Abstract
Background: Acne vulgaris is one of the most common skin diseases. One of the therapeutic options recommended for severe acne or acne that has not responded to previous therapies is isotretinoin. However, its use may lead to adverse changes in the serum lipid profile and increased levels of transaminases. In this study, we evaluated the effect of supplementation with evening primrose oil in acne vulgaris patients treated with isotretinoin on blood lipid parameters and transaminase activity. Methods: Study participants were randomly assigned to two treatments: conventional with isotretinoin (25 patients) and novel with isotretinoin combined with evening primrose oil (4 × 510 mg/day; 25 patients) for 9 months. Results: Compared to isotretinoin treatment, isotretinoin treatment combined with evening primrose oil had a positive effect on TCH concentrations (mean: 198 vs. 161, p < 0.001), LDL (95.9 vs. 60.2, p < 0.001), HDL (51.0 vs. 48.0, p < 0.001), TG (114 vs. 95.0, p < 0.001), ALT (24.0 vs. 22.0, p < 0.001), and AST (28.0 vs. 22.0, p < 0.001), but had no effect on the energy and ingredient content of the diets (p > 0.05) after treatment. Conclusion: Evening primrose oil was found to have beneficial effects on lipid profiles and transaminase activity during isotretinoin treatment. However, longer studies are needed to make more reliable decisions regarding the use of evening primrose oil and its safety in clinical practice. The evening primrose oil treatment group also showed a reduction in dietary energy due to a reduction in dietary protein and carbohydrates.
1. Introduction
Acne vulgaris, a chronic inflammatory disease of the sebaceous glands [1], is one of the most common skin diseases [2,3,4]. The incidence of acne vulgaris is highest during adolescence, but it can also occur at other times in life [2,4,5].
The primary mechanism of the disease involves excessive sebum secretion, keratinocyte hyperproliferation in the hair follicle, altered bacterial colonization, and a host inflammatory response [4,5,6]. The exact sequence of these events is not clear, but the main pathophysiological factor is probably an androgen-induced increase in sebum production and secretion, combined with qualitative changes in sebum [5]. Characteristic changes in sebum composition in patients with acne include decreased linoleic acid, increased sapienic acid, squalene, diacylglycerols (DG), and lipid peroxides, and an increased ratio of saturated to unsaturated fatty acids [7,8,9].
The occurrence of acne is associated with profound negative effects on mental health, including increased rates of mood disorders, psychiatric hospitalizations, school absenteeism, job loss, and suicide [10].
Treatment of acne depends on its form and severity. The mainstay of acne therapy is retinoids, which, depending on the form of the disease and its severity, can be used generally or systemically [11,12,13,14]. In light of recent studies, isotretinoin is recommended as a first-line treatment for moderate to severe inflammatory acne [2,11,15,16,17,18,19].
However, the use of isotretinoin may lead to a number of adverse effects, which include changes in the serum lipid profile, including total cholesterol (TCH), cholesterol in the LDL fraction (LDL), and triacylglycerols (TG) [20,21], as well as an increase in aminotransferases [22,23,24].
Studies have shown that evening primrose oil (EPO) supplementation may be effective in improving lipid profiles, but results have been inconsistent. For example, oral intake of EPO at a dose of ≤4 g/day was found to significantly decrease serum TG levels and significantly increase HDL levels in hyperlipidemic subjects [25].
The biological effect of evening primrose oil is due to its composition and the biological properties of its components. The most important, in terms of quantity, are polyunsaturated fatty acids (PUFAs), mainly linoleic acid (LA) and γ-linolenic acid (GLA), which belong to the group of omega-6 acids [26,27].
The available literature shows that linoleic acid lowers blood cholesterol by 13.8%, as predicted by the Keys equation [28], while γ-linolenic acid lowers TG levels and raises HDL levels [29]. In an animal model, diets containing γ-linolenic acid from evening primrose oil also significantly reduced serum TG and TCH levels compared to other oils (palm and safflower) [30]. A review by Hooper et al. [31] confirmed that increased intake of omega-6 fats reduces serum total cholesterol levels without affecting the other fractions.
The beneficial effect of evening primrose oil in combination with hemp oil and a hot-nature diet on liver enzyme levels was also demonstrated [32]. Higher dietary linoleic acid levels were also generally associated with lower serum liver enzyme levels [33].
Guided by the above rationale, a study was undertaken to evaluate the effect of evening primrose oil supplementation in acne vulgaris patients treated with isotretinoin on blood lipid parameters and transaminase activity and diet.
2. Materials and Methods
The study was designed as a randomized trial according to CONSORT standards (see Supplementary Material, Table S1) [34,35] and conducted according to the guidelines of the Declaration of Helsinki. The study protocol was approved by the Bioethics Committee of the Poznan University of Medical Sciences (ref 268/15). All participants received information about the study. All subjects were aware that their participation was voluntary and they could withdraw at any time without giving reasons.
2.1. Study Participants
A group of 50 patients aged 18 to 30 years (mean age 22.0 ± 2.07 years) with a diagnosis of moderate to severe acne vulgaris participated in the study after giving written consent. They were randomly divided into two groups: 25 subjects for the IOW study group (mean age 22.5 ± 1.92 years) and 25 subjects for the control group I (mean age 21.6 ± 2.14 years). There was no significant difference in age between groups (p = 0.116). Patients were eligible for the study on the basis of dermatological assessment. The inclusion criteria were a diagnosis of moderate to severe acne or lack of improvement after previous medication. Exclusion criteria included pregnancy or breastfeeding, metabolic diseases, liver failure, elevated blood cholesterol levels, use of statins, high vitamin A levels in the body, and hypersensitivity to vitamin A. Potential participants were recruited from patients treated at the outpatient clinic of the Department of Dermatology, Poznan University of Medical Sciences. After consenting to participate in the study, they were examined by a physician during the inclusion visit to meet protocol requirements.
2.2. Study Design
The study used randomization in a simple procedure (computerized random numbers). An observational study was carried out in patients treated at the outpatient clinic of the Department of Dermatology, Poznan University of Medical Sciences. The decision to introduce isotretinoin according to dermatological guidelines was made by experienced dermatologists.
Study participants were randomly assigned (allocation ratio: 1:1) to arms I and IOW. In arm I, an individually determined dose of oral isotretinoin (10 to 40 mg isotretinoin) was administered by a dermatologist. In arm IOW, subjects received isotretinoin plus encapsulated evening primrose oil (Oenothera paradoxa) in the amount of 2 capsules (2 × 510 mg) in the morning and 2 capsules (2 × 510 mg) in the evening. The duration of the intervention was 9 months and concurrently ran in both arms. All subjects took an individually determined dose of oral isotretinoin (10 to 40 mg of isotretinoin) by a dermatologist. Treatment was usually started at a dose of 0.5 mg/kg body weight/day. The therapeutic dose ranged from 0.5–1 mg/kg body weight/day. For isotretinoin, the cumulative dose for the entire treatment period was 120–150 mg/kg body weight. Of the 50 subjects included in the study, all completed 9 months of treatment (group I 25 subjects, group IOW 25 subjects). No side effects or adverse events were reported. The study analyzed the results of biochemical parameters at baseline and after 9 months (although patients were followed up more frequently by a dermatologist). Dietary assessment (dietary history) was performed twice in study participants before and after the study. Biochemical parameters—total cholesterol (TCH), LDL fraction cholesterol (LDL), HDL fraction cholesterol (HDL), triglycerides (TG), aspartate transaminase (ASP), and alanine transaminase (ALT) levels—were also assessed twice.
The encapsulated evening primrose oil used in the study was extracted from Oenothera paradoxa and manufactured by Adamed Consumer Healthcare S.A. The capsule contained evening primrose seed oil (Oenothera paradoxa) at 510 mg and unsaturated fatty acids at 390 mg, including: linoleic acid (LA) at 347 mg, gamma-linolenic acid (GLA) at 42.4 mg, gelatin, and humectants glycerol and sorbitol. According to the manufacturer’s recommendations, the recommended intake is not exceeded, which is for adolescents and adults 1–2 capsules 2 times a day. Composition of evening primrose oil capsule is shown in Table 1.

Table 1.
Composition of evening primrose oil capsule.
During the intervention, participants were instructed not to make changes to their previous dietary and physical activity habits. In addition, during the intervention, participants were supervised by telephone by a dietician to verify adherence to the study protocol, especially the regular intake of oil capsules. To verify adherence to the study protocol, participants regularly returned empty oil containers to the research team. The primary outcomes of the study were to compare the effects of treatment of acne vulgaris with isotretinoin or isotretinoin combined with evening primrose oil on lipid profiles and aminotransferase levels. Here, we present the results of endpoints such as TCH, LDL, HDL, TG, and ALT and AST levels. All outcome measurements were collected at the University of Poznan Medical School before and after each intervention period. Parameters related to aminotransferase levels and lipid profiles were analyzed at ALAB Laboratory (Poznań, Poland). All results were assessed by the same methods for both arms.
Randomization was performed by an independent researcher using computer software (Excel, Microsoft Corp, Redmond, WA, USA). The randomization list was generated and the allocation order was concealed until allocation to the intervention. Neither study participants nor research staff knew the allocation order.
The minimum sample size was calculated using Statistica StatSoft 13.3 data analysis software based on the following assumptions: a Type I probability of error of α = 0.05 and an allocation ratio of 1:1. As assumed, the minimum sample size was 23 subjects, but assuming a 10% dropout rate, we assumed that at least 25 subjects should be recruited for the study. We calculated the minimum sample size based on changes in TCH previously reported by Acmaz et al. [36], which were before isotretinoin treatment (133.2 ± 20.3 mg/dL) and after treatment (153.3 ± 20.3 mg/dL). The power for this size is 0.9072.
2.3. Experimental Procedure
2.3.1. Dietary Assessment
The assessment of nutrient intake was based on a dietary intake interview conducted according to the guidelines of the Institute of Food and Nutrition (IŻŻ) in Warsaw [37]. The study was conducted systemically, i.e., the interview on the intake of products and foods was conducted with each respondent for a period of 3 days before the start of treatment and 3 days after 9 months of treatment. The analysis of the questionnaire results concerning the qualitative and quantitative composition of whole food rations was performed using computer databases prepared on the basis of “Tables of composition and nutritional value of food” [38]. The assessment of the level of intake was carried out on the basis of an application prepared in the Diet 6.0 program [39].
2.3.2. Biochemical Tests
From each fasting patient, 12 h after the last meal, 10 mL of venous blood was collected from an elbow vein puncture. Roche COBAS INTEGRA 400+ analyzer was used for determination of total cholesterol TCH, HDL, and TG.
Determination of total cholesterol (TCH) in blood serum
An enzymatic and colorimetric method was used to determine total cholesterol, in which cholesterol esterase (CE) hydrolyzed cholesterol esters to free cholesterol and fatty acids. Cholesterol oxidase (CHOD) then catalyzed the oxidation of cholesterol to the cholest-4-en-3-one form and hydrogen peroxide. In the presence of hydrogen peroxide peroxidase (POD), oxidative coupling of phenol and 4-aminoantipyrine (4-AAP) occurred with the formation of red colored quinine. The intensity of the resulting color, directly proportional to the concentration of cholesterol in the sample, was determined by measuring the absorbance at 512 nm.
Determination of HDL-cholesterol in blood serum
A homogeneous colorimetric enzymatic method was used to determine serum HDL-cholesterol concentration.
HDL-cholesterol concentration was enzymatically determined using cholesterol esterase and cholesterol oxidase attached from PEG to amino groups. Cholesterol esters were quantitatively broken down into free cholesterol and fatty acids under the influence of cholesterol esterase. In the presence of oxygen, cholesterol was oxidized by oxidase to 4-Δ-cholestenone and hydrogen peroxide. In this method, water-soluble complexes with lipoprotein particles LDL, VLDL, and chylomicrons were formed in the presence of magnesium sickle cell and dextran sulfate and were resistant to polyethylene glycol-modified enzymes.
The intensity of the resulting blue quinonimine dye, directly proportional to the concentration of HDL cholesterol in the sample, was determined by measuring the increase in absorbance at a wavelength of 583 nm.
Calculation of LDL fraction cholesterol
The cholesterol concentration of the low-density lipoprotein (LDL) fraction was determined using the calculation method proposed by Friedewald et al. [40]. It assumes that the concentration of LDL cholesterol is the difference between the concentration of total cholesterol and the sum of the concentration of HDL fraction cholesterol and triacylglycerols.
This is represented by the following formula:
LDL (mg/dL) = TCH − HDL − (TG/5)
LDL (mmol/L) = TCH − HDL − (TG/2,2)
LDL − LDL – cholesterol
TCH − total cholesterol
HDL − HDL – cholesterol
TG − triglycerides
When serum triacylglycerols exceeded 4.00 mmol/L, in massive hypertriglyceridemia and in hyperlipoproteinemia (HLP) type III, a direct method was used [41].
Determination of serum triglycerides (TG)
A homogeneous colorimetric enzymatic and colorimetric (GPO/PAP) method with glycerophosphate oxidase and 4-aminophenazone was used. In this method, triacylglycerols were hydrolyzed by lipoprotein lipase (LPL) to glycerol and fatty acids. The glycerol, in a reaction catalyzed by glycerol kinase (GK), was then phosphorylated to glycerol-3-phosphate with ATP. Oxidation of glycerol-3-phosphate was catalyzed by glycerophosphate oxidase (GPO) with the formation of dihydroxyacetone phosphate and hydrogen peroxide. In the presence of POD peroxidase, oxidative coupling of 4-chlorophenol and 4-aminophenazone occurred under the influence of hydrogen peroxide with the formation of red-colored quinonimine, measured at 512 nm. The increase in absorbance was directly proportional to the concentration of triglycerides in the sample.
Determination of serum ALT and AST
For the determination of ALT activity, an immersion assay for quantification of specific ALT activity (GPT) was used with a Reflotron® reflectance photometer from Roche. For the determination of aspartate transaminase (AST) activity, an optimized modified method based on the recommendations of the International Federation of Clinical Chemistry (IFCC) was used, which employs the Karmen–Bergmeyer technique of coupling malate dehydrogenase (MDH) and the reduced form of nicotinamide adenine dinucleotide (NADH) during the detection of AST compound in serum [42,43]. The ratio of change in absorbance at light wavelengths of 340 nm and 405 nm caused by conversion of the NADH form to NAD+ is directly proportional to the amount of AST compound present in the sample.
Alanine aminotransferase (ALT) was determined by an enzymatic method based on studies by Wroblewski and LaDue [44], proposed as recommended by the International Federation of Clinical Chemistry, IFCC organization. In this reaction, the compound ALT catalyzes the transfer of an amino group from L-alanine to α-ketoglutarate to form L-glutamate and pyruvate. The change in absorbance difference for light wavelengths of 340 nm and 405 nm is due to the conversion of the NADH form to NAD+. It is directly proportional to the amount of ALT compound in the sample [45].
2.4. Statistic Methods
Compliance of analyzed quantitative variables with normal distribution was checked using the Shapiro–Wilk test. In order to check the differences in the studied parameters before and after taking the drug, the Wilcoxon test was applied. To check the differences between the study groups for changes in the studied parameters, Mann–Whitney U test was applied. McNemar’s test was also applied to check the differences for qualitative variables in the studied parameters before and after taking the drug. The level of statistical significance was taken at p < 0.05. STATISTICA 13.3 and PS IMAGO PRO 7 (IBM SPSS Statistics 27) were used for data analyses. Data are presented as a median with quartile deviation (Q).
3. Results
Recruitment began in 2015 and 2016 and ended in March 2020, and the intervention period also ended in March 2020. The flowchart of participants is shown in Figure 1. Of the 57 people who were assessed for eligibility, seven did not meet the inclusion criteria and were excluded from the study. A total of 50 participants were randomized to arm I (n = 25) and arm IOW (n = 25), received the allocated intervention, and completed the study. No major side effects were identified.
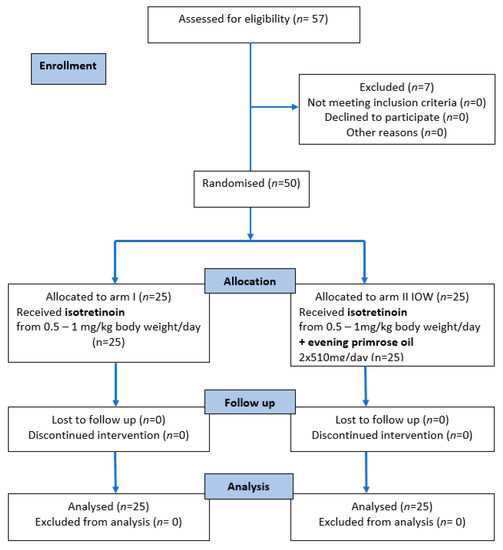
Figure 1.
The CONSORT 2010 flow diagram.
The baseline characteristics of the study population are shown in Table 2, and in most cases, no differences were observed between subjects assigned to arm I and arm IOW. Differences between groups were found only for HDL (p < 0.001) and LDL (p = 0.035) concentrations.

Table 2.
Baseline characteristics of the study population.
3.1. Evaluation of ALT, AST, TCH, LDL, HDL, and TG Parameters before and after Treatment with Isotretinoin or Isotretinoin Combined with Evening Primrose Oil
Before the study, all patients had normal ALT and AST test results that did not exceed the recommended reference values and showed no significant differences between groups I and IOW (AST p = 0.278, ALT p = 0.675). TCH, LDL, HDL, and TG levels were also within normal range in all patients in group I and IOW. For TC and TG values, no statistically significant differences were found between groups I and IOW (p > 0.05); however, a significantly higher median value for HDL was found in group I (p < 0.001) and for LDL in group IOW (p = 0.035).
After 9 months of isotretinoin treatment, patients showed a statistically significant increase in ALT (p < 0.001), AST (p < 0.001), TCH (p < 0.001), LDL (p < 0.001), and TG (p < 0.001), and a decrease in HDL (p = 0.013). The group treated with isotretinoin and supplemented with evening primrose oil after 9 months showed a statistically significant decrease in AST (p = 0.036), TCH (p = 0.025), and LDL (p = 0.003) levels, no change in ALT (p = 0.151), and an increase in HDL (p < 0.001) and TG (p = 0.025) levels. Significant differences between the isotretinoin-treated group and the isotretinoin combined with evening primrose group after 9 months were in AST (p = 0.001), TCH (p < 0.001), LDL (p = 0.003), and TG (p < 0.001) levels. In contrast, no differences were observed between the study groups for ALT (p = 0.217) and HDL (p = 0.366) concentrations. These results are presented in Table 3 and illustrated in Figure 2 and Figure 3.

Table 3.
Changes in ALT, AST, TCH, LDL, HDL, and TG levels or concentrations in groups using isotretinoin or isotretinoin with evening primrose oil.
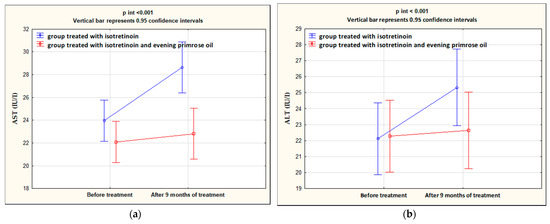
Figure 2.
Changes in AST (a) and ALT (b) levels in the groups treated with isotretinoin or isotretinoin and evening primrose oil.
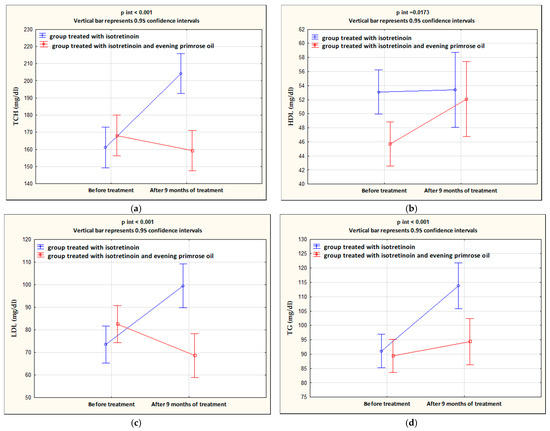
Figure 3.
Changes in concentrations of TCH (a), HDL (b), LDL (c), and TG (d) in the groups treated with isotretinoin or isotretinoin and evening primrose oil.
The analysis of the distribution of subjects in groups for which the analyzed parameters were within or outside the norm before and after 9 months of treatment showed that, in group I, the percentage of subjects whose TCH levels exceeded the norm significantly increased from 8% before treatment to 84% after treatment (p < 0.001). For the other parameters (HDL, LDL, TG, AST, and ALT), no significant changes in distribution were observed in groups I and IOW. These results are summarized in Table 4.

Table 4.
Percentage of subjects in groups I and IOW for which the analyzed parameters were within or outside the reference ranges.
3.2. Evaluation of Dietary Changes before and after Treatment with Isotretinoin or Isotretinoin Combined with Evening Primrose Oil
Before the treatments, no significant differences were found between the energy value of the studied rations of group I and IOW (p > 0.05), the content of protein, animal protein, vegetable protein, fat, carbohydrates, sucrose, and fiber, and the share of energy derived from these components (p > 0.05). There were also no differences between the contents of SFA (saturated fatty acids); MFA (monounsaturated fatty acids); EFA (essential fatty acids); n-3 and n-6 family fatty acids; oleic acid; gamma-linolenic acid and long-chain polyunsaturated fatty acids; vitamins A, E, C, B6, thiamine, and riboflavin; as well as iron, zinc, and copper in group I and IOW rations (for all p > 0.05).
After 9 months of treatment, no changes in nutritional parameters were observed in the isotretinoin-treated group (I) compared to the results obtained at the beginning of the study (p > 0.05) except for a decrease in mean vitamin E content (p = 0.028). However, in the group treated with isotretinoin combined with EPO supplementation, there was a significant decrease in the energy value of the diet (by 438 kcal, p = 0.005), a decrease in protein (p = 0.026), carbohydrates (p = 0.09), sucrose (p = 0.003), dietary fiber (p = 0.013), iron (p = 0.009), copper (p = 0.015), vitamin E (p = 0.042), riboflavin (p = 0.030), and vitamin B6 (p = 0.002). The niacin content of IOW rations also decreased by half (p = 0.002) and the vitamin C content decreased by two-thirds (p = 0.025). After 9 months of treatment, however, there were no significant differences between the groups for any of the parameters analyzed (p > 0.05 in all cases). There were also no significant differences for Δ before and after treatment between groups (p > 0.05 in all cases). These results are summarized in Table 5.

Table 5.
Content of analyzed nutrients in whole daily rations of the study group before and after treatment with isotretinoin (I) or isotretinoin combined with evening primrose oil (IOW).
4. Discussion
Oral isotretinoin (13-cis-retinoic acid) was synthesized in 1955 [46] and was first approved for the treatment of severe forms of acne by the U.S. Food and Drug Administration (FDA) in 1982 [47]. To date, isotretinoin remains a highly effective treatment for moderate, severe, and recurrent acne, reliably leading to dermatological improvement after treatment [2,15,16,17,18,19,48]. Isotretinoin is the only therapy that affects all major etiologic factors of acne [47,49].
However, there are side effects that limit the use of isotretinoin. Among the most worrying are the suspected teratogenic effects [2]. Studies confirm that long-term high-dose use of retinoid-containing drugs can cause liver dysfunction without liver damage [23,50]. Hepatic dysfunction occurs in up to 15% of patients taking isotretinoin [22]. In a study by Schulpis et al. [51], a statistically significant increase in liver enzymes was observed after isotretinoin administration, and in a study by Nazarian et al. [52], a difficult-to-reduce increase in alanine aminotransaminase (ALT) was observed after 20 weeks of isotretinoin treatment. However, a recent study by Pona et al. showed that isotretinoin does not increase liver enzyme levels in patients with severe acne [53]. Numerous studies have observed significant, up to 5-fold, increases in transaminase activity from baseline values induced by isotretinoin administration [23,24,54]. In our study, isotretinoin treatment also significantly increased hepatic aminotransferases ALT and AST in group I. However, no increase in the concentration of hepatic aminotransferases was observed in the group treated with isotretinoin in combination with evening primrose oil; moreover, a decrease in AST levels was demonstrated after 9 months of treatment (p < 0.05).
Several studies have also observed an adverse effect of isotretinoin on changes in lipid profiles. In a study by Zech et al. [20], men with acne treated with oral isotretinoin for four months showed isotretinoin-induced increases in plasma TG and TCH levels, increased very-low-density lipoprotein (VLDL) cholesterol, increased LDL levels, and decreased HDL levels compared to pretreatment values. A study by De Marchi et al. [21] confirmed isotretinoin treatment-induced changes in plasma lipid concentrations. In ten acne patients treated with isotretinoin at a dose of 0.8 mg/kg body weight, TCH, LDL, and TG increased after 4 weeks of treatment. An increase in TCH, TG, and LDL and a decrease in HDL were also observed in 30 patients treated with 0.5 mg/kg isotretinoin [55]. In patients with PCOS complicated by severe cystic acne, isotretinoin treatment also significantly increased TG and TCH levels [36].
A meta-analysis by Lee et al. supported the effect of isotretinoin on lipid profiles, but in a small proportion of patients and without high risk [56]. In our study, the isotretinoin-treated group showed an increase in TCH and LDL levels and a decrease in HDL levels. The opposite results were observed in patients treated with isotretinoin and evening primrose oil; here, there was a decrease in TCH and LDL levels and an increase in HDL levels. TG was increased in both groups. Moreover, after 9 months of isotretinoin treatment in group I, the percentage of patients whose TCH levels exceeded the norm significantly increased from 8% before treatment to 84% after treatment. The improvement of lipid parameters and aminotransferase levels in patients treated with isotretinoin combined with evening primrose oil suggests a protective effect of this oil. The beneficial effect of evening primrose oil is related to its composition and the biological properties of its components, mainly linoleic acid (LA) and γ-linolenic acid (GLA), which belong to the group of omega-6 acids [26,27].
A very common adverse effect of isotretinoin therapy is hypertriglyceridemia [57]. In our study, an increase in TG concentration was observed in both study groups, but in the group treated with isotretinoin with evening primrose oil (IOW), TG concentration increased to a lesser extent than in the control group (I).
Analysis of the results regarding energy value and the content and proportion of energy derived from selected nutrients after 9 months of treatment with isotretinoin or isotretinoin combined with evening primrose oil supplementation provided interesting information. In the group treated with isotretinoin, only a decrease in vitamin E content was found, whereas in the group that additionally took evening primrose oil, the energy value of the diet and the content of protein, carbohydrates, sucrose, dietary fiber, iron, and copper as well as vitamins E, B2, B3, B6, and C decreased.
The lower energy value of the diet in subjects treated with isotretinoin combined with evening primrose oil may have influenced, to some extent, the favorable changes in the lipid profile. Holloszy and Fontana [58] showed lower serum TCH and LDL and higher HDL concentrations in patients with a lower energy diet compared to the group following a typical Western diet. Similarly, a study by Maciejewska et al. [59] found that a 30% reduction in dietary energy resulted in significantly lower TCH, LDL, and TG concentrations.
The observed reduction in dietary energy in the group treated with isotretinoin and evening primrose oil is probably related to the properties of this oil, which are known to help the body to better utilize fat [60] and increase insulin sensitivity [61].
It seems that the gamma-linolenic acid contained in evening primrose oil is mainly responsible for the decrease in food intake in the IOW group and the decrease in dietary energy value. There is no available literature on the effect of gamma-linolenic acid on appetite reduction, but there are reports indicating its positive influence on body weight reduction. In an animal model study, Takada et al. [62] observed a significant weight loss in animals fed a GLA supplemented diet compared to a control group. The weight loss effect associated with gamma-linolenic acid intake has also been observed in human models. In subjects experiencing significant weight loss after taking 890 mg GLA/day for 1 year, weight gain was significantly impaired again. This was due to GLA stimulating brown adipose tissue and increasing metabolic rates. In addition, the prostaglandins that formed initiated fat burning in the mitochondria of brown adipose tissue, which could be an effective factor in promoting weight loss [63]. Some studies have shown that gamma-linolenic acid (GLA) can increase resting metabolic rate (RMR), which may help with weight control. However, no effect on RMR or body mass index was observed in overweight young adults with a family history of obesity [29]. A study by Khamaisi et al. also showed insulin-sensitizing properties of GLA-alpha-lipoic acid conjugate associated with antioxidant activity [64]. Nevertheless, it is possible that evening primrose oil reduces intake through other mechanisms.
The decrease in carbohydrates observed in the IOW group also may have improved TCH, TG, HDL, and LDL levels [65,66,67]. This effect mainly relates to products containing simple carbohydrates [68,69] and their content in the diet of the IOW group significantly decreased after the 9-month treatment. However, the decrease in dietary carbohydrates was also accompanied by a decrease in dietary fiber, which has a protective effect on the lipid profile [70,71] and on ALT levels [72].
Reducing the other components in the diet had less significant effects on lipid profiles and aminotransferase activity. Interestingly, there was no reduction in fat or energy derived from fat in the diets of patients in the IOW group. There were also no significant changes in the fatty acid composition of the diet before and after treatment, which could have a significant effect on the changes in lipid profiles observed in this group.
However, iron and zinc decreased in the diets of IOW patients after 9 months of treatment. If and how iron affects lipid profiles and progression of atherosclerosis remains highly debatable. In the Mendelian study by Zhou et al., it was shown that genetically predicted higher blood iron levels are associated with disorders of lipid metabolism including hyperlipidemia and hypercholesterolemia. They are also consistently associated with lower blood concentrations of TCH and LDL [73]. Thus, the decrease in dietary iron after treatment with isotretinoin combined with evening primrose oil appears to be positive in terms of improving the lipid profile. On the other hand, a decrease in zinc content did not have a beneficial effect on lipid profile parameters. A comprehensive systematic review and meta-analysis conducted by Asbaghi et al. [74] showed that lipid profiles may be affected by zinc supplementation. In women with migraines, serum LDL and TCH levels also decreased after zinc supplementation [75]. Zinc supplementation also decreased ALT and AST levels in rats with nonalcoholic steatohepatitis [76]. Similarly, in patients with minimal hepatic encephalopathy, zinc supplementation significantly decreased ALT and AST levels [77].
It seems that the decrease in vitamins had no effect on improving lipid parameters and aminotransferase levels in IOW patients. A study by Ashor et al. showed no overall effect of vitamin C on TCH and HDL levels [78]. In addition, no improvement in TCH, LDL, and HDL levels was observed under the influence of vitamin E supplementation in exercising rats [79]. The greatest effect on lipid profiles among B vitamins was observed for vitamin B12. A clinical study in women showed that low B12 levels were associated with increased TCH, LDL, and TCH to HDL ratio [80]. However, for our study, no significant differences in vitamin B12 levels were found between groups and before and after treatment. Such differences were also not shown for vitamin D, for which observational studies confirm the association between its increased serum levels and a favorable lipid profile [81,82].
Taking into account the results of our study, it can be concluded that during isotretinoin treatment, evening primrose oil has a protective effect on changes in lipid profiles and transaminase activity. It also affects the energy value of the diet and the content of some of the components in it. However, longer studies are needed to make more reliable decisions about the use of evening primrose oil and its safety in clinical practice.
The study had some strengths and limitations. The main strengths of this study were the randomized controlled design, lasting 9 months. Furthermore, this is one of the first human studies comparing the effects of evening primrose oil in combination with isotretinoin and isotretinoin alone on TCH, LDL, HDL, TG, ALT, and AST levels. The main limitation of this study was the small group size, which was sufficient for inference. The lack of output homogeneity between arms I and IOW in terms of HDL and LDL concentrations was also a limitation. We also did not assess other factors that could potentially influence the results, such as alcohol consumption, smoking, and physical activity. However, all participants were instructed not to make changes in dietary habits or physical activity. Furthermore, the doses of linoleic acid and gamma-linolenic acid were taken from the information available from the evening primrose oil producer. Multiple testing adjustment was also not calculated, which could reduce the significance of differences in results obtained before and after treatment with isotretinoin or isotretinoin and evening primrose oil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14071342/s1, Table S1: CONSORT 2010 checklist of information to include when reporting a randomised trial.
Author Contributions
Conceptualization, A.K. and J.P.; methodology, A.P. and P.J.; formal analysis, Z.A.; investigation, A.K. and A.D.-P.; resources, A.K. and G.K.; writing—original draft preparation, I.B.; writing—review and editing, J.P. and A.P. and Z.A.; visualization, I.B. and P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was approved by Resolution No. 268/15 of the Bioethics Committee of the Poznan University of Medical Sciences. The study was conducted according to the guidelines of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting the results obtained are deposited with the authors for review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nast, A.; Dréno, B.; Bettoli, V.; Degitz, K.; Erdmann, R.; Finlay, A.Y.; Ganceviciene, R.; Haedersdal, M.; Layton, A.; López-Estebaranz, J.L.; et al. European Evidence-Based (S3) Guidelines for the Treatment of Acne. J. Eur. Acad. Dermatol. Venereol. JEADV 2012, 26 (Suppl. 1), 1–29. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.S.; Shin, D.B.; Wang, S.; Margolis, D.J.; Takeshita, J. The Clinical Utility of Laboratory Monitoring During Isotretinoin Therapy for Acne and Changes to Monitoring Practices over Time. J. Am. Acad. Dermatol. 2020, 82, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, H. Acne, the Skin Microbiome, and Antibiotic Treatment. Am. J. Clin. Dermatol. 2019, 20, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Eichenfield, L.F.; Tan, J. Acne and Rosacea. Dermatol. Ther. 2017, 7, 43–52. [Google Scholar] [CrossRef]
- O’Neill, A.M.; Gallo, R.L. Host-Microbiome Interactions and Recent Progress into Understanding the Biology of Acne Vulgaris. Microbiome 2018, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New Developments in Our Understanding of Acne Pathogenesis and Treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef]
- Zouboulis, C.C. Acne and Sebaceous Gland Function. Clin. Dermatol. 2004, 22, 360–366. [Google Scholar] [CrossRef]
- Camera, E.; Ludovici, M.; Tortorella, S.; Sinagra, J.-L.; Capitanio, B.; Goracci, L.; Picardo, M. Use of Lipidomics to Investigate Sebum Dysfunction in Juvenile Acne. J. Lipid Res. 2016, 57, 1051–1058. [Google Scholar] [CrossRef]
- Habeshian, K.A.; Cohen, B.A. Current Issues in the Treatment of Acne Vulgaris. Pediatrics 2020, 145, S225–S230. [Google Scholar] [CrossRef]
- Haller, H.; Anheyer, D.; Cramer, H.; Dobos, G. Complementary Therapies for Clinical Depression: An Overview of Systematic Reviews. BMJ Open 2019, 9, e028527. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A Journey from the Molecular Structures and Mechanisms of Action to Clinical Uses in Dermatology and Adverse Effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.S.; Pecone, D.; Pona, A.; Cline, A.; Feldman, S.R. Topical Retinoids in Acne Vulgaris: A Systematic Review. Am. J. Clin. Dermatol. 2019, 20, 345–365. [Google Scholar] [CrossRef]
- Barbieri, J.S.; Spaccarelli, N.; Margolis, D.J.; James, W.D. Approaches to Limit Systemic Antibiotic Use in Acne: Systemic Alternatives, Emerging Topical Therapies, Dietary Modification, and Laser and Light-Based Treatments. J. Am. Acad. Dermatol. 2019, 80, 538–549. [Google Scholar] [CrossRef]
- Bagatin, E.; Costa, C.S. The Use of Isotretinoin for Acne—An Update on Optimal Dosing, Surveillance, and Adverse Effects. Expert Rev. Clin. Pharmacol. 2020, 13, 885–897. [Google Scholar] [CrossRef]
- Landis, M.N. Optimizing Isotretinoin Treatment of Acne: Update on Current Recommendations for Monitoring, Dosing, Safety, Adverse Effects, Compliance, and Outcomes. Am. J. Clin. Dermatol. 2020, 21, 411–419. [Google Scholar] [CrossRef]
- Lee, J.W.; Yoo, K.H.; Park, K.Y.; Han, T.Y.; Li, K.; Seo, S.J.; Hong, C.K. Effectiveness of Conventional, Low-Dose and Intermittent Oral Isotretinoin in the Treatment of Acne: A Randomized, Controlled Comparative Study. Br. J. Dermatol. 2011, 164, 1369–1375. [Google Scholar] [CrossRef]
- Borghi, A.; Mantovani, L.; Minghetti, S.; Giari, S.; Virgili, A.; Bettoli, V. Low-Cumulative Dose Isotretinoin Treatment in Mild-to-Moderate Acne: Efficacy in Achieving Stable Remission. J. Eur. Acad. Dermatol. Venereol. JEADV 2011, 25, 1094–1098. [Google Scholar] [CrossRef]
- Zech, L.A.; Gross, E.G.; Peck, G.L.; Brewer, H.B. Changes in Plasma Cholesterol and Triglyceride Levels after Treatment with Oral Isotretinoin. A Prospective Study. Arch. Dermatol. 1983, 119, 987–993. [Google Scholar] [CrossRef]
- De Marchi, M.A.; Maranhão, R.C.; Brandizzi, L.I.V.; Souza, D.R.S. Effects of Isotretinoin on the Metabolism of Triglyceride-Rich Lipoproteins and on the Lipid Profile in Patients with Acne. Arch. Dermatol. Res. 2006, 297, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Retinoids. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Kizilyel, O.; Metin, M.S.; Elmas, O.F.; Cayir, Y.; Aktas, A. Effects of Oral Isotretinoin on Lipids and Liver Enzymes in Acne Patients. Cutis 2014, 94, 234–238. [Google Scholar] [PubMed]
- Zane, L.T.; Leyden, W.A.; Marqueling, A.L.; Manos, M.M. A Population-Based Analysis of Laboratory Abnormalities during Isotretinoin Therapy for Acne Vulgaris. Arch. Dermatol. 2006, 142, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, M.; Zarezadeh, M.; Moradi Moghaddam, O.; Emami, M.R.; Kord-Varkaneh, H.; Mousavi, S.M.; Alizadeh, S.; Heshmati, J.; Olang, B.; Aryaeian, N. Effect of Evening Primrose Oil Supplementation on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Phytother. Res. PTR 2020, 34, 2628–2638. [Google Scholar] [CrossRef]
- Mahboubi, M. Evening Primrose (Oenothera biennis) Oil in Management of Female Ailments. J. Menopausal Med. 2019, 25, 74–82. [Google Scholar] [CrossRef]
- Timoszuk, M.; Bielawska, K.; Skrzydlewska, E. Evening Primrose (Oenothera biennis) Biological Activity Dependent on Chemical Composition. Antioxidants 2018, 7, 108. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.R.; Broste, S.K.; Frantz, R.P.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-Evaluation of the Traditional Diet-Heart Hypothesis: Analysis of Recovered Data from Minnesota Coronary Experiment (1968–1973). BMJ 2016, 353, i1246. [Google Scholar] [CrossRef]
- DiSilvestro, R.A.; Olivo Marston, S.; Zimmerman, A.; Joseph, E.; Boeh McCarty, C. Borage Oil Intake by Overweight Young Adults: No Effect on Metabolic Rate; Beneficial Effects on Plasma Triglyceride and HDL Cholesterol Readings. Food Funct. 2021, 12, 8882–8886. [Google Scholar] [CrossRef]
- Ide, T.; Origuchi, I. Physiological Effects of an Oil Rich in γ-Linolenic Acid on Hepatic Fatty Acid Oxidation and Serum Lipid Levels in Genetically Hyperlipidemic Mice. J. Clin. Biochem. Nutr. 2019, 64, 148–157. [Google Scholar] [CrossRef]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 Fats for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2018, 2018, CD011094. [Google Scholar] [CrossRef]
- Rezapour-Firouzi, S.; Arefhosseini, S.R.; Ebrahimi-Mamaghani, M.; Baradaran, B.; Sadeghihokmabad, E.; Torbati, M.; Mostafaei, S.; Chehreh, M.; Zamani, F. Activity of Liver Enzymes in Multiple Sclerosis Patients with Hot-Nature Diet and Co-Supplemented Hemp Seed, Evening Primrose Oils Intervention. Complement. Ther. Med. 2014, 22, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Pertiwi, K.; Küpers, L.K.; Geleijnse, J.M.; Zock, P.L.; Wanders, A.J.; Kruger, H.S.; van Zyl, T.; Kruger, I.M.; Smuts, C.M. Associations of Linoleic Acid with Markers of Glucose Metabolism and Liver Function in South African Adults. Lipids Health Dis. 2020, 19, 138. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; Mayo-Wilson, E.; Montgomery, P.; Macdonald, G.; Michie, S.; Hopewell, S.; Moher, D. CONSORT-SPI 2018 Explanation and Elaboration: Guidance for Reporting Social and Psychological Intervention Trials. Trials 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, H.; Altman, D.G.; Hammerschlag, R.; Youping, L.; Taixiang, W.; White, A.; Moher, D. STRICTA Revision Group Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT Statement. PLoS Med. 2010, 7, e1000261. [Google Scholar] [CrossRef] [PubMed]
- Acmaz, G.; Cinar, L.; Acmaz, B.; Aksoy, H.; Kafadar, Y.T.; Madendag, Y.; Ozdemir, F.; Sahin, E.; Muderris, I. The Effects of Oral Isotretinoin in Women with Acne and Polycystic Ovary Syndrome. Biomed. Res. Int. 2019, 7, 2513067. [Google Scholar] [CrossRef]
- Gronowska-Senger, A. Methodological Guide to Dietary Surveys. Committee on Human Nutrition of the Polish Academy of Sciences, Warszawa 2013. Available online: http://www.knozc.pan.pl/images/Przewodnik_metodyczny_calosc.pdf (accessed on 17 January 2022).
- Tables of Composition and Nutritional Value of Foods. Available online: https://pzwl.pl/Tabele-skladu-i-wartosci-odzywczej-zywnosci,56121347,p.html (accessed on 17 January 2022).
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybarczuk, M.; Charzewska, J. Instructions for Using the Diet 6.D Programme for Planning and Ongoing Assessment of Individual and Collective Nutrition. Available online: https://docplayer.pl/144985512-Instrukcja-korzystania-z-programu-dieta-6-d-do-planowania-i-biezacej-oceny-zywienia-indywidualnego-i-zbiorowego.html (accessed on 1 February 2021).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Langlois, M.R.; Descamps, O.S.; van der Laarse, A.; Weykamp, C.; Baum, H.; Pulkki, K.; von Eckardstein, A.; De Bacquer, D.; Borén, J.; Wiklund, O.; et al. Clinical Impact of Direct HDLc and LDLc Method Bias in Hypertriglyceridemia. A Simulation Study of the EAS-EFLM Collaborative Project Group. Atherosclerosis 2014, 233, 83–90. [Google Scholar] [CrossRef]
- Bergmeyer, H.U.; Bowers, G.N.; Horder, M.; Moss, D.W. Provisional Recommendations on IFCC Methods for the Measurement of Catalytic Concentrations of Enzymes. Part 2. IFCC Method for Aspartate Aminotransferase. Clin. Chim. Acta Int. J. Clin. Chem. 1976, 70, F19–F29. [Google Scholar] [CrossRef]
- Schumann, G.; Canalias, F.; Joergensen, P.J.; Kang, D.; Lessinger, J.-M.; Klauke, R. IFCC Reference Procedures for Measurement of the Catalytic Concentrations of Enzymes: Corrigendum, Notes and Useful Advice. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)--IFCC Scientific Division. Clin. Chem. Lab. Med. 2010, 48, 615–621. [Google Scholar] [CrossRef]
- Wroblewski, F.; Ladue, J.S. Serum Glutamic Pyruvic Transaminase in Cardiac with Hepatic Disease. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. 1956, 91, 569–571. [Google Scholar] [CrossRef]
- Journal of the Polish Chemical Society. Chemical News. Wrocław. 2016. Available online: https://www.dbc.wroc.pl/Content/32070/WCh-1-2-2016.pdf (accessed on 17 January 2021).
- Bojarowicz, H.; Płowiec, A. Effect of vitamin A on skin condition. Probl. Hig. Epidemiol. 2010, 91, 352–356. [Google Scholar]
- Layton, A. The Use of Isotretinoin in Acne. Dermatoendocrinol 2009, 1, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.S.; Besarwal, R.K.; Bhola, K. Oral Isotretinoin in Different Dose Regimens for Acne Vulgaris: A Randomized Comparative Trial. Indian J. Dermatol. Venereol. Leprol. 2011, 77, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.; Freiman, A. Management of Acne. CMAJ Can. Med. Assoc. J. 2011, 183, E430–E435. [Google Scholar] [CrossRef]
- Roenigk, H.H. Liver Toxicity of Retinoid Therapy. Pharmacol. Ther. 1989, 40, 145–155. [Google Scholar] [CrossRef]
- Schulpis, K.H.; Georgala, S.; Papakonstantinou, E.D.; Michas, T.; Karikas, G.A. The Effect of Isotretinoin on Biotinidase Activity. Skin Pharmacol. Appl. Skin Physiol. 1999, 12, 28–33. [Google Scholar] [CrossRef]
- Nazarian, R.S.; Zheng, E.; Halverstam, C.; Cohen, S.R.; Wolkoff, A.W. Prolonged Serum Alanine Aminotransferase Elevation Associated with Isotretinoin Administration. Case Rep. Hepatol. 2019, 2019, 9270827. [Google Scholar] [CrossRef]
- Pona, A.; Garza, J.; Haidari, W.; Cline, A.; Feldman, S.; Taylor, S. Abnormal Liver Function Tests in Acne Patients Receiving Isotretinoin. J. Dermatolog. Treat. 2019, 32, 469–472. [Google Scholar] [CrossRef]
- Cyrulnik, A.A.; Viola, K.V.; Gewirtzman, A.J.; Cohen, S.R. High-Dose Isotretinoin in Acne Vulgaris: Improved Treatment Outcomes and Quality of Life. Int. J. Dermatol. 2012, 51, 1123–1130. [Google Scholar] [CrossRef]
- Yaldiz, M.; Kara, A.; Güven, M.; Solak, B.; Kara, R.; Erdem, M.T. Assessment of Auditory Function and Lipid Levels in Patients Receiving Oral Isotretinoin (13-Cis Retinoid) Therapy for Acne Vulgaris. Postepy Dermatol. Alergol. 2020, 37, 360–363. [Google Scholar] [CrossRef]
- Lee, Y.H.; Scharnitz, T.P.; Muscat, J.; Chen, A.; Gupta-Elera, G.; Kirby, J.S. Laboratory Monitoring During Isotretinoin Therapy for Acne: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2016, 152, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Pile, H.D.; Sadiq, N.M. Isotretinoin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Holloszy, J.O.; Fontana, L. Caloric Restriction in Humans. Exp. Gerontol. 2007, 42, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, D.; Michalczyk, M.; Czerwińska-Rogowska, M.; Banaszczak, M.; Ryterska, K.; Jakubczyk, K.; Piotrowski, J.; Hołowko, J.; Drozd, A.; Wysokiński, P.; et al. Seeking Optimal Nutrition for Healthy Body Mass Reduction among Former Athletes. J. Hum. Kinet. 2017, 60, 63–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shannon, M. An Empathetic Look at Overweight. CCL Fam. Found. 1993, 20, 3–5. [Google Scholar]
- Mert, H.; İrak, K.; Çibuk, S.; Yıldırım, S.; Mert, N. The Effect of Evening Primrose Oil (Oenothera biennis) on the Level of Adiponectin and Some Biochemical Parameters in Rats with Fructose Induced Metabolic Syndrome. Arch. Physiol. Biochem. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Takada, R.; Saitoh, M.; Mori, T. Dietary Gamma-Linolenic Acid-Enriched Oil Reduces Body Fat Content and Induces Liver Enzyme Activities Relating to Fatty Acid Beta-Oxidation in Rats. J. Nutr. 1994, 124, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.A.; Phinney, S.D. Gamma-Linolenate Reduces Weight Regain in Formerly Obese Humans. J. Nutr. 2007, 137, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Khamaisi, M.; Rudich, A.; Beeri, I.; Pessler, D.; Friger, M.; Gavrilov, V.; Tritschler, H.; Bashan, N. Metabolic Effects of Gamma-Linolenic Acid-Alpha-Lipoic Acid Conjugate in Streptozotocin Diabetic Rats. Antioxid. Redox Signal. 1999, 1, 523–535. [Google Scholar] [CrossRef]
- Volek, J.S.; Sharman, M.J.; Forsythe, C.E. Modification of Lipoproteins by Very Low-Carbohydrate Diets. J. Nutr. 2005, 135, 1339–1342. [Google Scholar] [CrossRef]
- Sharman, M.J.; Kraemer, W.J.; Love, D.M.; Avery, N.G.; Gómez, A.L.; Scheett, T.P.; Volek, J.S. A Ketogenic Diet Favorably Affects Serum Biomarkers for Cardiovascular Disease in Normal-Weight Men. J. Nutr. 2002, 132, 1879–1885. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Long-Term Effects of a Very-Low-Carbohydrate Weight Loss Diet Compared with an Isocaloric Low-Fat Diet after 12 Mo. Am. J. Clin. Nutr. 2009, 90, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Haslam, D.E.; Peloso, G.M.; Guirette, M.; Imamura, F.; Bartz, T.M.; Pitsillides, A.N.; Wang, C.A.; Li-Gao, R.; Westra, J.M.; Pitkänen, N.; et al. Sugar-Sweetened Beverage Consumption May Modify Associations between Genetic Variants in the CHREBP (Carbohydrate Responsive Element Binding Protein) Locus and HDL-C (High-Density Lipoprotein Cholesterol) and Triglyceride Concentrations. Circ. Genomic Precis. Med. 2021, 14, e003288. [Google Scholar] [CrossRef] [PubMed]
- Kuklina, E.V.; Park, S. Sugar-Sweetened Beverage Consumption and Lipid Profile: More Evidence for Interventions. J. Am. Heart Assoc. 2020, 9, e015061. [Google Scholar] [CrossRef]
- Vitaminol, J.N.S. Effects of High-Fiber Biscuits on Lipid and Anthropometric Profile of Patients with Type 2 Diabetes. J. Nutr. Sci. Vitaminol. 2020, 66, S391–S397. [Google Scholar] [CrossRef]
- Velázquez-López, L.; Muñoz-Torres, A.V.; García-Peña, C.; López-Alarcón, M.; Islas-Andrade, S.; Escobedo-de la Peña, J. Fiber in Diet Is Associated with Improvement of Glycated Hemoglobin and Lipid Profile in Mexican Patients with Type 2 Diabetes. J. Diabetes Res. 2016, 2016, 2980406. [Google Scholar] [CrossRef] [PubMed]
- Mollahosseini, M.; Daneshzad, E.; Rahimi, M.H.; Yekaninejad, M.S.; Maghbooli, Z.; Mirzaei, K. The Association between Fruit and Vegetable Intake and Liver Enzymes (Aspartate and Alanine Transaminases) in Tehran, Iran. Ethiop. J. Health Sci. 2017, 27, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, C.; Francis, M.; Sun, Y.; Ryu, M.-S.; Grider, A.; Ye, K. The Causal Effects of Blood Iron and Copper on Lipid Metabolism Diseases: Evidence from Phenome-Wide Mendelian Randomization Study. Nutrients 2020, 12, 3174. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Fouladvand, F.; Panahande, B.; Nasiri, M.; Khodadost, M.; Shokri, A.; Pirouzi, A.; Sadeghi, O. Effects of Zinc Supplementation on Lipid Profile in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. NMCD 2020, 30, 1260–1271. [Google Scholar] [CrossRef]
- Mazaheri, M.; Aghdam, A.M.; Heidari, M.; Zarrin, R. Assessing the Effect of Zinc Supplementation on the Frequency of Migraine Attack, Duration, Severity, Lipid Profile and Hs-CRP in Adult Women. Clin. Nutr. Res. 2021, 10, 127–139. [Google Scholar] [CrossRef]
- Mousavi, S.N.; Faghihi, A.; Motaghinejad, M.; Shiasi, M.; Imanparast, F.; Amiri, H.L.; Shidfar, F. Zinc and Selenium Co-Supplementation Reduces Some Lipid Peroxidation and Angiogenesis Markers in a Rat Model of NAFLD-Fed High Fat Diet. Biol. Trace Elem. Res. 2018, 181, 288–295. [Google Scholar] [CrossRef]
- Mousa, N.; Abdel-Razik, A.; Zaher, A.; Hamed, M.; Shiha, G.; Effat, N.; Elbaz, S.; Elhelaly, R.; Hafez, M.; El-Wakeel, N.; et al. The Role of Antioxidants and Zinc in Minimal Hepatic Encephalopathy: A Randomized Trial. Ther. Adv. Gastroenterol. 2016, 9, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Brown, R.; Keenan, P.D.; Willis, N.D.; Siervo, M.; Mathers, J.C. Limited Evidence for a Beneficial Effect of Vitamin C Supplementation on Biomarkers of Cardiovascular Diseases: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutr. Res. 2019, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Asha Devi, S.; Prathima, S.; Subramanyam, M.V.V. Dietary Vitamin E and Physical Exercise: I. Altered Endurance Capacity and Plasma Lipid Profile in Ageing Rats. Exp. Gerontol. 2003, 38, 285–290. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Finer, S.; Voyias, P.D.; McCarthy, C.M.; Vatish, M.; Moore, J.; Smart-Halajko, M.; Bawazeer, N.; Al-Daghri, N.M.; McTernan, P.G.; et al. Vitamin B12 Insufficiency Induces Cholesterol Biosynthesis by Limiting S-Adenosylmethionine and Modulating the Methylation of SREBF1 and LDLR Genes. Clin. Epigenet. 2015, 7, 14. [Google Scholar] [CrossRef]
- Challoumas, D. Vitamin D Supplementation and Lipid Profile: What Does the Best Available Evidence Show? Atherosclerosis 2014, 235, 130–139. [Google Scholar] [CrossRef]
- Dibaba, D.T. Effect of Vitamin D Supplementation on Serum Lipid Profiles: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).