence glycemic variability in humans. Nutr. Res. 2011, 31, 452–459. [Google Scholar] [CrossRef]
- Barrett, D.M.; Garcia, E.; Wayne, J.E. Textural modification of processing tomatoes. Crit. Rev. Food Sci. Nutr. 1998, 38, 173–258. [Google Scholar] [CrossRef]
- Van Eck, A.; Wijne, C.; Fogliano, V.; Stieger, M.; Scholten, E. Shape up! How shape, size and addition of condiments influence eating behavior towards vegetables. Food Funct. 2019, 10, 5739–5751. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T.M. Effect of blood sampling schedule and method of calculating the area under the curve on validity and precision of glycaemic index values. Br. J. Nutr. 2004, 91, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borer, K.T.; Lin, P.J.; Wuorinen, E. Timing of Meals and Exercise Affects Hormonal Control of Glucoregulation, Insulin Resistance, Substrate Metabolism, and Gastrointestinal Hormones, but Has Little Effect on Appetite in Postmenopausal Women. Nutrients 2021, 13, 4342. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Brand-Miller, J.C.; Abernethy, J.; Astrup, A.; Atkinson, F.; Axelsen, M.; Bjorck, I.; Brighenti, F.; Brown, R.; Brynes, A.; et al. Measuring the glycemic index of foods: Interlaboratory study. Am. J. Clin. Nutr. 2008, 87, 247S–257S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo, F.; Tomas-Barberan, F.A.; Ferreres, F. Characterisation of flavonols in broccoli (Brassica oleracea L. var. italica) by liquid chromatography-uV diode-array detection-electrospray ionisation mass spectrometry. J. Chromatogr. A 2004, 1054, 181–193. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Ota, T. Glucoraphanin: A broccoli sprout extract that ameliorates obesity-induced inflammation and insulin resistance. Adipocyte 2018, 7, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [Green Version]
- Bahadoran, Z.; Tohidi, M.; Nazeri, P.; Mehran, M.; Azizi, F.; Mirmiran, P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: A randomized double-blind clinical trial. Int. J. Food Sci. Nutr. 2012, 63, 767–771. [Google Scholar] [CrossRef]
- Tonni, I.; Riccardi, G.; Piancino, M.G.; Stretti, C.; Costantinides, F.; Paganelli, C. The influence of food hardness on the physiological parameters of mastication: A systematic review. Arch. Oral Biol. 2020, 120, 104903. [Google Scholar] [CrossRef]
- Bolhuis, D.P.; Forde, C.G.; Cheng, Y.; Xu, H.; Martin, N.; de Graaf, C. Slow food: Sustained impact of harder foods on the reduction in energy intake over the course of the day. PLoS ONE 2014, 9, e93370. [Google Scholar] [CrossRef]
- Zijlstra, N.; Mars, M.; Stafleu, A.; de Graaf, C. The effect of texture differences on satiation in 3 pairs of solid foods. Appetite 2010, 55, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.; Goh, A.T.; Chatonidi, G.; Ponnalagu, S.; Wee, S.; Stieger, M.; Forde, C.G. Impact of food texture modifications on oral processing behaviour, bolus properties and postprandial glucose responses. Curr. Res. Food Sci. 2021, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Goh, A.T.; Chatonidi, G.; Choy, M.; Ponnalagu, S.; Stieger, M.; Forde, C.G. Impact of Individual Differences in Eating Rate on Oral Processing, Bolus Properties and Post-Meal Glucose Responses. Physiol. Behav. 2021, 238, 113495. [Google Scholar] [CrossRef] [PubMed]
- McArthur, B.M.; Mattes, R.D.; Considine, R.V. Mastication of Nuts under Realistic Eating Conditions: Implications for Energy Balance. Nutrients 2018, 10, 710. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Ranawana, D.V.; Leow, M.K.; Henry, C.J. Effect of chicken, fat and vegetable on glycaemia and insulinaemia to a white rice-based meal in healthy adults. Eur. J. Nutr. 2014, 53, 1719–1726. [Google Scholar] [CrossRef]
- Hatonen, K.A.; Virtamo, J.; Eriksson, J.G.; Sinkko, H.K.; Sundvall, J.E.; Valsta, L.M. Protein and fat modify the glycaemic and insulinaemic responses to a mashed potato-based meal. Br. J. Nutr. 2011, 106, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Ballance, S.; Knutsen, S.H.; Fosvold, O.W.; Wickham, M.; Trenado, C.D.; Monro, J. Glyceamic and insulinaemic response to mashed potato alone, or with broccoli, broccoli fibre or cellulose in healthy adults. Eur. J. Nutr. 2018, 57, 199–207. [Google Scholar] [CrossRef]
- Shokraei, S.; Khandouzi, N.; Sina, Z.; Nasrollahzadeh, J. The acute effect of incorporating lettuce or watercress into a moderately high-fat meal on postprandial lipid, glycemic response, and plasma inflammatory cytokines in healthy young men: A randomized crossover trial. Lipids Health Dis. 2021, 20, 66. [Google Scholar] [CrossRef]
- Maruyama, C.; Kikuchi, N.; Masuya, Y.; Hirota, S.; Araki, R.; Maruyama, T. Effects of green-leafy vegetable intake on postprandial glycemic and lipidemic responses and alpha-tocopherol concentration in normal weight and obese men. J. Nutr. Sci. Vitaminol. 2013, 59, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Vallejo, F.; Tomas-Barberan, F.; Garcia-Viguera, C. Health-promoting compounds in broccoli as influenced by refrigerated transport and retail sale period. J. Agr. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active compounds, antioxidant activity and alpha-glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Satsu, H.; Watanabe, H.; Fukaya, M.; Tsukamoto, Y.; Miyamoto, Y.; Shimizu, M. Acetic acid suppresses the increase in disaccharidase activity that occurs during culture of caco-2 cells. J. Nutr. 2000, 130, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Che, C.T.; Lau, C.B.; Leung, P.S.; Cheng, C.H. Inhibition of intestinal and renal Na+-glucose cotransporter by naringenin. Int. J. Biochem. Cell Biol. 2006, 38, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Ke, M.Y.; Li, W.H.; Zhang, S.Q.; Fang, X.C. The impact of soluble dietary fibre on gastric emptying, postprandial blood glucose and insulin in patients with type 2 diabetes. Asia Pac. J. Clin. Nutr. 2014, 23, 210–218. [Google Scholar]
- Irmela, S.; Michelle, V.D.K.; Teresa, O.; Matthijs, D.; Ruud, V. The effect of chewing on oral glucoraphanin hydrolysis in raw and steamed broccoli. J. Funct. Foods 2018, 45, 306–312. [Google Scholar]
- Evans, J.L. Antioxidants: Do they have a role in the treatment of insulin resistance? Indian J. Med. Res. 2007, 125, 355–372. [Google Scholar]
- Katsarou, V.; Tsolaki, M. Trends in Personalized Nutrition. In Personalized Nutrition by Predicting Glycemic Responses; Galanakis, C.M., Ed.; Academic Press Ltd.: London, UK, 2019; pp. 55–79. [Google Scholar]
- Xu, J.; Xiao, X.; Li, Y.; Zheng, J.; Li, W.; Zhang, Q.; Wang, Z. The effect of gum chewing on blood GLP-1 concentration in fasted, healthy, non-obese men. Endocrine 2015, 50, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Lasschuijt, M.; Mars, M.; de Graaf, C.; Smeets, P. How oro-sensory exposure and eating rate affect satiation and associated endocrine responses—A randomized trial. Am. J. Clin. Nutr. 2020, 111, 1137–1149. [Google Scholar] [CrossRef]
- Just, T.; Pau, H.W.; Engel, U.; Hummel, T. Cephalic phase insulin release in healthy humans after taste stimulation? Appetite 2008, 51, 622–627. [Google Scholar] [CrossRef]
- Smeets, P.A.; Erkner, A.; de Graaf, C. Cephalic phase responses and appetite. Nutr. Rev. 2010, 68, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Teff, K.L.; Mattes, R.D.; Engelman, K.; Mattern, J. Cephalic-phase insulin in obese and normal-weight men: Relation to postprandial insulin. Metabolism 1993, 42, 1600–1608. [Google Scholar] [CrossRef]
- Imai, S.; Fukui, M.; Ozasa, N.; Ozeki, T.; Kurokawa, M.; Komatsu, T.; Kajiyama, S. Eating vegetables before carbohydrates improves postprandial glucose excursions. Diabet. Med. 2013, 30, 370–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, S.; Fukui, M.; Kajiyama, S. Effect of eating vegetables before carbohydrates on glucose excursions in patients with type 2 diabetes. J. Clin. Biochem. Nutr. 2014, 54, 7–11. [Google Scholar] [CrossRef] [Green Version]



| Sample | Rice (g) | Broccoli (g) | AC 2 (g) | Protein (g) | Fat (g) | Dietary Fiber (g) | Weight 3 (g) | Energy (kcal) |
|---|---|---|---|---|---|---|---|---|
| R | 171.5 | - | 50.0 | 7.0 | 0.6 | 1.00 | 478.9 | 845.6 |
| SR | 171.5 | 283.9 | 56.0 | 13.9 | 0.6 | 11.57 | 478.9 | 897.1 |
| HR | 171.5 | 307.4 | 56.0 | 13.9 | 0.6 | 11.57 | 478.9 | 897.1 |
| S+R | 171.5 | 283.9 | 56.0 | 13.9 | 0.6 | 11.57 | 478.9 | 897.1 |
| H+R | 171.5 | 307.4 | 56.0 | 13.9 | 0.6 | 11.57 | 478.9 | 897.1 |
| Characteristics | Mean ± SD |
|---|---|
| Age (years) | 22.8 ± 2.11 |
| Weight (kg) | 54.49 ± 7.05 |
| Height (cm) | 161.67 ± 4.22 |
| BMI (kg/m2) | 20.83 ± 2.31 |
| Waist circumference (cm) | 65.67 ± 3.75 |
| Body fat (%) | 27.93 ± 3.35 |
| Fat-free mass (%) | 67.88 ± 3.17 |
| Systolic blood pressure (mmHg) | 103.20 ± 8.65 |
| Diastolic blood pressure (mmHg) | 63.53 ± 7.58 |
| Fasting blood glucose level (mmol/L) | 4.73 ± 0.33 |
| Fasting insulin level (µU/mL) | 4.69 ± 0.92 |
| Fasting HOMA-IR | 0.99 ± 0.22 |
| Texture Characteristics | Raw Broccoli (R) | Soft Broccoli (SB) | Hard Broccoli (HB) |
|---|---|---|---|
| Puncture force (N) | 8.48 ± 0.68 a | 0.79 ± 0.42 c | 4.47 ± 1.92 b |
| Flexibility (mm) | 1.50 ± 0.20 b | 2.06 ± 0.53 a | 1.84 ± 0.48 ab |
| Shear force (N) | 4.18 ± 0.93 b | 4.77 ± 0.86 b | 8.44 ± 1.70 a |
| Toughness (N·mm) | 14.06 ± 1.83 a | 13.18 ± 3.08 ab | 10.26 ± 3.02 b |
| Brittleness | 5.22 ± 0.97 a | 1.73 ± 0.59 c | 3.27 ± 0.79 b |
| Oral Processing Behaviors | Rice (R) | Soft Broccoli (SB) | Hard Broccoli (HB) |
|---|---|---|---|
| Total mastication time (s) | 23.36 ± 2.53 b | 29.45 ± 2.48 b | 37.11 ± 3.05 a |
| Chew rate (chews/s) | 1.54 ± 0.07 | 1.53 ± 0.04 | 1.59 ± 0.05 |
| Number of chews (no.) | 37.00 ± 4.69 b | 45.50 ± 4.43 b | 62.90 ± 6.16 a |
| Chews per gram (no.) 2 | 7.44 ± 0.94 a | 4.37 ± 0.43 b | 5.89 ± 0.58 ab |
| Eating rate (g/min) | 14.37 ± 1.51 b | 22.35 ± 1.80 a | 17.51 ± 1.49 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Fan, Z.; Lou, X.; Zhao, W.; Lu, X.; Hu, J.; Han, Y.; Liu, A. Combination of Texture-Induced Oral Processing and Vegetable Preload Strategy Reduced Glycemic Excursion but Decreased Insulin Sensitivity. Nutrients 2022, 14, 1318. https://doi.org/10.3390/nu14071318
Wu Y, Fan Z, Lou X, Zhao W, Lu X, Hu J, Han Y, Liu A. Combination of Texture-Induced Oral Processing and Vegetable Preload Strategy Reduced Glycemic Excursion but Decreased Insulin Sensitivity. Nutrients. 2022; 14(7):1318. https://doi.org/10.3390/nu14071318
Chicago/Turabian StyleWu, Yixue, Zhihong Fan, Xinling Lou, Wenqi Zhao, Xuejiao Lu, Jiahui Hu, Yue Han, and Anshu Liu. 2022. "Combination of Texture-Induced Oral Processing and Vegetable Preload Strategy Reduced Glycemic Excursion but Decreased Insulin Sensitivity" Nutrients 14, no. 7: 1318. https://doi.org/10.3390/nu14071318
APA StyleWu, Y., Fan, Z., Lou, X., Zhao, W., Lu, X., Hu, J., Han, Y., & Liu, A. (2022). Combination of Texture-Induced Oral Processing and Vegetable Preload Strategy Reduced Glycemic Excursion but Decreased Insulin Sensitivity. Nutrients, 14(7), 1318. https://doi.org/10.3390/nu14071318






