Incidence and Impact of Refeeding Syndrome in an Internal Medicine and Gastroenterology Ward of an Italian Tertiary Referral Center: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Committee Approval
2.2. Patients
2.3. Protocol Description
2.3.1. Protocol Algorithm
2.3.2. Determination of RS Risk
- BMI was between 16 and 18.5 kg/m2;
- A weight loss of 5% of habitual weight was reported;
- There was no or negligible oral intake for 5–6 days OR < 75% of estimated energy requirement for >7 days during an acute illness or injury OR < 75% of estimated energy requirement for >1 month;
- There were low levels of potassium, phosphorus, magnesium, or normal current levels and recent low levels necessitating minimal or single-dose supplementation;
- There was evidence of moderate subcutaneous fat loss;
- There was evidence of mild or moderate muscle loss;
- In presence of higher-risk comorbidities (moderate disease).
- BMI was <16 kg/m2;
- A weight loss of 7.5% in 3 months or >10% in 6 months was reported;
- There was no or negligible oral intake for >7 days OR < 50% of estimated energy requirement for >5 days during an acute illness or injury OR < 50% of estimated energy requirement for >1 month;
- There were moderately/significantly low levels of potassium, phosphorus, magnesium, or minimally low or normal levels and recent low levels necessitating significant or multiple-dose supplementation;
- There was evidence of severe subcutaneous fat loss;
- There was evidence of severe muscle loss;
- In presence of higher-risk comorbidities (severe disease).
2.3.3. Diagnosis of RS
- A decrease in serum phosphorus, potassium, and/or magnesium levels by 10–20% (mild RS), 20–30% (moderate RS), or >30%, and/or organ dysfunction resulting from a decrease in any of these and/or due to thiamin deficiency (severe RS).
- The decrease occurs within 5 days of reinitiating or substantially increasing energy provision.
2.4. Outcomes Measures
2.5. Sample Size Calculation
2.6. Data Collection and Statistical Analysis
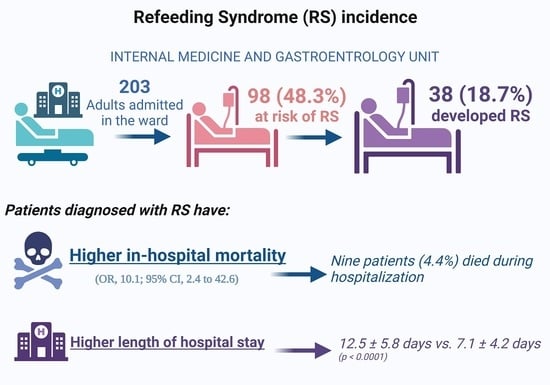
3. Results
3.1. Baseline Patients’ Characteristics
3.1.1. Nutritional Evaluation
3.1.2. Refeeding Syndrome
3.1.3. Length of Hospital Stay
3.1.4. In-Hospital Mortality and Hospital Readmission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedli, N.; Stanga, Z.; Sobotka, L.; Culkin, A.; Kondrup, J.; Laviano, A.; Mueller, B.; Schuetz, P. Revisiting the refeeding syndrome: Results of a systematic review. Nutrition 2017, 35, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehanna, H.M.; Moledina, J.; Travis, J. Refeeding syndrome: What it is, and how to prevent and treat it. BMJ 2008, 336, 1495–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioffi, I.; Ponzo, V.; Pellegrini, M.; Evangelista, A.; Bioletto, F.; Ciccone, G.; Pasanisi, F.; Ghigo, E.; Bo, S. The incidence of the refeeding syndrome. A systematic review and meta-analyses of literature. Clin. Nutr. 2021, 40, 3688–3701. [Google Scholar] [CrossRef] [PubMed]
- Stanga, Z.; Brunner, A.; Leuenberger, M.; Grimble, R.F.; Shenkin, A.; Allison, S.P.; Lobo, D.N. Nutrition in clinical practice-the refeeding syndrome: Illustrative cases and guidelines for prevention and treatment. Eur. J. Clin. Nutr. 2008, 62, 687–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedli, N.; Odermatt, J.; Reber, E.; Schuetz, P.; Stanga, Z. Refeeding syndrome: Update and clinical advice for prevention, diagnosis and treatment. Curr. Opin. Gastroenterol. 2020, 36, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195, Epub 2020 Mar 2. Erratum in: Nutr. Clin. Pract. 2020, 35, 584–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.strobe-statement.org/ (accessed on 28 February 2022).
- Kraaijenbrink, B.V.; Lambers, W.M.; Mathus-Vliegen, E.M.; Siegert, C.E. Incidence of refeeding syndrome in internal medicine patients. Neth. J. Med. 2016, 74, 116–121. [Google Scholar] [PubMed]
- Rinninella, E.; Cintoni, M.; De Lorenzo, A.; Addolorato, G.; Vassallo, G.; Moroni, R.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Risk, prevalence, and impact of hospital malnutrition in a Tertiary Care Referral University Hospital: A cross-sectional study. Intern. Emerg. Med. 2018, 13, 689–697. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Clinical Excellence. Nutrition Support in Adults Clinical Guideline CG32. 2006. Available online: www.nice.org.uk/page.aspx?o=cg032 (accessed on 28 February 2022).
- De Vargas Cony, K.; de Magalhães Francesconi, C.F. An unexpectedly high incidence of refeeding syndrome in patients with total parenteral nutrition in a reference university hospital. Clin. Nutr. 2021, 40, 3702–3707. [Google Scholar] [CrossRef] [PubMed]
- Doig, G.S.; Simpson, F.; Heighes, P.T.; Bellomo, R.; Chesher, D.; Caterson, I.D.; Reade, M.C.; Harrigan, P.W. Refeeding Syndrome Trial Investigators Group. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: A randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Res. Med. 2015, 3, 943–952. [Google Scholar] [CrossRef]


| Variables | |
|---|---|
| Male (n, %) | 127 (62.6) |
| Age, in years (mean ± SD) | 66.05 ± 14.08 |
| Admission (n, %) | |
| Elective | 62 (30.5) |
| Emergency | 139 (68.5) |
| Other | 2 (1) |
| CCI score (mean ± SD) | 3.02 ± 2.43 |
| Body weight, in kg (mean ± SD) | 71.76 ± 16.29 |
| Height, in cm (mean ± SD) | 169.03 ± 8.56 |
| BMI, in kg/m2 (mean ± SD) | 25.02 ± 4.88 |
| NRS-2002 > 3 | 70 (34.5) |
| MUST score (n, %) | |
| 0 | 73 (36.0) |
| 1 | 31 (15.3) |
| ≥2 | 99 (48.7) |
| Risk of RS (n, %) | 98 (48.3) |
| Medium (n, %) | 44 (21.7) |
| High (n, %) | 54 (26.6) |
| RS diagnosis (n, %) | 38 (18.7) |
| Nutrition team support (n, %) | 24 (11.8) *(24.5) ** |
| Nutritional supplementation within 48 h (n, %) | 74 (36) * (75.5) ** |
| Oral nutritional supplementation (n, %) | 63 (31) * (64.3) ** |
| Parenteral nutrition (n, %) | 13 (6.4%) *(13.26) ** |
| LOS, in days (mean ± SD) | 8.24 ± 5.75 |
| In-hospital mortality (n, %) | 9 (4.4) |
| Readmission within 30 days (n, %) | 13 (6.4) |
| Absence of RS (n = 60) | Diagnosis of RS (n = 38) | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Male (n, %) | 35 (58.3) | 22 (57.9) | 1.02 (0.45–2.31) | 0.97 |
| Age in years (mean ± SD) | 68.2 ± 12.7 | 69.9 ± 14.0 | 1.01 (0.97–1.04) | 0.51 |
| ER admission (n, %) | 52 (88.1) | 33 (89.2) | 1.11 (0.30–4.08) | 0.87 |
| CCI score (mean ± SD) | 3.4 ± 2.6 | 3.2 ± 2.7 | 0.93 (0.76–1.09) | 0.41 |
| Baseline body weight in kg, (mean ± SD) | 67.3 ± 15.9 | 68.8 ± 14.4 | 1.00 (0.98–1.04) | 0.61 |
| Body height in cm, (mean ± SD) | 168.4 ± 9.1 | 167.4 ± 9.6 | 0.98 (0.94–1.03) | 0.57 |
| BMI in kg/m2 (mean ± SD) | 23.6 ± 4.4 | 24.6 ± 5.1 | 1.05 (0.96–1.15) | 0.27 |
| NRS-2002 > 3 (n, %) | 39 (65.0) | 22 (57.9) | 0.74 (0.32–1.71) | 0.48 |
| MUST ≥ 2 (n, %) | 48 (80.0) | 30 (78.9) | 0.93 (0.34–2.55) | 0.90 |
| High RS Risk (n, %) | 32 (54.2) | 21 (55.3) | 1.04 (0.45–2.36) | 0.92 |
| Nutrition team support (n, %) | 14 (25.0) | 9 (25.0) | 1.01 (0.38–2.62) | 0.98 |
| Nutritional supplementation within 48 h (n, %) | 41 (70.7) | 32 (84.2) | 2.21 (0.78–6.25) | 0.13 |
| Oral nutritional supplementation (n, %) | 35 (58.3) | 27 (71.1) | 1.75 (0.73–4.18) | 0.20 |
| Parenteral nutrition (n, %) | 8 (13.3) | 4 (10.5) | 0.76 (0.21–2.73) | 0.68 |
| Na T0, in mmol/L (mean ± SD) | 139.2 ± 4.8 | 139.4 ± 4.1 | 1.01 (0.92–1.10) | 0.81 |
| K T0, in mmol/L (mean ± SD) | 3.9 ± 0.4 | 3.8 ± 0.6 | 0.83 (0.37–1.86) | 0.66 |
| Ca T0, in mg/dL (mean ± SD) | 8.9 ± 0.7 | 9.1 ± 0.7 | 1.47 (0.81–2.69) | 0.206 |
| Albumin T0, in g/L (mean ± SD) | 29.5 ± 6.7 | 28.1 ± 5.9 | 0.96 (0.90–1.03) | 0.28 |
| P T0, in mg/dL (mean ± SD) | 3.3 ± 0.6 | 3.4 ± 0.7 | 1.16 (0.61–2.199 | 0.64 |
| Mg T0, in mg/dL (mean ± SD) | 2.0 ± 0.3 | 2.1 ± 0.4 | 3.42 (0.91–12.81) | 0.07 |
| Na 48 h, in mmol/L (mean ± SD) | 138.4 ± 4.7 | 139.0 ± 4.3 | 1.03 (0.92–1.15) | 0.58 |
| K 48 h, in mmol/L (mean ± SD) | 3.9 ± 0.4 | 3.5 ± 0.6 | 0.21 (0.07–0.61) | 0.004 |
| P 48 h, in mg/dL (mean ± SD) | 3.3 ± 0.7 | 2.8 ± 0.6 | 0.24 (0.10–0.60) | 0.002 |
| Mg 48 h, in mg/dL (mean ± SD) | 1.9 ± 0.3 | 1.9 ± 0.3 | 0.97 (0.21–4.45) | 0.97 |
| Na 5 days, in mmol/L (mean ± SD) | 131.5 ± 3.6 | 138.7 ± 4.1 | 1.05 (0.91–1.22) | 0.49 |
| K 5 days, in mmol/L (mean ± SD) | 3.7 ± 0.4 | 3.6 ± 0.5 | 0.69 (0.17–2.769 | 0.60 |
| P 5 days, in mg/dL (mean ± SD) | 3.4 ± 0.5 | 2.5 ± 0.6 | 0.04 (0.01–0.32) | 0.002 |
| Mg 5 days, in mg/dL (mean ± SD) | 1.8 ± 0.2 | 1.9 ± 0.4 | 2.10 (0.26–16.629 | 0.48 |
| HR (95% CI) | p-Value | |
|---|---|---|
| Male | 0.89 (0.66–1.19) | 0.45 |
| Age | 0.99 (0.98–1.01) | 0.34 |
| ED admission | 0.38 (0.28–0.54) | <0.0001 |
| CCI score | 1.01 (0.95–1.07) | 0.65 |
| Baseline body weight | 1.00 (0.99–1.01) | 0.12 |
| Body height | 1.00 (0.98–1.02) | 0.60 |
| Baseline BMI | 1.02 (0.99–1.05) | 0.08 |
| Baseline NRS-2002 > 3 | 0.67 (0.49–0.92) | 0.01 |
| Baseline MUST ≥ 2 | 0.51 (0.37–0.71) | <0.0001 |
| RS risk | 0.66 (0.50–0.88) | 0.005 |
| High RS risk | 1.02 (0.67–1.53) | 0.92 |
| RS | 0.45 (0.31–0.66) | <0.0001 |
| Nutritional team support | 0.70 (0.43–1.13) | 0.14 |
| Nutritional supplementation within 48 h | 1.34 (0.84–2.16) | 0.21 |
| Oral nutritional supplementation | 1.00 (0.74–1.37) | 0.96 |
| Parenteral nutrition | 0.59 (0.34–1.04) | 0.07 |
| OR (95% CI) | p-Value | |
|---|---|---|
| Male | 0.46 (0.09–2.29) | 0.34 |
| Age | 1.04 (0.98–1.10) | 0.14 |
| ED admission | 3.72 (0.46–30.44) | 0.22 |
| CCI score | 1.12 (0.87–1.45) | 0.34 |
| Weight | 0.95 (0.89–1.01) | 0.06 |
| Height | 1.00 (0.92–1.08) | 0.91 |
| Baseline BMI | 0.82 (0.69–0.97) | 0.02 |
| Baseline NRS-2002 > 3 | 2.48 (0.64–9.55) | 0.18 |
| Baseline MUST ≥ 2 | 0.37 (0.05–3.17) | 0.37 |
| RS | 10.1 (2.4–42.6) | 0.002 |
| Nutrition team support | 0.83 (0.16–4.30) | 0.82 |
| Nutritional supplementation within 48 h | 0.12 (0.02–0.56) | 0.006 |
| Oral nutritional supplementation | 0.36 (0.03–2.17) | 0.21 |
| Parenteral nutrition | 4.75 (0.89–25.6) | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinninella, E.; D’Angelo, M.; Borriello, R.; Galasso, T.; Cintoni, M.; Raoul, P.; Impagnatiello, M.; Annicchiarico, B.E.; Gasbarrini, A.; Mele, M.C. Incidence and Impact of Refeeding Syndrome in an Internal Medicine and Gastroenterology Ward of an Italian Tertiary Referral Center: A Prospective Cohort Study. Nutrients 2022, 14, 1343. https://doi.org/10.3390/nu14071343
Rinninella E, D’Angelo M, Borriello R, Galasso T, Cintoni M, Raoul P, Impagnatiello M, Annicchiarico BE, Gasbarrini A, Mele MC. Incidence and Impact of Refeeding Syndrome in an Internal Medicine and Gastroenterology Ward of an Italian Tertiary Referral Center: A Prospective Cohort Study. Nutrients. 2022; 14(7):1343. https://doi.org/10.3390/nu14071343
Chicago/Turabian StyleRinninella, Emanuele, Marco D’Angelo, Raffaele Borriello, Tiziano Galasso, Marco Cintoni, Pauline Raoul, Michele Impagnatiello, Brigida Eleonora Annicchiarico, Antonio Gasbarrini, and Maria Cristina Mele. 2022. "Incidence and Impact of Refeeding Syndrome in an Internal Medicine and Gastroenterology Ward of an Italian Tertiary Referral Center: A Prospective Cohort Study" Nutrients 14, no. 7: 1343. https://doi.org/10.3390/nu14071343
APA StyleRinninella, E., D’Angelo, M., Borriello, R., Galasso, T., Cintoni, M., Raoul, P., Impagnatiello, M., Annicchiarico, B. E., Gasbarrini, A., & Mele, M. C. (2022). Incidence and Impact of Refeeding Syndrome in an Internal Medicine and Gastroenterology Ward of an Italian Tertiary Referral Center: A Prospective Cohort Study. Nutrients, 14(7), 1343. https://doi.org/10.3390/nu14071343